?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rabies is the most lethal zoonotic, vaccine-preventable viral disease in the world. Its treatment is complicated by insufficient vaccine supply and the requirement for four to five repeated injections, as commercially available inactivated rabies lack adjuvant and have low immunogenicity. In this study, we focused on the role of a Krebs cycle intermediate, succinate dehydrogenase (SDH), in the innate immune response to cytokine production. We formulated a novel nanoemulsion adjuvant, Golden03, which stabilizes mouse SDH activity and contains more coenzyme Q10 and succinic acid than the classic MF59 adjuvant. Mice were immunized on days 1, 3, and 7, with seroconversion rate results suggesting that Golden03 significantly enhanced vaccine-stimulated antibody production against the rabies virus. Neutralizing antibody concentration testing by RFFIT indicated that treatment with Golden03 could result in antibody levels of up to 0.74 IU/mL 5 days post infection (DPI). ELISPOT for IFN-γ in mouse spleen cells showed that Golden03 enhanced immune responses at 14 DPI, inducing a rapid and powerful cellular response compared to the control group. Furthermore, the Vaccine-Golden03 group displayed no obvious weight loss or death after intracranial injection with CVS-11. An additional advantage is that Golden03 allowed for a three-quarter reduction in dose, while maintaining its efficacy and rapid stimulation effect. We suggest that Golden03 could be developed as a potential adjuvant for use in human rabies vaccine.
Introduction
Rabies is a zoonosis caused by the rabies virus, which is a negative single stranded RNA virus which is distributed widely worldwide. If scratched or bitten by an infected animal (e.g. dog, wolf, cat, or bat), humans may be infected by rabies. It can also be transmitted from infected animals to the human body through the eyes, mouth, or nasal mucosa pathway.Citation1,Citation2
Classically, from mucosal wound infection, the rabies virus travels quickly along the neural pathways of the peripheral nervous system. Retrograde axonal transport of the rabies virus to the central nervous system (CNS) is the key step of pathogenesis during natural infection.Citation3 The virus then spreads to other organs from the CNS.Citation4 Because of the blood-brain barrier, rabies can easily escape the control of the immune system and eventually kill the host.Citation5
Critically, rabies isn’t usually diagnosed until the patient has clinical symptoms, and the rabies virus may remain inactive in its hosts body for extended periods, becoming reactivated only after a long period of time.Citation5 Death can occur from two days to five years from the time of initial infection.Citation5,Citation6 In humans, rabies is almost invariably fatal once clinical symptoms have developed.
Rabies caused approximately 17,400 deaths worldwide in 2015.Citation7 More than 95% of human deaths caused by rabies occur in Africa and Asia.Citation6,Citation7 About 40% of these deaths occur in children under the age of 15.Citation8
Rabies vaccine is used to prevent rabies before or after exposure to the virus and, following a full course of vaccination, the immunity effect is long-lasting. Doses are usually given by injection into the skin or muscle and, until now have proven very effective in protecting humans against rabies.Citation9 Globally, millions of people have been vaccinated and it is estimated that this saves more than 250,000 people a year. Rabies vaccines are on the World Health Organization’s List of Essential Medicines, which contains the most effective and safe medicines needed in effective health systems.Citation10 However, the economic burden of vaccination is heavy. The wholesale cost in developing countries was between 44 and 78 USD for a course of treatment in 2014. In the United States, a full course of rabies vaccine costs more than 750 USD.Citation11,Citation12
To prevent the onset of rabies and death, rabies vaccine must be administered as quickly as possible following exposure. Commercially available rabies vaccines are inactivated vaccines without adjuvant, and have limited immunogenicity.Citation13 Therefore, four to five repeated injections are required for post-exposure prophylaxis, which adds to the cost of vaccination, and may also lead to vaccine failure if vaccination is incomplete. The development of a new generation of adjuvant might, therefore, be an effective method for enhancing the immune effect of current rabies vaccines.
