ABSTRACT
Increased measles immunization has led to a significant decline in measles incidence and mortality. During 2016 it is estimated that fewer than 100,000 died from measles for the first time in recorded history. In highly immunized countries measles epidemiology has changed. Threats to national elimination goals and public health include aging cohorts of naïve people that exist from imperfect vaccination rates during the early years of immunization programs. This may be complemented by some loss of immunity in vaccinated populations. While childhood immunization must remain a focus for control efforts, due to higher mortality in the very young, these naïve adolescents and adults also accumulate as they age and add to the pool of susceptible people, perhaps beyond the view of those that are focused on childhood immunization. Here, features of measles epidemiology and control in highly immunized populations are reviewed, providing global data where necessary, to highlight why countries with high immunization coverage are still threatened by measles outbreaks and how changing dynamics may alter disease control.
Introduction
The measles virus is one of the most infectious pathogens known to man. In the pre-vaccination era measles infected more than 90% of children before they reached 15 years old, causing more than two million deaths.Citation1 In 2016 measles was estimated to infect fewer than 7 million people globally and kill less than 90,000 people, mostly children.Citation2 These deaths are entirely preventable through immunization. Immunization has caused the decline in measles in 2000 from 145 to 19 cases per million people in 2016. During the same period the estimated deaths declined correspondingly from 550,100 to 89,780, dropping below 100,000 deaths per annum for the first time since records began.Citation2 Measles immunization is estimated to have prevented 20.4 million deaths in this 16-year period.
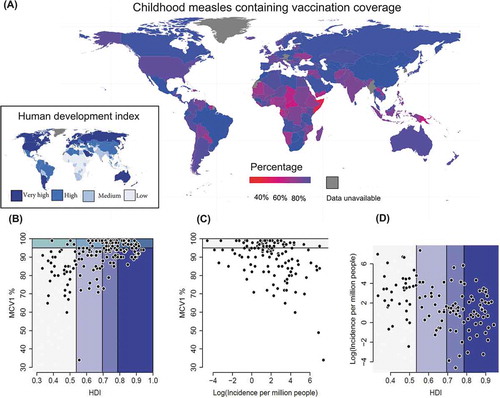
Due to changing human demographics, increasing immunization and declining incidence, measles epidemiology is changing.Citation2,Citation3 Globally, indicators of human development are increasing.Citation4 As human development index (HDI) scores increase, so is a country’s likelihood of increasing the childhood single dose measles-containing vaccine (MCV1) coverage (). This transition, however, is yet to fully manifest itself in concomitant declines in measles cases and incidences (). The rest of this review looks at aspects of why that may be, using most recent or complete data from a range of sources.
Figure 1. The relationship between human development index, vaccination and measles. A: a map of global single measles-containing vaccine (MCV1) coverage in children with the global human development index (HDI) shown (inset). The relationship between B: national childhood MCV1% and HDI, C: MCV1% and measles incidence, and D: measles incidence and HDI. Data are from WHO and UNDP for 195 countries in 2012 (most complete data). HDI colors (A, inset map) are the same in A-D.
