ABSTRACT
Introduction: Pneumococcal diseases caused by Streptococcus pneumoniae represent a significant health and economic burden. Mexico has benefited from the inclusion of the 7-valent (PCV7) and 13-valent pneumococcal conjugate vaccines (PCV13) since their inclusion in the National Immunization Program (NIP) in 2006 and 2010, respectively. The objective of this study is to estimate the impact of the existing program and predict future implications of a change in the current program.
Methods: A previously published model was updated to estimate the historic impact of the PCV programs relative to pre-PCV implementation. Future disease trends were forecasted based on historical serotype behaviors for each PCV13 serotype and non-vaccine serotypes across different age groups. Costs and outcomes were estimated over a 10-year period based on continued use of PCV13 compared to a switch to PCV10.
Results: The PCV7 and subsequent PCV13 NIP were estimated to prevent over 1.5 million cases of pneumococcal disease and 1,854 deaths, corresponding to a net savings of $34.50 Billion MXN. Continued use of PCV13 was estimated to save over 300 thousand cases of pneumococcal disease and 373 deaths compared to switching to PCV10 over a 10-year period. Despite a higher vaccine cost, maintaining PCV13 was cost-saving compared to PCV10, saving $6.71 billion MXN over 10 years.
Conclusion: The PCV program in Mexico has provided a significant return on investment. Sustained PCV13 use was estimated to provide the greatest healthcare and economic impact in Mexico. Changes to the pneumococcal vaccination program could result in serotype replacement and reduction in herd effects.
Introduction
Streptococcus pneumoniae is a gram-positive bacterium with more than 90 serotypes and is a pathogen known to cause invasive pneumococcal disease (IPD) such as bacteremia, and meningitis, as well as non-invasive infections such as pneumonia and otitis media (OM). Since the early 2000s, pneumococcal vaccines, in which capsular polysaccharides are conjugated to carrier proteins (PCVs), have been used in children to enhance immunogenicity. Pneumococcal conjugate vaccines (PCVs) containing 7 (PCV7, Prevnar®, Wyeth Lederle Vaccines), 10 (PCV10, Synflorix®, GlaxoSmithKline Biologicals S.A.), and 13 (PCV13, Prevnar 13®, Wyeth/Pfizer Vaccines) pneumococcal polysaccharide antigens have been licensed and implemented in routine vaccination programs across the world (Supplementary Material S1). The use of these PCVs has substantially reduced the burden of vaccine-type pneumococcal disease in both vaccinated and unvaccinated populations through robust herd effects.Citation1–Citation3
In 2006 the vaccine was introduced as a pilot vaccination strategy for some of the poorest regions in Mexico, expanding the program to a full National Immunization Program (NIP) in 2008 using a 2 + 1 schedule. In 2010, a switch to a higher valent PCV was made gradually. By the end of 2011, all children less than 2 years of age in Mexico were receiving PCV13 through the NIP. A study based on the National Health and Nutrition Survey in 2012Citation4 indicated that full schedule coverage for PCV13 was 80.8% for children below one year of age and 88% for children 15–23 months old.Citation5 The introduction of pneumococcal conjugate vaccines in the NIP in Mexico has been very successful in reducing the burden of disease, especially in children under 5 years of age. Decreased incidence of all-cause pneumonia (60.5% reduction), all-cause meningitis (59%) and OM (49%) were observed over a 10 year period.Citation6,Citation7
Even though immunization programs have proven to reduce the incidence of disease, they represent a significant budget impact for the government. For that reason, vaccine program funding bodies continuously look for ways to reduce expenditures. Currently, there are two pneumococcal conjugate vaccines registered in Mexico: PCV13 and PCV10. PCV13 has been in the NIP for almost 7 years, while PCV10 is available in the private market. While a change of the vaccine included in the NIP may result in lower vaccine acquisition costs, PCV10 covers fewer serotypes which could lead to disease re-emergence of unprotected serotypes and result in an overall increase in incidence. This combined with lower herd effects could result in an additional overall net cost to the health system.
Several studies have evaluated the cost-effectiveness of pneumococcal vaccines in Mexico, but none of them have considered the economic impact that a switch on the immunization program strategy from a higher to a lower valent vaccine would have.Citation8,Citation9 The aim of this study was to estimate the retrospective public health and economic impact that pneumococcal vaccination with PCVs has had in Mexico from 2006 to 2014, the last year complete data were available, and to prospectively estimate the potential public health and economic impact of changing vaccine programs from a higher to lower valent vaccine.