There are many types of adjuvants, which may be based on a variety of inorganic compounds including alum, aluminum hydroxide, and aluminum phosphate. It has been reported that aluminum-based adjuvants, which are included in precipitated form in some vaccines, form antigenic libraries in vivo after antigen adsorption, and slowly release antigens. Because they stimulate the immune system by inducing the release of uric acid as an immunological signal, they strongly attract certain types of monocytes, which differentiate into dendritic cells. The dendritic cells pick up the antigen, carry it to lymph nodes, and stimulate T cells and B cells.Citation14 This appears to contribute to the induction of a powerful Th2 response, and so is useful for immunizing against pathogens that are blocked by antibodies. However, aluminum-based adjuvants have little capacity to stimulate cellular (Th1) immune responses, which are important for protection against many intracellular pathogens, nor is it useful when the antigen is peptide-based.Citation14,Citation15
Furthermore, when aluminum adjuvant was used in combination with a rabies vaccine, it was found to have no advantages. Lin et al. suggested that vaccines without it had a better effect, indicating that aluminum adjuvant should be eliminated from rabies vaccine for human use.Citation16
Bacterial DNA comprising palindromic sequences and containing unmethylated CpG is recognized by toll-like receptor 9 of plasmacytoid dendritic cells (pDCs), and induces the production of interferon-α and chemokines, leading to the activation of Th1 immune responses. Therefore, synthetic equivalents of bacterial DNA (CpG oligodeoxynucleotides) have been developed for clinical applications. They are usually phosphorothioated for in vivo use, although this approach has been reported to lead to adverse effects in mouse models.Citation17
Liu et al (2016). evaluated the safety of repeated dose of CpG rabies vaccine in mice.Citation18 CpG 684 rabies vaccine caused severe allergic reactions, with increases in serum globulin and spleen weight, and decreases in serum alkaline phosphatase (ALP). It also induced decreases in platelet count, and increases in monocytes and serum total cholesterol. Treatment-related microscopic findings were cell hyperplasia in spleen white pulp and elevated tingling body macrophage counts in the germinal center of white pulp, and a marked inflammatory reaction and hyperplasia of fibrous tissue in injection site muscle. Most of these changes had recovered 3 weeks after administration of the final dose.
More promising adjuvants have, however, been developed and applied to rabies vaccines, e.g. PICKCa, which is a conjugate of poly I, poly C, kanamycin (K), and calcium chloride (Ca), PICKCa is a safe and effective interferon inducer and, according to a phase II randomized study, had safety and immunogenicity characteristics similar to that of a novel PIKA rabies vaccine using an accelerated regimen.Citation19 All subjects achieved the target titter by Day 14. The accelerated regimen using the investigator PIKA rabies vaccine was well-tolerated and demonstrated immunogenicity comparable to the classic regimen using the commercially available vaccine in healthy adults.
In this study, we focused on the role of a Krebs cycle intermediate, succinate dehydrogenase (SDH), in the innate immune response to cytokine production. Golden03 is a novel nanoemulsion adjuvant for rabies vaccine which stabilizes SDH, which is a potent adjuvant to enhance immune responses against virus infection. Here we investigated the efficacy of Golden03 as an adjuvant to improve humoral and cellular immune responses to rabies vaccine in BALB/c mice. Supplementation of the rabies vaccine with Golden03 significantly accelerated the production of specific antibodies 5 days after initial immunization compared to the original vaccines. Furthermore, Golden03 promoted the induction of stronger cellular immune responses by the rabies vaccine, including the production of IL-1β, IFN-γ, and the activation of CD8 + T cells. Here, our findings indicated that Golden03 enhances humoral and cellular immunity, and is a promising adjuvant for the development of more effective rabies vaccines in the future.
Results
Seroconversion rates
At 14 days post injection (DPI), animals’ seroconversion rates of IgG were calculated for each group using fractional doses (), with Golden03-stimulated BALB/c mice being shown to produce the highest level of IgG antibody during this study.