Measles virus and its clinical disease
Measles virus is a single-stranded, negative-sense, enveloped RNA Morbillivirus in the family Paramyxoviridae and is spread through a range of mechanisms, from airborne droplets to direct contact.Citation5,Citation6 Symptoms typically start 10 to 12 days after exposure to the virus, however, crucially for disease transmission and control, people may be infectious up to four days before the start of the classical rash.Citation6 Initial clinical signs include fever, coughing, nasal discharge, and inflamed eyes. Small white spots form inside the mouth, followed by a rash on the face and body from two through to five days after the start of symptoms.Citation1 Overall symptoms typically last seven to ten days and following recovery most people are immune for life.Citation6 Complications, ranging from pneumonia, blindness, and meningitis, occur in approximately a third of cases. Measles can cause mental retardation as well as a fatal progressive neurologic disorder, subacute sclerosing pan-encephalitis. These complications are the reason measles kills and why mortality has declined with increased global measles immunization.Citation1,Citation6
Global eradication efforts
The World Health Assembly (WHA) aimed to facilitate global measles eradication (zero cases worldwide) through achieving ≥ 90% national level and ≥ 80% district level MCV1 administration among children aged 1 year. This would effectively reduce global annual measles incidence to < 5 cases per million and global mortality by 95% from the 2000 estimate. These targets were missed, with > 19 cases per million estimated in 2016 and an 84% decline in mortality.Citation2
Global eradication is facilitated through regional elimination. Measles elimination is defined as “the absence of endemic measles virus transmission in a region or other defined geographic area for ≥ 12 months, in the presence of a high quality surveillance system that meets targets of key performance indicators”.Citation2 The Global Vaccine Action Plan aimed to eliminate measles in four of six World Health Organization (WHO) regions by 2015 and five by 2020, with all countries aiming for measles elimination by or before 2020. Dabbagh, et al. Citation2 and Orenstein, et al. Citation7 recently reviewed the progress towards these targets. Among the WHO regions, only the Region of the Americas (AMR) has eliminated measles. The AMR was verified free of endemic measles in September 2016.Citation8 This elimination was achieved through control programs established in the early 1990s, often coordinated through the Pan American Health Organization (PAHO), and comprised high routine vaccine coverage and mass campaigns along with case-based surveillance.Citation9 By 2017 countries verified endemic measles free comprised 24 in the European Region (EUR), six in the Western Pacific Region (WPR), and two in the South-East Asia Region (SEAR). However, with the exception of the Western Pacific Region (WPR) no region has sustained MCV1 coverage > 95% since 2008.Citation2 For those countries in which endemic measles is eliminated, high population immunization coverage and surveillance are essential, because introductions can cause significant outbreaks.Citation10–Citation13 The reintroduction and establishment of endemic measles in Mongolia following elimination is evidence of the real risk to countries.Citation14
Immunization and population immunity
Two statistics are important for immunization and population immunity, when considering measles endemicity. The first is the proportion of a population that is immune, with a target of 95% set by WHO, because of measles’ high infectivity.Citation1 The other is the absolute number of naïve people, as measles has a critical community size that allows population level persistence due to its immunizing infection.Citation15
Global MCV1 coverage reached 85% in 2009, but has remained around this proportion. In 2016 the African Region (AFR, 72%) and Eastern Mediterranean Region (EMR, 77%) regions had the lowest coverage, followed by the SEAR (87%), AMR (92%), EUR (93%) and WPR (> 95%).Citation2 In the EUR MCV1 coverage was greater in 2012 (95%) than 2016 and district-level declines have occurred in highly immunized (> 90% MCV1) countries.Citation2,Citation12
Globally, coverage with a second measles containing vaccine (MCV2) reached 64% in 2016, substantially up from 15% in 2000.Citation2 MCV2 may be crucial to induce and maintain immunity, as a range of factors can affect vaccine efficacy and people receiving both MCV doses are less often reported as cases.Citation11 In China a third MCV dose is given, with eight-month, 18 month and 6 year-old children the target age classes for vaccination. A similar strategy was used in Japan from 2008 for five years, with high school children receiving a third MCV.Citation13