Results
Retrospective analysis
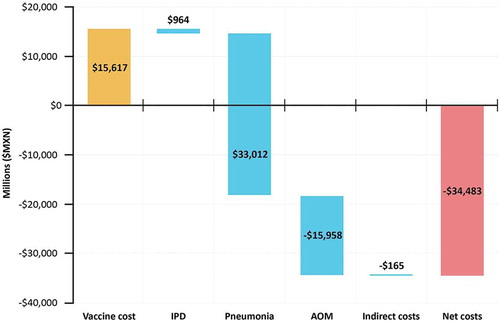
Results of the retrospective analysis demonstrated that between 2006 and 2014, the PCV7 and subsequent PCV13 program, averted over 1.5 million cases of pneumococcal disease, and prevented 1,840 deaths over a nine-year period (). This corresponded to a net cost-savings of $34.5 Billion MXN (). Therefore, over the nine-year period where Mexico had a PCV7 and PCV13 program, there was a $2.21 MXN return on investment for each MXN invested in the PCV program.
Figure 1. Historic cost of PCV program and cost-savings from cases of disease averted (millions of $MXN). summarizes the historic vaccine investment costs and the cost savings from observed reductions in IPD, Pneumonia, OM, and indirect costs between 2006 and 2014 in Mexico.
Results are presented in $MXN ($19.7 MXN = $1 USD)OM = Otitis Media; IPD = invasive pneumococcal disease
Prospective analysis
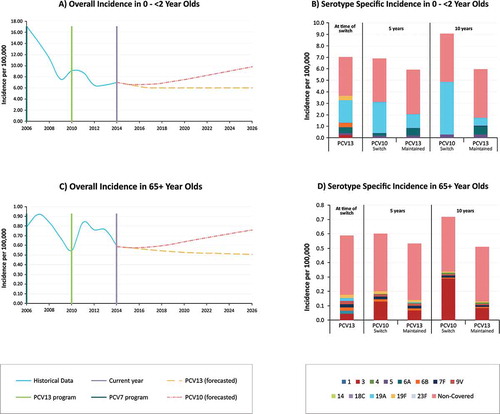
In 2014, the most recent data that was available for the model, incidence of disease caused by PCV13 serotypes was 3.7 per 100,000 in children < 2 year old and represented 52% of the total burden of IPD. Based on our forecasting model, after 10 years, in children < 2 years old PCV13-type IPD was estimated to increase to 4.9 per 100,000 when switching to PCV10, representing 54% of the total IPD burden, but was estimated to drop to 1.7 per 100,000 while maintaining PCV13 ( and ). While serotype replacement of non-vaccine type disease occurred in the PCV13 estimation, this was smaller than the PCV13-10 type (serotypes 3, 6A, and 19A) replacement in the PCV10 scenario, specifically due to serotype 19A. After 10 years, incidence of IPD in < 2 year olds caused by any serotype was estimated at 9.1 and 6.0 per 100,000 with PCV10 and PCV13, respectively. A similar trend was observed in other age groups, with a higher level of replacement from serotype 3 observed in 65+ in the PCV10 arm compared to the PCV13 arm ( and ).
Figure 2. Forecasted incidence of invasive pneumococcal disease in 0–2 and 65 year Olds. presents the (A) forecasted all-cause IPD in 0–2 year olds using trend line estimates from Mexico, (B) the incidence of IPD in 0–2 year olds by serotype at the time of switch, and 5 and 10 years post switch with PCV10 or PCV13,(C) the forecasted all-cause IPD in 65+ year olds using trend line estimates from Mexico, (D) and the incidence of IPD in 65+ year olds by serotype at the time of switch, and 5 and 10 years post switch with PCV10 or PCV13.
Based on the most recent incidence of pneumococcal disease, in the hypothetical scenario where Mexico switched to a PCV10 vaccination program, over the next 10 years there would be an estimated 310,034 additional cases of pneumococcal disease and 373 additional deaths compared to maintaining PCV13 (). Maintaining use of PCV13 in Mexico was estimated to be a cost-saving strategy compared to switching to PCV10. Despite the higher vaccine cost, maintained use of PCV13 was estimated to save the Mexican government over $6.71 Billion MXN over a 10 year period due to greater disease averted.
Table 1. Historic impact of pneumococcal vaccination programs.
Table 2. Prospective impact of maintaining PCV13 versus switching to PCV10 over 10 years.
Sensitivity analysis
PCV13 remained cost saving compared to PCV10 across a number of scenario analyses (). These included: varying the trend line estimates used to forecast vaccine impact given experiences in different countries, changing the time horizon (5 and 20 years), lengthening the time before serotype replacement began with PCV10 (2 and 3 years), and reducing the total level of replacement that occurs (See ). In only one scenario was PCV13 more costly than PCV10 (assuming trend lines from the UK for PCV13 and the Netherlands for PCV10). However, in this scenario, PCV13 remained highly cost-effective. Results were also robust in one-way and probabilistic sensitivity analyses, as PCV13 remained cost-saving in all individual parameter variation and in 100% of simulations (Supplementary Material S3).