Table 1. The seroconversion rate of each experimental group by fractional doses at 14 days post injection (DPI).
More effective vaccine international unit (IU) (2.5 IU) can stimulate the body to produce more antibody (IgG). MF59 adjuvant allows for vaccines to be administered at 1/2 dose, but Golden03 allows for further reduction to 1/4 dose, compared to original vaccine administered at full dose. Additionally, when vaccine-Golden03 was used at 1/6 dose, more than half of the animals tested displayed significant seroconversion. The Golden03 adjuvant had a good fractional dose effect at 1/4 dose.
Furthermore, the OD values from individual mice were converted into antibody concentrations (pg/mL) using the standard serum curve (NICPBP) and used to construct a scatter plot. When used at 1/4 dose, shows that both MF59 and Golden03 adjuvant had higher IgG titer responses to rabies than the original vaccine, with the Vaccine-Golden03 group having the best performance.
Figure 1. Distribution of IgG antibody levels in each group mouse following stimulation by different adjuvants (**P < 0.01, *P < 0.05).
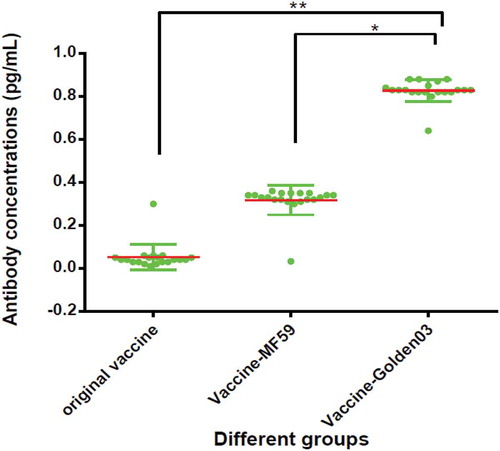
Collectively, these results indicated that Golden03 might be a promising adjuvant in rabies vaccines for humans, boosting their antibody responses against specific antigens during the incubation period of the rabies virus.
Rapid fluorescent focus inhibition test (RFFIT)
The rapid fluorescent focus inhibition test (RFFIT) was selected as the pharmacodynamic marker assay, and is regarded as the standard rabies virus neutralization assay in diagnostic laboratories, vaccine and bio-therapeutic characterization, and rabies-related clinical studies.
Serum results of neutralization titers are listed in . Neutralizing antibody concentrations ≥ 0.5 IU/mL were first observed in mice in the Vaccine-Golden03 groups on the 5th day (up to 0.74), but only on the 14th day in the Vaccine-MF59 and original vaccine groups (up to 0.86 and 0.67, respectively). Additionally, Golden03 had a 3/4 reduction in dosage, while still maintaining its effect and rapid stimulation.
Table 2. The dynamic detection of neutralizing antibody titers in each adjuvant group with suitable dose by rapid fluorescent focus inhibition testing.
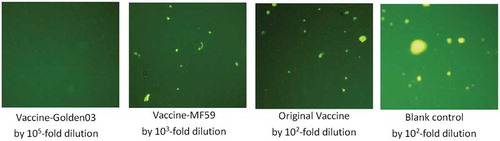
We collected fluorescent foci images, shown in , using a fluorescence microscope (400×). This magnification was found to be effective for different serum groups at different dilutions. Fluorescent foci units (FFU) were calculated at 5 DPI, and were found to be effectively suppressed in the vaccine-Golden03 group (667), compared with blank control group (500,000). At this point only a single immunization had been performed, without a booster, which resulted in low suppression in the original vaccine (360,000) and vaccine-MF59 groups (220,000). Golden03, however, was able to achieve adequate antibody levels by the fifth day.