Measles immunization strategies that vaccinate only the very young (< 1 year-old) are not efficient.Citation11,Citation16,Citation17 Mathematical models have suggested vaccination coverage as low as 85% of children aged 1 to 7 years at five-yearly intervals is sufficient in Israel, but the majority of studies in Europe suggest a minimum of 87% coverage is essential to prevent endemic measles circulation and most estimates are higher than 90%.Citation18–Citation21 In New Zealand it was estimated that approximately 92% immunity was required.Citation22 In Japan immunity levels have been measured up to 92%, but age-structure estimates have suggested herd immunity may be above 80% but lower than the 90 to 95% required to prevent ongoing transmission.Citation23,Citation24
Even within regions with high immunization coverage naïve populations may exceed the critical community size for measles, estimated at 250,000 to 500,000, allowing the virus to persist at the population level.Citation15,Citation25–Citation27 Dabbagh, et al. Citation2 estimated globally 20.8 million infants did not receive MCV1 in 2016. Citation2 The majority of those (53%) are in countries with lower than 95% MCV1 immunization rates and large birth cohorts. In addition, evidence of some vaccine ‘failure’, waning immunity and pockets of susceptible populations suggest that relying on minimum percentages to be vaccinated alone may leave areas, cohorts, or communities susceptible to outbreaks in countries with highly immunized populations.Citation11,Citation28,Citation29 Here, spatial and contact structure will play key roles in determining measles epidemiology (see below).Citation30–Citation31
Trentini and colleagues estimated residual susceptibility to measles ranges from 3% in the UK to more than 10% in Kenya and Ethiopia.Citation32 They estimated that in high-income, well immunized countries (> 90% MCV1), such as Italy, Singapore, and South Korea, only approximately 20% of susceptible individuals are < 5 years old.Citation5 This change is partly due to reduced fertility and the authors estimated that that change alone “contributed to almost half of the reduction in measles incidence” in those countries through a reduction in naïve young populations. This is similar to recent estimates from New Zealand,Citation11,Citation22 Europe,Citation33 and China, where the age distribution of measles cases has changed in response to both demographic and vaccination processes.Citation3
A major cause of many susceptible people in an immunized population is due to the transition period. When most countries establish immunization programs, there is a transition period from the introduction of MCV to high childhood immunization levels. This transition period leads to cohorts of youths and young adults with lower immunity than their parents, who gained immunity through natural infection when young, and the very young children in which high MCV1 immunization rates have been reached. These cohorts with lower immunity track through time to increase the proportion of susceptible adults. Reduced population immunity due to waning vaccine immunity may further increase the numbers of older people with lower immunity (see below). Interestingly, the transition period is often longer in countries with longer histories of MCV usage, such as New Zealand.Citation11 This should be less important in countries that have rapidly transitioned from low to high immunization, because the transition from natural infection to vaccine induced immunity does not allow for such cohorts to exist.
Disease incidence and transmission in highly immunized populations
In 2016 the reported measles cases numbered 132,137, which corresponds to an estimated reduction to 19 cases per million people globally. In the AMR the estimated measles incidence was less than five cases per million. In highly immunized countries where endemic measles is eliminated, outbreaks are initiated by measles importations.Citation13,Citation34–Citation36 In the USA from 2009 to 2014, 275 importations from 58 countries caused 66 outbreaks totaling > 1264 cases. These were larger figures than the previous 7 years,Citation34 and a similar pattern is observed in other countries, such as New Zealand,Citation11 Japan, Citation13,Citation35,Citation36 and China.Citation25 In 2017, the European Union (EU) experienced a resurgence of measles with several outbreaks and 37 fatalities.Citation33 Twenty-eight EU countries reported 14,600 measles cases, equivalent to 28.3 cases per million people, with Romania (5,608), Italy (5,098), Greece (967) and Germany (929) reporting the greatest numbers.