Table 3. Scenario analyses of maintaining PCV13 compared to switching to PCV10.
Discussion
This study highlights the significant health benefit that PCV7 and PCV13 have provided in Mexico. Our results indicate that over an 9 year period, PCV7 and PCV13 have saved 1,840 lives and provided an overall net savings to the Mexican Health System of approximately $34.5 Billion MXN. Despite a lower vaccine acquisition cost, changing the NIP to PCV10 was estimated to cause a higher burden of disease in Mexico due to serotype replacement, specifically due to higher circulation of non-covered serotypes such as serotypes 3 and 19A. The health resource utilization associated with these additional disease cases negated the savings from a lower priced vaccine, therefore maintaining PCV13 in the NIP was estimated to result in better health outcomes at a lower cost to the Mexican health system. This result was consistent across all sensitivity analyses, and PCV13 remained cost-effective or cost-saving in all scenario analyses.
This analysis is important for decision makers given that the majority of disease replacement was estimated to be caused by serotype 19A. This outcome has been seen in a number of settings which use PCV10 as the vaccine does not provide protection against 19A carriage.Citation10 For example, Belgium switched from PCV13 to PCV10 between 2015 and 2016, and has since seen an increase in cases of 19A from 2 cases in 2016 to 21 cases in 2017, suggesting our results may underestimate potential replacement.Citation11 This is important because disease caused by serotype 19A has often been shown to be more invasive and highly resistant to antibiotics.Citation12,Citation13 Given the increasing risk of antibiotic resistance, using a vaccine with greater serotype coverage is paramount to ensure the broadest protection of infants in Mexico reducing overall antibiotic use and associated costs of resistant episodes. While some early case-control studies did demonstrate that PCV10 may have cross-protection against 19A,Citation14,Citation15 more recent studies have shown that cases of 19A have been increasing in both vaccinated and unvaccinated individuals in countries using PCV10.Citation10,Citation16,Citation17 Our analysis mitigates for any uncertainty around cross-protection by using data from Finland and the Netherlands, countries that use PCV10, in sensitivity analysis. These scenarios thus would capture any observed cross protection. Despite this, PCV13 remained cost-saving across the majority of scenarios.
Previous economic evaluations of PCVs in Mexico have a number of important limitations that this analysis improves upon.Citation8,Citation9 An early study by Mucino-Ortega and colleagues (2010) determined PCV13 was cost-saving compared to PCV10. However this analysis did not consider the impact of herd effects.Citation8 Furthermore this study evaluated the impact of replacing PCV7 with a higher valent PCV program (PCV10 or PCV13) rather than switching from a higher to lower-valent vaccine as was done in this analysis.
A more recent analysis by Gomez and colleagues (2016) determined that PCV10 would be the cost-saving option compared to PCV13.Citation9 The results from this study however were driven entirely by the benefits of PCV10 preventing OM caused by non-typable Haemophillus influenza (NTHi) based on evidence from an investigational PCV11 vaccine. However, recent studies have shown PCV10 did not demonstrate any statistically significant effect on NTHi-clinically confirmed OM.Citation18,Citation19 This study also did not fully account for the benefits of PCV13 on serotype 3 and assumed cross- protection for PCV10 protecting against IPD and OM caused by serotype 6A and 19A. These assumptions are not consistent with the most up to date literatureCitation20 and so the results should be interpreted with caution.
Both of these cost-effectiveness analyses in Mexico have also underestimated the potential impact of serotype replacement and indirect effects due to the static nature of their design.Citation8,Citation9 They also rely on assumptions around the efficacy of specific serotypes and cross-reactivity. The study presented here improves upon previous estimates by taking into consideration the potential for serotype replacement due to changes in vaccine pressure as well as any potential cross-reactivity by using real world data as opposed to data from case control studies which may not reflect how serotypes behave in the real world.Citation15,Citation21 This has been shown to be of significant importance, as examples exist around the world where reduction of vaccine pressure of important serotypes such as 19A results in increases in disease in both vaccinated and unvaccinated populations.Citation10 Similarly, recent studies in Spain have shown rapid return of both invasive and non-invasive disease when vaccine pressure is reduced,Citation22,Citation23 further justifying the need to maintain sustained high levels of uptake with the broadest coverage.