ELISPOT for ifn-γ and IL-4
The influence of Golden03 on IFN-γ and IL-4 levels in immunized mice was testing. To analyze the types of cellular immune responses and cytokine levels stimulated by Golden03, spleen cells from experimental mice were isolated on the 14th day after the first injection, following three immunizations on the 1st, 3rd, and 7th days, respectively. Spleen cells were tested using IFN-γ and IL-4 ELISPOT assays.
Single lymphocyte cell suspensions were stimulated with the full dose of rabies vaccine. ELISPOT results are shown in . In the Vaccine-Golden03 group, the Golden03 adjuvant combined with a 1/4 dose of vaccine caused a four-fold higher number of IFN-γ producing cells (P < 0.05) when compared to the original vaccine group. In the case of the MF59 adjuvant, its combination induced the same degree of IFN-γ and IL-4 producing cells as were found with the original vaccine alone. No significant differences was detected between any of the experimental groups with respect to the number of IL-4 producing cells (P > 0.05).
Figure 3. Induction of IIFN-γ and IL-4 production by Golden03 adjuvant in immunized mice, measured by enzyme-linked immunospot (ELISPOT) assay.
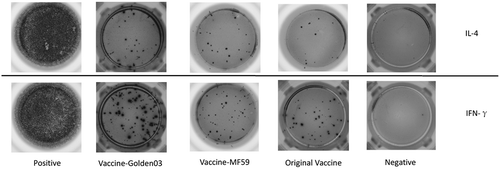
These results suggested that Golden03 could enhance IFN-γ immune responses at 14 DPI.
Expression of inflammatory genes
To further study the possible molecular immune mechanisms of Golden03 adjuvant, the expression of several immune response genes was investigated. The mRNA levels of interferon (IFN-γ), pro-inflammatory cytokines (TNF-α,IL-1β, IL-12, IL-23), and T cell markers (CD4 and CD8) were measured in the muscle tissue of experimental mice at different time points following immunization and compared with mRNA levels in the control group.
As shown in , no upregulation of IL-12, IL-23, or CD4+ was detected from 5 DPI to 10 DPI. However, three important cytokines were found to be highly expressed when compared to the control group, including IFN-γ (140 ± 32.2 pg/mL), IL-1β (107 ± 22.9 pg/mL), and TNF-α (8.3 ± 1.3 pg/mL). The higher expression of these pro-inflammatory cytokines could aid in the rapid production of neutralizing antibodies. It was interesting to note that upregulation of expression was found in CD8 + T cells at 10 DPI, but not at 5 DPI, compared with the control group. Two booster immunizations were needed for full activation of inflammatory cytokines.
Figure 4. Adjuvant and rabies vaccine associated with decreased infiltration and activation of inflammatory and immune cells.
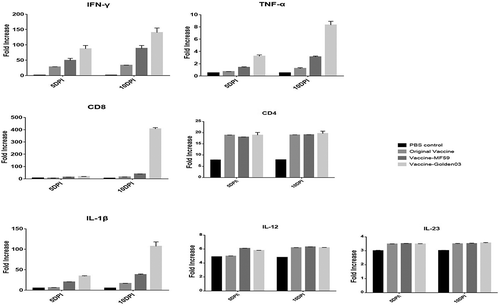
These results suggested that the early stage of first booster immunizations (5 DPI) was important during the rapid immune response after exposure, which involved inflammatory immune responses with IFN-γ and IL-1β. The use of Golden03 adjuvant induced a faster and stronger cellular response than was seen in the PBS control group.
Activity of succinate dehydrogenase (SDH)
Following two booster immunizations at 10 DPI, muscle tissue from the right lower limbs of immunized mice (i.e. the injection site) was dissected and isolated. After tissue homogenization, intracellular mitochondria were isolated following kit instructions.
Changes in SDH activity, which differed significantly in each group (P < 0.05), are listed in . The Golden03 adjuvant enhanced SDH activity following the third booster shot, but this enhancement was not observed in the original vaccine or Vaccine-MF59 groups.