Gastañaduy and colleagues estimated the reproduction number, the average number of secondary cases per infection, R, to assess the transmissibility of measles in the USA from 2001 to 2014.Citation37 Measles elimination requires R at < 1. The authors used four approaches, each suggested R was well below 1 (range 0.72 to 0.45, with no 95% confidence interval (CI) > 1). However, they noted year-to-year variability in the values of R and an increase in transmissibility in recent years. These reflect recent estimates from other highly immunized countries. In New Zealand, after years of very low incidence, analyses suggest that measles R often includes or exceeds one (0.18 to 3.92) despite high levels of population immunity. Similarly, R has been estimated as high as 9 (2 to 151 95% CI) in Japan from outbreaks and 1.5 to 3 from serological analyses.Citation24,Citation35
The age distribution of New Zealand measles cases reflected the age distribution of naïve individuals, with increased case age in more recent years.Citation11 A similar increase in case age may be seen in other countries such as China,Citation3,Citation25,Citation38,Citation39 and may be occurring in an ongoing outbreak in Japan.Citation40 In Japan there has been a pronounced shift in ages affected, with the majority of cases (range 50 to 100%) in each of five years experiencing measles outbreaks since 2011 being in > 20 year-old people.Citation36 In EU countries children under five comprised 37% of all 14,600 cases in 2017 and had the highest incidences (366 and 164 cases per million population in < 1 year and 1‒4 year-olds respectively).Citation33 However, adults ≥ 20 years old were 38% of cases in 2017. The majority of cases were unvaccinated, ranging from 72% in 25‒29 year-olds, to 96% in < 1 year-old children too young to receive the vaccination.Citation33
Australia has an established measles serosurveillance program.Citation41 The measles-specific IgG seroprevalence and R were estimated for 2012 to 2013, and compared with previous serosurveys (1996 to 1999, 2002 and 2007). Seronegative and equivocal sero-status individuals in age groups increased through time for all ages groups, from 2 to 39 years old.Citation41 Like the New Zealand situation, in Australia R has increased from 0.57 in 1999 to 1.7 in 2012 to 2013.Citation41 Increases in R in highly immunized populations may reflect a series of issues, including spatial heterogeneity in population immunity,Citation29 but studies also suggest vaccine induced measles-specific IgG antibodies decline with time since vaccination and maternally-derived immunity may wane faster if vaccine derived.Citation41–Citation44 Vaccinated people may be susceptible to measles for a range of reasons, including maternally-derived antibody interference, infection before an immune response has been developed, and poor vaccine storage and handling affecting vaccine efficacy,Citation45–Citation47 but waning immunity may increasingly play a role.Citation41 This poses numerous questions and requires further study, and yet supports the case for sustaining a highly immunized population.,Citation42
Disease and mortality in highly immunized populations
Measles deaths declined globally from 550,100 (95% CI = 374,000 to 896,500) in 2000 to 89,780 (95% CI = 45,700 to 269,600) in 2016.Citation2 Vaccination is estimated to have prevented approximately 20.4 million deaths from 2000 to 2016.Citation2 Deaths in developed countries are less common than in developing countries. In 2017, 37 measles deaths were reported by eight European countries, comprising 26 in Romania, four in Italy, two in Greece, and one each in Bulgaria, France, Germany, Portugal and Spain.Citation33 Recent examples of deaths include a fatal case in Italy, during a measles outbreak that started in early January 2017 causing 2,851 cases, of which 73% were greater than 15 years old. Most of the cases (89%) were unvaccinated and 6% received just one dose. Complications were reported in 35% of patients and one 9 year-old patient died.Citation10 In Romania, a large outbreak is ongoing, with 55 deaths reported from over 13,700 since the epidemic began in 2016,Citation48 despite previous efforts to improve vaccination rates, which have led to high (e.g. 94% in 2014) childhood immunization levels.Citation49,Citation50
Measles cost estimates in highly immunized populations
In developing countries, the cost to society from measles (e.g. through disability-adjusted life years, DALYs) is largely due to premature deaths. However, in highly immunized and developed nations the economic impact of cases is still high. The containment of a single case of measles in Iowa, USA, was estimated to cost > US$142,000 in 2004.Citation51 Sixteen outbreaks in the USA in 2011 involving a total of 107 people, lasting an average of 22 days, required between 42,600 and 83,100 personnel hours, at a cost of between US$2.7 and US$5.3 million.Citation52 Significant effort was spent on 8,900 to 17,500 contacts, each requiring 4.7 personnel hours at a cost of US$298 per contact.Citation52 Recent analyses of the public health and economic consequences of reduced vaccination in the USA suggest a 5% decline in MCV coverage would lead to an estimated 3-fold increase in measles cases for children aged 2 to 11 years and an additional US$2.1 million in costs per annum.Citation53
In New Zealand the cost of 187 confirmed and probable measles cases in 2014 was estimated to be > US$864,000 due to earnings lost, case management and hospitalization costs.Citation22 In China, a measles outbreak among office workers lead to a total of 7,930 contacts being identified, with household costs estimated at US$605 per case and control costs of US$17,481 per case.Citation38 Mental retardation cases and deaths were not reported among the cases in New Zealand or China, but such tragic cases would significantly increase the direct and societal costs.