As with any modelling exercise, the model is subject to some limitations. The most important assumption is that the historical serotype trends determine disease incidence prospectively. In other words, we assumed that disease will behave similarly to what was observed in the past. This assumption has the benefit of being a conceptually simple and intuitive approach, but it is subject to limitations. Furthermore, because IPD is relatively rare, in most cases the predictive trends were estimated using small sample sizes and therefore could be over or under estimated. These trends in Mexico are also limited in that they rely on passive surveillance data and that, depending on where isolates originated, may over or underestimate the historic serotype distribution.Citation24–Citation28 This could be a reason for 52% vaccine type disease in the baseline year of this analysis, therefore potentially overestimating future disease impact if vaccine type disease were actually lower. However, this would reduce the impact and replacement for both vaccines and thus would unlikely change the incremental difference significantly.
Second, the model does not allow trends in serotype emergence and reductions to vary over time. It is possible that disease could behave differently over different periods of time when vaccination uptake changes, or due to epidemiologic shifts. For example, following the introduction of PCV7, replacement with 19A was observed given the opening of the pneumococcal niche to other serotypes. Trends developed immediately after the introduction of PCV7 would not have predicted emergence of 19A. Comparably, the model is unable to account for the possible emergence of a serotype that has not yet started to increase. To date, no serotype(s) have emerged in the several years following introduction of PCV13 in Mexico in the way that 19A did after the introduction of PCV7. Thus, this limitation may not have substantial impact for several more years. However, as more data become available on emerging serotypes, these could be modelled separately.
Third, our model assumes that the proportion of pneumococcal OM and pneumococcal pneumonia change proportionally with IPD . This methodology may over or underestimate the impact on OM and pneumonia if the serotypes causing OM and pneumonia are not consistent with IPD; however it has been used in several studies to extrapolate non-invasive disease outcomes associated with PCV introduction.Citation29–Citation32 Previous economic evaluations have instead used clinical effectiveness data from PCV10 and PCV13 given that both vaccines have demonstrated an impact on all-cause OM and all-cause pneumonia.Citation33–Citation38 However, translating these vaccine effectiveness parameters from other countries to Mexico may be more vulnerable to overestimation given differences in underlying epidemiology, vaccine uptake, and antibiotic use.Citation20 Therefore our proportional approach may be conservative in that it only accounts for changes in disease caused by S. pneumoniae.
Finally, as of 2018, Belgium is the only example of a switch from a higher to lower valent vaccine.Citation11 Historical vaccine use, antibiotic prescriptions, vaccine uptake, schedules, and catch-up vaccination policy choices all may have an impact on both the time to and extent of serotype replacement. We have attempted to mitigate this by evaluating several trend lines and how these might impact disease re-emergence. Further we test a number of constraints and limitations on potential serotype replacement. However, further research is necessary to understand the potential parameters that may influence changes in serotype replacement. The time to and degree of disease re-emergence may vary in each country depending on a number of factors.
Conclusion
This modelling exercise shows the public health and economic impact the introduction of PCVs has had in Mexico. It is the first evaluation in Mexico to estimate the impact of serotype replacement when considering a change in pneumococcal vaccination allowing decision makers to understand what may happen when changing from a higher to lower valent vaccine. This type of model has not been used in the past in Mexico but can help in providing the scenarios that could be faced if considering a change in the NIP. Previous steady state models did not fully account for the impact of changes in serotype dynamics, therefore underestimate the potential risks involved in changing vaccination policy. This is important, as past experience has shown that removal of vaccine pressure has severe effects on both vaccinated and unvaccinated populations. Specifically, serotypes 3, 6A, and 19A remain a burden in Mexico; therefore continued vaccination with PCV13 will continue to provide an important public health benefit. As non-vaccine serotypes continue to increase, it will be essential to protect populations against all pneumococcal serotypes with higher valent PCVs.
Methods
Model structure
A published decision analytic model was updated to estimate the historic public health and economic impact of the PCV7 and PCV13 NIP in Mexico, as well as evaluating a potential change from the current immunization program using PCV13 to using PCV10.Citation29 The structure is therefore split in two parts: a retrospective impact analysis and a prospective forecasting model.
In the retrospective analysis, we estimated the total impact of pneumococcal vaccination compared with a hypothetical scenario in which no vaccine program existed. To do this, the number of cases of IPD, pneumonia, and OM as observed during the retrospective period using real world data (described below) were estimated. The observed cases were compared to an estimated incidence of disease based on pre-PCV incidence. The cumulative vaccine impact was estimated based on the resulting difference between pre-PCV incidence carried forward and the observed incidence in the presence of PCVs. To ensure we do not overestimate the historic impact of the vaccines, and that some change is due to natural fluctuation, we assume that only the 8.2% of pneumonia cases caused by S. pneumoniae are due to the introduction of PCVs (discussed below).Citation24
The prospective forecasting model used historic real world passive surveillance data on IPD in Mexico to estimate the potential future disease scenarios with either PCV13 or PCV10. For each serotype contained within PCV13, a trend was estimated based on the historical behavior when it was covered or uncovered by a vaccine (Supplementary Material S2). A similar approach has been proposed in the literature to examine changes in serotype dynamics in CanadaCitation39 and the Netherlands.Citation31 In Mexico, these trends were estimated between 2006 to 2010 for PCV7 serotypes, and 2010 to 2014 for PCV13 serotypes. Because PCV10 does not contain the 3 additional serotypes in PCV13 (3, 6A, and 19A), in the base case we assumed that these serotypes behaved as they did prior to the introduction of PCV13 (2006–2010) in the event of a switch to PCV10. Given these forecasts, the incidence of IPD, pneumococcal pneumonia, and pneumococcal OM were estimated for a 10 year time horizon into the future maintaining PCV13 or switching to PCV10. It was assumed that serotype replacement would not begin until 1 year following the switch of a program given historic PCV13 use and remaining stock of the vaccine. Total life years, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios were estimated.