Table 3. Results of succinate dehydrogenase (SDH) activity in muscle tissue of experimental mice following adjuvant and vaccine stimulation.
CVS-11 challenge to experimental animals
Protective immune responses induced by Golden03 in immunized mice were analyzed. To evaluate the protective effect of Vaccine-Golden03, immunized mice were challenged with 50 LD50 of CVS-1114 days after initiation of booster immunization. As shown in , the mice immunized with PBS (as negative control), original vaccine (with 1/4 dose) and vaccine-MF59 (with 1/4 dose) showed serious weight loss and signs of illness, including listlessness and neurological disorders or death. In contrast, the Vaccine-Golden03 group displayed no obvious weight loss following intracranial injection with CVS-11. Very slight signs of illness were observed in some mice from the Vaccine-Golden03 group.
Figure 5. Weight changes and survival in mice after lethal challenge. (A) Weight changes recorded for 14 days following challenge in each group. (B) Survival curves for the immunized groups, using Kaplan-Meier test.
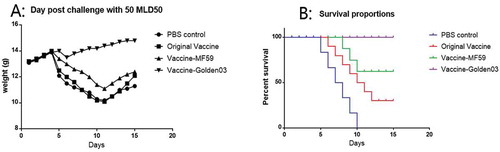
As shown in , significantly more survivors were observed among the mice immunized with Vaccine-Golden03 (100%) or Vaccine-MF59 (62.5%) than among the original vaccine with no-adjuvant (12.5%) and control groups (0%).
Discussion
Rabies is a fatal infectious disease caused by the rabies virus and, untreated, has an almost 100% fatality rate. Approximately 55,000 people die from rabies annually, with more than 50% of these deaths occurring in Asia.Citation6,Citation9,Citation20 It predominantly affects poor and vulnerable populations living in remote rural locations in tropical regions. The World Health Organization (WHO), the World Organization for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO) and the Global Alliance for Rabies Control (GARC) have established a global “United Against Rabies” collaboration to provide a common strategy to achieve “Zero human rabies deaths by 2030”.
Treatment of rabies is difficult, and may be further complicated by insufficient vaccine supply, and the economic burden of vaccines. Additionally, the rabies vaccination protocol in wide use is difficult, and involves prompt vaccination after exposure, injection of rabies immunoglobulin and thorough wound washing with soap and water. Vaccination after exposure cannot, by itself, provide sufficient protection. Antibody production is delayed, the resulting antibody titer is low, and the maintenance time is short.Citation21 Highly efficient, non-toxic, safe, and inexpensive rabies vaccine adjuvants capable of generating an immediate protective immune response and induce potent cellular and humoral immunity are, therefore, an important field of vaccine research.
Many kinds of adjuvant are used in conjunction with rabies vaccines, e.g. immunomodulatory adjuvants (including monophosphoryl lipid A, flagellin, CpG, ODN, and PIKA), delivery system adjuvants (including liposome, MF-59TM, and AS03), and compound adjuvants (including ISCOM). All of these adjuvants provide some degree of advantage in rabies vaccine use. Their mechanism of function is closely linked to toll-like receptor signaling pathways (summarized in supplementary Figure S1). Notably, the PICKCa adjuvant has been licensed for clinical study, as it shows a better protection effect compared with commercial adjuvant-free rabies vaccine in post-exposure immunization, even when no anti-rabies immunoglobulin is used. PICKCa adjuvant can be used as a candidate therapeutic vaccine for immunization of patients with exposure of degree III, in whom protection by immunoglobulin has failed.Citation19 This adjuvant has been found to stimulate non-specific immune function, humoral, and cellular immune responses in animals.