Supplementary immunization activities
In lower income countries with typically high fertility, susceptible individuals are very young, for example, in Ethiopia ~ 60% susceptible were < 10 years old.Citation32 These can often be the target of supplementary immunization activities (SIAs) and Dabbagh, et al. Citation2 reported approximately 119 million people received supplementary MCV in 2016. These SIAs were undertaken during 33 mass immunization campaigns in 31 countries, including Ethiopia. Citation2 The coverage was high (≥ 95% in 20 SIAs) and in these countries SIAs were responsible for more than 25% of immunized individuals, including ~ 45% in Ethiopia.Citation32
The necessity of SIAs in highly immunized populations may be questioned (). In Beijing, China, since 2006 migrant college students have been required to have MCV administered, presumably because of cohorts of poorly immunized young adults.Citation3,Citation25 As discussed above, altered age structure of measles cases in high income/immunization coverage countries is now likely reflecting changing distributions of naïve people in the populations. This feature, and possible waning vaccine induced immunity, suggests SIAs may be required for older cohorts even when childhood immunization rates are high.Citation3,Citation6,Citation11,Citation12,Citation22,Citation30,Citation32,Citation35,Citation54 In Japan higher vaccination coverage is estimated to have decreased natural immunity boosting through declining incidence and reduced measles exposure, leading to higher case numbers in 20 to 29- and 30 to 39-year-old age groups and suggestions that immunization of “these age groups might be important for eliminating imported viruses”.Citation44
The societal return on investment suggests measles vaccination programs are hugely beneficial financially.Citation16,Citation55,Citation56 In highly immunized countries SIAs can still be beneficial. Estimates from the Republic of Korea and New Zealand suggest SIAs are cost effective with benefit-cost ratio of between 1.03 and 1.27 in Korea,Citation57 and must cost more than US$66 (and potentially up to US$1877) per immunized person in New Zealand before the financial costs outweigh the benefits.Citation22
Discussion
Globally MCV1 coverage of children has plateaued, despite improvements in human development. Aging cohorts of naïve people often exist as imperfect immunization programs take time to implement.Citation22,Citation24 These older naïve populations may have different disease outcomes to younger cases and may pose different case management issues than the very young. Complemented by some loss of vaccine-derived immunity, these older cohorts may increasingly contribute to the local, national and international transmission of measles, threatening regional elimination and global eradication efforts.Citation11,Citation25,Citation58,Citation59 Cost-benefit analyses are required to determine the economic and societal benefits of SIAs among older cohorts. Future studies must continue to monitor changing seroprevalence of measles antibodies in aging and highly immunized populations,Citation23,Citation41 and control efforts may need to be consider including older cohort SIAs,Citation44 in addition to maintaining high child immunization rates if global measles eradication is to be achieved.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author.
Acknowledgments
Thanks to members of mEpiLab and IDReC at Massey University and Tomasz Kiedrzynski on the Ministry of Health, New Zealand and an anonymous reviewer.
References
- World Health Organization. Global measles and rubella strategic plan; Geneva, Switzerland: WHO Press. 2012.
- Dabbagh A, Patel MK, Dumolard L, Gacic-Dobo M, Mulders MN, J-M O-B, Kretsinger K, Papania MJ, Rota PA, Goodson JL. Progress toward regional measles elimination—worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep. 2017;66(42):1148.