Population
According to Consejo Nacional de Población (CONAPO), in 2014 the Mexican population was 120,285,089, with a birth cohort of 4,425,280 eligible for pneumococcal vaccination (see ).Citation40 Vaccine coverage was assumed for 85% of infants using a 3-dose (2 + 1) vaccination schedule. The remaining individuals comprised the non-vaccinated cohorts. The population was stratified into 6 age groups: 0-< 5 years, 5–17 years, 18–34 years, 35–49 years, 50–64 years, and 65+ years.
Epidemiology
Ipd
Incidence of IPD was retrieved from data published by the Epidemiological Surveillance System Platform (SUIVECitation7 for the years 2004 to 2014 (See ). This incidence data were weighted based on the serotype distribution data provided by SIREVA to estimate the trends of both covered and uncovered serotypes for PCV10 and PCV13 forecasts.Citation24–Citation28
Table 4. Population and economic parameters.
Table 5. Epidemiologic parameters (per 100,000 population).
IPD surveillance data included all invasive disease and the model considered IPD as combination of meningitis and bacteremia. To estimate the economic and clinical impact of IPD, the proportion of IPD due to meningitis was estimated based on the average number of cases reported during 2011 to 2014 from the SIREVA (Secretaría de Salud) passive surveillance system.Citation24–Citation28
Pneumonia
The incidence of all-cause inpatient pneumonia was obtained from the Epidemiological Surveillance System Platform (SUIVE.Citation7 Due to a lack of data on non-hospitalized pneumonia in Mexico, cases were estimated to occur relative to hospitalized pneumonia at a rate of 7:1 based on data used in other studies.Citation8
Assuming the ratios of all-cause inpatient pneumonia to IPD and outpatient pneumonia to IPD were constant over time, we estimated the prospective change in the number of pneumococcal hospitalized and non-hospitalized pneumonia cases by multiplying these ratios by the forecasted number of IPD cases each year. Rates of pneumococcal pneumonia were assumed to change proportionally to IPD based on the assumption that comparable serotypes would cause both invasive and non-invasive disease. This assumption has been used in previous evaluations and documented in the literature.Citation30–Citation32 The proportion of all-cause pneumonia that was assumed to be pneumococcal was estimated at 19% for the retrospective analysis and 8.2% in the most recent year based on data from SIREVA.Citation24 The historic percentage was used as a weight to estimate the proportion of pneumonia that was assumed to decrease due to PCV implementation. The direct and indirect effects of vaccination are implicitly considered in the serotype data for the 0-< 5 age group.
Otitis media
Incidence rates of OM were retrieved from the Epidemiological Surveillance System Platform (SUIVE).Citation7 Because no serotype specific data for OM was available in Mexico, rates of pneumococcal OM were assumed to proportionally change relative to IPD similar to pneumonia. Historically, all changes in OM incidence were assumed to be due to PCV implementation.
Mortality
A general risk of mortality was assumed for the entire population based on published estimates ().Citation41 For each case of disease, there is a risk of mortality based on case fatality rates. These rates were sourced from the published literature for meningitis, bacteremia, and hospitalized pneumonia.Citation42–Citation45 No mortality risk was included for non-hospitalized pneumonia and OM.
Disease sequelae
As a result of contracting pneumococcal disease, clinical sequelae such as neurologic impairment and hearing loss may occur. For meningitis, we assumed hearing loss and neurological impairment for 13% and 7% of patients, respectively.Citation46,Citation47 For OM, 5% of patients were assumed to require myringotomy procedures.Citation47 Sequelae of bacteremia or pneumonia were not considered in this analysis.