Toll-like receptors (TLRs) belong to a class of proteins that plays a key role in the innate immune system. Basically, PICKCa is activated by TLR3, recognizes dsRNA associated with viral infection, and induces the activation of IRF3 and NF-κB. However, the innate immune signaling pathways also include other possible means of stimulation. TNF and IFN-γ are the two most important signals for classical macrophage activation.Citation22
The Krebs cycle is an amphibolic pathway operating in the mitochondrial matrix of mouse. In response to pro-inflammatory stimuli, macrophages and dendritic cells undergo profound metabolic remodeling to support the biosynthetic and bioenergetic requirements of the cell. Recently, it has been discovered that this metabolic shift also involves the rewiring of the Krebs cycle to regulate cellular metabolic flux and the accumulation of Krebs cycle intermediates, notably, citrate, succinate, and fumarate.Citation23,Citation24
We chose succinate as the breakthrough material in the new adjuvant, Golden03, and used succinate-sodium citrate buffer as a basic buffer in all formulations. A new role for Krebs cycle intermediates as signaling molecules and immunomodulators was therefore demonstrated in this study.
When the effect of interleukin-1β inhibition with canakinumab on incident lung cancer was analyzed in patients with atherosclerosis, it was found that the SDH inhibitor blocked induction of IL-1β and boosted IL-10 production in vivo, which had a positive effect on treatment.Citation25Applying this practical theory to the development of rabies adjuvants, we propose that the SDH stabilizer might have a positive effect in adjuvants or on anti-rabies immunity.
Researchers from Harbin Medical University have found that coenzyme Q10 can increase the succinate dehydrogenase activity of mitochondrial in cells.Citation26 Anti-oxidant coenzyme Q10 has also been found to help activate innate immunity in patients with type 1 and type 2 diabetes mellitus.Citation27,Citation28 We used coenzyme Q10 as an important component of Golden03, finding that, in addition to is other functions, it produced the adjuvant’s golden color (Figure S2). The core role of SDH activity in production of inflammatory factor in Krebs cycle is summarized in supplementary Figure S3.
Because coenzyme Q10 is fat-soluble, we dissolved it in squalene, producing a saturated solution. During our experiment, succinate dehydrogenase (SDH) activity was measured, and we found that Golden03 adjuvant enhanced SDH activity following the third injection. This result is attributed to the protective effect of coenzyme Q10 on the vaccine’s stability, and the substrate effect provided by succinic acid.
As long as the SDH is sufficiently active, it is conducive to the expression of early inflammatory factors. This, in turn, aids production of neutralizing antibodies in vivo. The most important cytokines were interferon (IFN) gamma and interleukin (IL) 1 beta. According to Wang et al., interferon as adjuvant enables higher efficiency of both of humoral and cellular immunity.Citation29 The rabies vaccine using IFN as adjuvant showed an improved immune effect. In their study, they mixed rabies vaccine for human use with IFN at a ratio of 1:1, and then administered injections on days 4, 7, 14, 30, and 60. The lymphocyte transformation rate induced by rabies vaccine containing the IFN adjuvant was significantly higher than that induced by adjuvant-free vaccine, or the vaccine containing aluminum hydroxide adjuvant. This finding was confirmed by the results of our present study.
Using the rapid fluorescent focus inhibition test (RFFIT), neutralizing antibody concentrations ≥ 0.5 IU/mL were first observed in mouse in the Vaccine-Golden03 groups on the 5th day (up to 0.74), but were only observed in the Vaccine-MF59 and original vaccine groups on the 14th day (up to 0.86 and 0.67, respectively).
To our knowledge, interferon has a positive effect on protection against the rabies virus in mice. These effects were very important in the early stages of infection. In a study by Zhuo et al., IFN-α was found to protect about 50% of mice from rabies when administered subcutaneously two weeks before infection with the CVS strain.Citation30 However, it failed to protect mice if injected after infection. The simultaneous administration of IFN-α and rabies vaccine (as a mixture) was found to elicit a better response when compared with either IFN or rabies vaccine used on their own. This mixture allowed for a 5-fold reduction in the amount of vaccine required for immunization. Furthermore, it showed no significant difference in efficacy between 5 × 103 IU dose group and 5 × 102 IU dose group of IFN-α.