- Li S, Ma C, Hao L, Su Q, An Z, Ma F, Xie S, Xu A, Zhang Y, Ding Z. Demographic transition and the dynamics of measles in six provinces in China: A modeling study. PLoS Med. 2017;14(4):e1002255.
- United Nations Development Programme. 2016 Human Development Report. 2016.
- Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87(10):2767–2779.
- de Vries RD, Mesman AW, Geijtenbeek TB, Duprex WP, de Swart RL. The pathogenesis of measles. Curr Opin Virol. 2012;2(3):248–255.
- Orenstein WA, Cairns L, Hinman A, Nkowane B, J-M O, Reingold AL. Measles and rubella global strategic plan 2012–2020 midterm review report: background and summary. Vaccine. 2018;36:A35–A42.
- Pan American Health Organization. Region of the Americas is declared free of measles. Washington, DC: Regional Office for the Americas of the World Health Organization; 2016.
- Siqueira MM, Brown DW. Measles and rubella in the Americas: the path to elimination. Human Virology in Latin America: Springer. 2017;p 291–306.
- Lancella L, Di Camillo C, Vittucci A, Boccuzzi E, Bozzola E, Villani A. Measles lessons in an anti-vaccination era: public health is a social duty, not a political option. Ital J Pediatr. 2017;43(1):102.
- Hayman D, Marshall J, French N, Carpenter T, Roberts M, Kiedrzynski T. Global importation and population risk factors for measles in New Zealand: a case study for highly immunized populations. Epidemiol Infect. 2017;145(9):1875–1885.
- Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, LeBaron CW. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics. 2010;125(4):747–755.
- Tanaka-Taya K. [Current situation of measles in Japan, 2017]. Uirusu. 2017;67(1):17–24.
- Hagan JE, Greiner A, Luvsansharav U-O, Lake J, Lee C, Pastore R, Takashima Y, Sarankhuu A, Demberelsuren S, Smith R. Use of a diagonal approach to health system strengthening and measles elimination after a large nationwide outbreak in Mongolia. Emerg Infect Dis. 2017;23(Suppl 1):S77.
- Bartlett MS. Mealses periodicity and community size. J Royal Stat Soc Ser a-General. 1957;120(1):48–70.
- Pelletier L, Chung P, Duclos P, Manga P, Scott J. A benefit-cost analysis of two-dose measles immunization in Canada. Vaccine. 1998;16(9–10):989–996.
- Organization WH. Measles vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(35):349–360.
- Agur Z, Cojocaru L, Mazor G, Anderson RM, Danon YL. Pulse mass measles vaccination across age cohorts. Proc Natl Acad Sci U S A. 1993;90(24):11698–11702.
- Babad HR, Nokes DJ, Gay NJ, Miller E, Morgan-Capner P, Anderson RM. Predicting the impact of measles vaccination in England and Wales: model validation and analysis of policy options. Epidemiol Infect. 1995;114(2):319–344.
- Wallinga J, Levy-Bruhl D, Gay N, Wachmann C. Estimation of measles reproduction ratios and prospects for elimination of measles by vaccination in some Western European countries. Epidemiol Infect. 2001;127(02):281–295.
- Edmunds WJ, Gay NJ, Kretzschmar M, Pebody R, Wachmann H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiol Infect. 2000;125(03):635–650.
- Hayman D, Marshall J, French N, Carpenter T, Roberts M, Kiedrzynski T. Cost-benefit analyses of supplementary measles immunisation in the highly immunized population of New Zealand. Vaccine. 2017;35(37):4913–4922.
- Kumakura S, Shibata H, Onoda K, Nishimura N, Matsuda C, Hirose M. Seroprevalence survey on measles, mumps, rubella and varicella antibodies in healthcare workers in Japan: sex, age, occupational-related differences and vaccine efficacy. Epidemiol Infect. 2013;142(1):12–19.