Costs
Mexican vaccine procurement requires confidential pricing, therefore prices for PCV10 ($12.85 USD) and PCV13 ($14.50 USD) were derived from published Pan American Health Organization (PAHO) Expanded Program of Immunization Vaccine Prices for 2017.Citation48
Direct medical costs were considered from the perspective of the public Mexican health system using clinical tabulators costs published by the Instituto Mexicano de Seguro Social (IMSS), a social security health system, in Mexico in 2014 (the latest edition.Citation49 These lists include the average fees that a patient could go through per disease case, such as, laboratory analyses, hospital stay, specialists’ visits, nurse fees, etc. The last version was published on 2014, therefore the amounts where inflated to 2018 dollars.Citation49 Indirect costs were estimated based on the numbers of hours productivity lost for each case of disease multiplied by the estimated average hourly wage of a working adult ($18.70 MXN).Citation50
Utility inputs
Utility weights range from 0.0 to 1.0, where 1.0 represents perfect health and 0.0 represents death. We assumed an age-specific baseline utility weight for individuals in each age group who do not experience a case of pneumococcal disease.Citation51 Utility decrements were applied for each occurrence of disease as well as long term sequelae. Specifically, decrements of 0.0070 and 0.0232 were assumed for a case of bacteremia and meningitis respectivelyCitation52 . Decrements of 0.0050, 0.0040, and 0.0060 were assumed for OM, inpatient pneumonia, and outpatient pneumonia respectively.Citation53 Finally, a lifetime decrement of 0.40 and 0.20 were included for neurological impairment or hearing loss, respectively.Citation47
Sensitivity analysis
To test the robustness of results, several sensitivity analyses and scenarios were tested. First, given the variation in PCV implementation and the potential variation in trend line estimations, trend forecasts were applied from countries with established PCV programs and robust surveillance. In these analyses, disease cases were forecasted based on historical surveillance data from the United Kingdom and the United States in which PCV13 is used in the infant immunization program following PCV7 in a 2 + 1 and 3 + 1 schedule, respectively, as well as the Netherlands and Finland, in which PCV10 is used in the infant vaccination program, both in a 2 + 1 schedule.Citation1,Citation2,Citation54–Citation56 These scenarios were meant to reflect potentially varying serotype dynamics under different situations to ascertain the robustness of serotype forecasts. For example, any potential cross-protection or changes in indirect effect that could be observed with either vaccine would be captured within these trend lines. Since Mexico has never used PCV10 in the NIP, trends observed from the Netherlands and Finland were of specific importance to extrapolate the impact of the vaccine. Additional scenarios were also tested varying the time horizon and the impact of a lag in serotype re-emergence.
In addition to scenario analyses, one-way, and probabilistic sensitivity analyses (PSA) (second order monte-Carlo simulations with 10,000 simulations) were undertaken to evaluate specific parameter uncertainty. These results are included in the Supplementary Material.
Disclosure of potential conflicts of interest
MWasserman, MGP, AGG, and RF are employees of Pfizer Inc. MWilson and CM are employees of RTI health solutions which recieved consulting fees from Pfizer Inc. FBB was an employee of Pfizer Inc. when this study was conducted but is no longer an employee.
Supplemental Material
Download MS Word (128.1 KB)Additional information
Funding
References
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. The Lancet Infectious Diseases; 2018.
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. The Lancet Global Health. 2017;5(1):e51–e59. doi:10.1016/S2214-109X(16)30306-0.
- Gutiérrez JP R-DJ, Shamah-Levy T, Villalpando-Hernández S, Franco A, Cuevas-Nasu L, and H.-Á.M. Romero-Martínez M. ENSANUT2012 resultados nacionales.pdf. Editor, 2012 ed.: Instituto Nacional de Salud Pública; 2012.
- Carnalla-Barajas MN, Soto-Noguerón A, Sánchez-Alemán MA, Solórzano-Santos F, Velazquez-Meza ME, Echániz-Aviles G, et al. Changing trends in serotypes of S. pneumoniae isolates causing invasive and non-invasive diseases in unvaccinated population in Mexico (2000-2014). Int J Infect Dis. 2017;58:1–7. doi:10.1016/j.ijid.2017.02.005.
- Palacios MG, Cané AG,A, Curcio D. Changes In Childhood Meningitis, Pneumonia And Acute Otitis Media After Universal Vaccination With Pneumococcal Conjugate Vaccines In Mexico (2004-2014). In: International Society of Pneumococci and Pneumococcal Diseases. Scotland: Glasgow; 2016.
- Epidemiología DGD Anuario de Morbilidad 1984-2016. 1984-2016. 2017. Available From: http://www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html.
- Mucino-Ortega E, Mould-Quevedo JF, Farkouh R, Strutton D. [Economic evaluation of an infant immunization program in Mexico, based on 13-valent pneumococcal conjugated vaccines]. Value Health. 2011;14(5 Suppl 1):S65–70. doi:10.1016/j.jval.2011.05.025.
- Gómez JA, Villaseñor-Sierra A, Aguilar GM, Manjarrez RC, Cervantes-Apolinar MY. Estimación de la relación costo-efectividad de las vacunas neumocócicas conjugadas Prevenar-13 y Synflorix®, utilizadas en los programas de vacunación de población infantil Mexicana. Value in Health Regional Issues. 2016;11:76–84. doi:10.1016/j.vhri.2016.07.009.
- Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert R-R, Jodar L. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines. 2017;16(10):1007–1027. doi:10.1080/14760584.2017.1362339.
- Desmet S, Verhaegen J, Van Ranst M, Peetermans W, Lagrou K. Switch in a childhood pneumococcal vaccination programme from PCV13 to PCV10: a defendable approach?. Lancet Infect Dis. 2018. DOI:10.1016/S1473-3099(18)30346-3.
- Janoir C, Lepoutre A, Gutmann L, Varon E. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. Open Forum Infect Dis. 2016;3(1):ofw020. doi:10.1093/ofid/ofw020.
- Castañeda E, Agudelo CI, De Antonio R, Rosselli D, Calderón C, Ortega-Barria E, et al. Streptococcus pneumoniae serotype 19A in Latin America and the Caribbean: a systematic review and meta-analysis, 1990–2010. BMC Infect Dis. 2012;12(1):124. doi:10.1186/1471-2334-12-166.
- Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2+ 1 infant schedule in Quebec, Canada. Pediatr Infect Dis J. 2010;29(6):546–549. doi:10.1097/INF.0b013e3181cffa2a.
- Domingues CMAS, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2(6):464–471. doi:10.1016/S2213-2600(14)70060-8.
- Brandileone M-CC, Almeida SC, Minamisava R, Andrade A-L. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018;36(19):2559–2566. doi:10.1016/j.vaccine.2018.04.010.
- Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, et al. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–1940. doi:10.1016/j.vaccine.2018.03.001.
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: A double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657. doi:10.1371/journal.pmed.1001657.
- Moffatt M, Sings H, Hilton B, Wasserman M, Farkouh R, editors. A review of higher-valent pneumococcal conjugate vaccine (PCV) impact on acute otitis media (AOM) and nasopharyngeal carriage (NPC) due to nontypeable haemophilus influenzae (NTHI). In: European Society for Paediatric Infectious Diseases (ESPID) 2017; Madrid (Spain): ESPID. p. 23-27.
- Wasserman M, Sings HL, Jones D, Pugh S, Moffatt M, Farkouh R. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17(1):71–78. doi:10.1080/14760584.2018.1409116.
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, et al. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children–a population-based study. PLoS One. 2015;10(3):e0120290. doi:10.1371/journal.pone.0120290.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Negreira S, Baquero F, Hernández-Sampelayo T, et al. Effect of the different 13-valent pneumococcal conjugate vaccination uptakes on the invasive pneumococcal disease in children: analysis of a hospital-based and population-based surveillance study in Madrid, Spain, 2007-2015. PloS One. 2017;12(2):e0172222. doi:10.1371/journal.pone.0172222.
- Tagarro A, Benito A, Sanchez A, Aznar E, Otheo E, Sanz-Rosa D, et al. Bacteremic pneumonia before and after withdrawal of 13-valent pneumococcal conjugate vaccine from a public vaccination program in spain: a case-control study. J Pediatr. 2016;171:111–115. e1-3. doi:10.1016/j.jpeds.2015.12.031.
- Organización Panamericana de la Salud O. Informe Regional SIREVA II 2011: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Washington DC: Organización Panamericana de la Salud, OMS, 2011. Available at: https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html
- Organización Panamericana de la Salud O. Informe Regional SIREVA II 2012: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores. Washington DC: 2012. Available at: https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html
- Instituto Nacional de Salud Pública SdS. Reporte de serotipos y susceptibilidad antimicrobiana de S. pneumoniae y H. influenzae. Cuernavaca, Morelos: Secretaría de Salud, 2013. Available at: https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html
- Instituto Nacional de Salud Pública SdS. Reporte de serotipos y susceptibilidad antimicrobiana de S. pneumoniae y H. influenzae. Cuernavaca, Morelos: Secretaría de Salud, 2014. Available at: https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html
- Instituto Nacional de Salud Pública SdS. Reporte de serotipos y susceptibilidad antimicrobiana de S. pneumoniae, H. influenzae y S. aureus. Cuernavaca, Morelos, México: Secretaría de Salud, 2015 Contract No.: 2015. Available at: https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html
- Wilson M, Wasserman M, Jadavi T, Postma M, Breton M-C, Peloquin F, et al. Clinical and Economic Impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infectious Diseases and Therapy; Chester (United Kingdom), 2018.
- van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–7213. doi:10.1016/j.vaccine.2012.10.017.