- Kinoshita R, Nishiura H. Assessing age-dependent susceptibility to measles in Japan. Vaccine. 2017;35(25):3309–3317.
- Chen M, Zhang Y, Huang F, Wang H, Liu D, Li J, Rodewald L, Wu J, Deng Y, Xu W. Endemic and imported measles virus–associated outbreaks among adults, Beijing, China, 2013. Emerg Infect Dis. 2015;21(3):477.
- Keeling MJ, Grenfell B. Disease extinction and community size: modeling the persistence of measles. Science (80-). 1997;275(5296):65–67.
- Black FL. Measles endemicity in insular populations: critical community size and its evolutionary implication. J Theor Biol. 1966;11(2):207–211.
- Glass K, Kappey J, Grenfell B. The effect of heterogeneity in measles vaccination on population immunity. Epidemiol Infect. 2004;132(04):675–683.
- Hens N, Abrams S, Santermans E, Theeten H, Goeyvaerts N, Lernout T, Leuridan E, van Kerckhove K, Goossens H, Damme V. P and others. Assessing the risk of measles resurgence in a highly vaccinated population: belgium anno 2013. Euro Surveill. 2015;20(1): 1–10.
- Theeten H, Hutse V, Hens N, Yavuz Y, Hoppenbrouwers K, Beutels P, Vranckx R, van Damme P. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiol Infect. 2011;139(4):494–504.
- Pinchoff J, Chipeta J, Banda GC, Miti S, Shields T, Curriero F, Moss WJ. Spatial clustering of measles cases during endemic (1998–2002) and epidemic (2010) periods in Lusaka, Zambia. BMC Infect Dis. 2015;15(1):121.
- Trentini F, Poletti P, Merler S, Melegaro A. Measles immunity gaps and the progress towards elimination: a multi-country modelling analysis. Lancet Infect Dis. 2017;17(10):1089–1097.
- European Centre for Disease Prevention and Control. Measles and rubella surveillance – 2017. Stockholm, Sweden: ECDC; 2018. 2018 Apr.
- Fiebelkorn AP, Redd SB, Gastañaduy PA, Clemmons N, Rota PA, Rota JS, Bellini WJ, Wallace GSA. Comparison of postelimination measles epidemiology in the United States, 2009–2014 Versus 2001–2008. J Pediatric Infect Dis Soc. 2017;6(1):40–48.
- Nishiura H, Mizumoto K, Asai Y. Assessing the transmission dynamics of measles in Japan, 2016. Epidemics. 2017;20:67–72.
- Kaida A, Iritani N, Kanbayashi D, Yamamoto SP, Hirai Y, Hakui N, Fujimori R, Mori H, Hirokawa H, Ogasawara J. Ten-year surveillance of measles virus from 2007–2016 in Osaka City, Japan. Jpn J Infect Dis. 2018;71(2):152–154.
- Gastañaduy PA, Paul P, Fiebelkorn AP, Redd SB, Lopman BA, Gambhir M, Wallace GS. Assessment of the status of measles elimination in the United States, 2001–2014. Am J Epidemiol. 2017;185(7):562–569.
- Ma R, Lu L, Suo L, Li X, Yang F, Zhou T, Zhai L, Bai H, Pang X. An expensive adult measles outbreak and response in office buildings during the era of accelerated measles elimination, Beijing, China. Vaccine. 2017;35(8):1117–1123.
- Tang L, Zhou Y, Pan Y, Zhu H. Measles epidemics and seroepidemiology of population in Wujin, Changzhou city, Jiangsu province, China 2015. Vaccine. 2017;35(22):2925–2929.
- Hwai LS. Measles outbreak in Japan, Taiwan puts thousands in quarantine, delays travel plans, Strait Times 2018.
- Gidding HF, Quinn HE, Hueston L, Dwyer DE, McIntyre PB. Declining measles antibodies in the era of elimination: australia’s experience. Vaccine. 2018;36(4):507–513.