- Thorrington D, van Rossum L, Knol M, de Melker H, Rümke H, Hak E, et al. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PloS One. 2018;13(2):e0192640. doi:10.1371/journal.pone.0192640.
- Thorrington D, Andrews N, Stowe J, Miller E, van Hoek AJ. Elucidating the impact of the pneumococcal conjugate vaccine programme on pneumonia, sepsis and otitis media hospital admissions in England using a composite control. BMC Med. 2018;16(1):13. doi:10.1186/s12916-018-1004-z.
- Lewnard JA, Givon-Lavi N, Weinberger DM, Lipsitch M, Dagan R. Pan-serotype reduction in progression of Streptococcus pneumoniae to otitis media after rollout of pneumococcal conjugate vaccines. Clin Infect Dis. 2017;65(11):1853–1861. doi:10.1093/cid/cix673.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611–618. doi:10.1093/cid/ciw347.
- Lau WC, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–5079. doi:10.1016/j.vaccine.2015.08.022.
- Sigurdsson S, Eythorsson E, Hrafnkelsson B, Erlendsdóttir H, Kristinsson KG, Haraldsson Á. Reduction in all-cause acute otitis media in children< 3 years of age in primary care following vaccination with 10-valent pneumococcal haemophilus influenzae protein-d conjugate vaccine: a whole-population study. Oxford (UK): Clinical Infectious Diseases. 2018. p. ciy233.
- Griffin MR, Mitchel E, Moore MR, Whitney CG, Grijalva CG, Centers for Disease C, et al. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines - Tennessee, 1998-2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):995–998.
- Becker-Dreps S, Amaya E, Liu L, Moreno G, Rocha J, Briceno R, et al. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J. 2014;33(6):637–642.
- Zhou Z, Deceuninck G, Lefebvre B, De Wals P. Forecasting trends in invasive pneumococcal disease among elderly adults in Quebec. Can J Infect Dis Med Microbiol. 2017;2017.
- CONAPO. Consejo nacional de población: 2004-2014. Available from: https://www.gob.mx/conapo.
- Instituto Nacional de Estadísticas Geografía e Informática [INEGI] (1986). Estadísticas Históricas de México Tomo I.: http://www.beta.inegi.org.mx/app/biblioteca/ficha.html?upc=702825460238, p. 145
- Buzzo AR, Roberts C, Mollinedo LG, Quevedo JM, Casas GL, Soldevilla JMS. Morbidity and mortality of pneumonia in adults in six Latin American countries. Int J Infect Dis. 2013;17(9). e673-e677 doi:10.1016/j.ijid.2013.02.015.
- Valenzuela MT, O'Loughlin R, De La Hoz F, Gomez E, Constenla D, Sinha A, et al. The burden of pneumococcal disease among Latin American and Caribbean children: review of the evidence. Revista Panamericana De Salud Pública. 2009;25:270–279.
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998–2007. New England J Med. 2011;364(21):2016–2025. doi:10.1056/NEJMoa1005384.
- Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. Jama. 2008;299(17):2048–2055. doi:10.1001/jama.299.17.2048.
- Mclntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, et al. Dexamethasone as adjunctive therapy in bacterial meningitis: a meta-analysis of randomized clinical trials since 1988. Jama. 1997;278(11):925–931.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–127.
- Organization PAH. Expanded program of immunization vaccine prices for year 2017. Washington DC (USA): Pan American Health Organization; 2017.
- Arroyave Loaiza María Gilma, Aburto Mejía Rocío. Grupos Relacionados con el Diagnóstico: Producto Hospitalario. GRD-IMSS: 2014. ISBN: 978-607-9464-50-9.
- SNTSS. Sindicato nacional de trabajadores del seguro social. 2017; Available from: http://www.sntss.org.mx/phocadownload/Comunicados/tabulador.pdf.
- Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics. 1999;15(4):369–376.
- Bennett JE, Sumner W, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–48.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–4214. doi:10.1016/j.vaccine.2004.05.003.
- Knol MJ, Berbers GA, Bootsma H, van der Ende A, Kaaijk P, Rots N, et al. 7.9 Pneumococcal disease. In: The national immunisation programme in the netherlands surveillance and developments in 2015-2016. 2016. cited 2017 26 February RIVM Report 2016-0141:[Available from http://www.rivm.nl/bibliotheek/rapporten/2016-0141.pdf.
- Knol MJ, de Melker HE, Sanders EAM, van der Ende A, editors. Incidence of IPD in the Netherlands up to five years after introduction of PCV10. Bilthoven (Netherlands): National Institute for Public Health and the Environment; 2016.
- National Institute for Health and Welfare Incidence of invasive pneumococcal disease in Finland. 2016 2016 Jun 23; Available from: https://www.thl.fi/en/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland.