- Winter A, Martinez M, Cutts F, Moss W, Ferrari M, McKee A, Lessler J, Hayford K, Wallinga J, Metcalf C. 2018. Benefits and challenges in using sero-prevalence data to inform models for measles and rubella elimination. J Infect Dis. 218(3):355–364.
- Waaijenborg S, Hahné SJ, Mollema L, Smits GP, Berbers GA, van der Klis FR, de Melker HE, Wallinga J. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis. 2013;208(1):10–16.
- Inaida S, Matsuno S, Kobune F. Measles elimination and immunisation: national surveillance trends in Japan, 2008–2015. Epidemiol Infect. 2017;145(11):2374–2381.
- Ahmed A, Sahota A, Stephenson I, Brown KE, Tang JW. Measles–A tale of two sisters, vaccine failure, and the resurgence of an old foe. J Infect. 2017;74(3):318–320.
- Breakwell L, Moturi E, Helgenberger L, Gopalani SV, Hales C, Lam E, Sharapov U, Larzelere M, Johnson E, Measles outbreak MC. Associated with vaccine failure in adults–federated states of Micronesia, February-August 2014. MMWR Morb Mortal Wkly Rep. 2015;64(38):1088–1092.
- Fowotade A, Okonko I, Nwabuisi C, Bakare R, Fadeyi A, Adu F. Measles vaccine potency and sero-conversion rates among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. Journal of Immunoassay and Immunochemistry. 2015;36(2):195–209.
- Associated Press. Romania’s measles outbreak kills dozens of children, 2018.
- Stanescu A, Janta D, Lupulescu E, Necula G, Lazar M, Molnar G, Pistol A. Ongoing measles outbreak in Romania, 2011. Eurosurveillance. 2011;16(31):19932.
- Pistol A, Hennessey K, Pitigoi D, Ion‐Nedelcu N, Lupulescu E, Walls L, Bellini W, Strebel P. Progress toward measles elimination in Romania after a mass vaccination campaign and implementation of enhanced measles surveillance. J Infect Dis. 2003;187(Supplement_1):S217–S222.
- Dayan GH, Ortega-Sánchez IR, LeBaron CW, Quinlisk MP. The cost of containing one case of measles: the economic impact on the public health infrastructure—iowa, 2004. Pediatrics. 2005;116(1):e1–e4.
- Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, Wallace GS. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine. 2014;32(11):1311–1317.
- Lo NC, Hotez PJ. Public health and economic consequences of vaccine hesitancy for measles in the United States. JAMA Pediatr. 2017;171(9):887–892.
- Jansen VA, Stollenwerk N, Jensen HJ, Ramsay M, Edmunds W, Rhodes C. Measles outbreaks in a population with declining vaccine uptake. Science (80-). 2003;301(5634):804.
- Thompson KM, Odahowski CL. Systematic review of health economic analyses of measles and rubella immunization interventions. Risk Anal. 2016;36(7):1297–1314.
- Hinman AR, Zhou F, Reef S, Massoudi M, Papania MJ, Yusuf HR, Bardenheier B, Zimmerman L, McCauley MM. An economic analysis of the current universal 2-dose measles-mumps-rubella vaccination program in the United States. J Infect Dis. 2004;189(Supplement 1):S131–S145.
- Bae GR, Choe YJ, Go UY, Kim YI, Lee JK. Economic analysis of measles elimination program in the Republic of Korea, 2001: a cost benefit analysis study. Vaccine. 2013;31(24):2661–2666.
- Ferrari MJ, Grais RF, Bharti N, Conlan AJ, Bjørnstad ON, Wolfson LJ, Guerin PJ, Djibo A, Grenfell BT. The dynamics of measles in sub-Saharan Africa. Nature. 2008;451(7179):679.
- Bharti N, Tatem AJ, Ferrari MJ, Grais RF, Djibo A, Grenfell BT. Explaining seasonal fluctuations of measles in Niger using nighttime lights imagery. Science (80-). 2011;334(6061):1424–1427.