If detected the lymphocyte transformation rate at 15 DPI, it induced by rabies vaccine containing IFN adjuvant was significantly higher and earlier than that induced by adjuvant-free vaccine and the vaccine containing aluminum hydroxide adjuvant.Citation29 Wang et al. showed that interferon as adjuvant has higher efficient to both of the humoral immune and cellular immune. The rabies vaccine using IFN as adjuvant shows the better immune effect.
In our study, we found that Golden03 adjuvant combined with a quarter dose of vaccine caused a four-fold higher number of IFN-γ producing cells (P < 0.05) compared with the original vaccine group. These results were supported by RT-PCR, which confirmed elevated IFN expression levels. Based on above findings, we concluded that the Golden03 adjuvant maintained the stability of SDH, which helped the mice to rapidly produce interferon. The resultant IFN was of great benefit to the production of neutralizing antibodies against the rabies virus.
Materials and methods
Virus source
The rabies vaccine virus was provided by the Institute of Medical and Biology provided the rabies vaccine, in the form of an inactivated vaccine made from CTN-1 V25 strains (cultured on KMB17 cells). Rabies virus seed was purchased from the National Institute of Chinese Pharmaceutical and Biological Products (NICPBP). Rabies virus CTN-1 V35 was cultured on KMB17 cells for 10 generations, and used to construct a working seed bank at our institute. Quality control required its virulence should be above 7.0 log LD50/mL. The virus was inactivated by β-propiolactone, and the vaccine was prepared following a series of concentration and purification processes. It was then dispensed and stored at −80 °C until further use. The CVS-11 (ATC VR959) strain, which was also purchased from NICPBP, was used as a challenge virus.
Cell source
Baby hamster kidney (BHK)-21 cells and KunMing Biology (KMB17) cells were obtained from the American Type Culture Collection (ATCC) (as ATCC 0661 and ATCC-5058, respectively). They were cultured in DMEM/F12 (Gibco) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS, Gibco).
Animal source
BALB/c mice aged 6–8 weeks were purchased from Beijing Vital River Laboratory Animal Technology Co., and reared under pathogen-free conditions. All mice were housed at least 2 weeks before the experiment. The experimental manipulation of mice was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Peking Union Medical College (DWLL201605024).
Adjuvants
Details of the manufacturing process for MF59 were acquired from the Technical Services Department of Beijing Novartis Pharmaceutical Co., Ltd. The reagents and formulations included 4.3% v/v squalene, 0.5% v/v Tween 80, and 0.5% v/v Span 80 in citrate buffer (10 mM, pH 7.0). The coarse emulsion was passed repeatedly through a micro-fluidizer to produce an oil/water emulsion of uniform small droplet size (160 nm), which was filter sterilized and dispensed into vials.
Golden03 was produced by the Institute of Medical and Biology. Coenzyme Q10 was purchased from Aladdin Industrial Corporation (Shanghai) Co., Ltd (Cat. No,. B1728018). Coenzyme Q10 (0.024 g) was weighed and then mixed in 6 mL of double distilled H2O in order to form a suspension. It was then extracted in squalene (6 mL) by vortexing for 15 min. After centrifugation at 5,000 rpm for 30 min, the oily supernatant was collected and used for the next preparation step. Golden03 was prepared as an emulsion consisting of 5.0% v/v squalene (containing coenzyme Q10), 0.5% v/v Tween 80, and 0.5% v/v Span 80 in succinic acid-sodium citrate buffer (10 mM, pH 6.5). These mixtures were prepared by homogenization at 8,000 psi with a high-pressure homogenizer (Emolsiflex-C3, Avestin, Canada).
Golden03 and MF59 adjuvants were stored as suspensions at 4 °C until use. Vaccine was mixed with Golden03 or MF59 adjuvant at a ratio of 1:1 (v/v) for 2 hours at room temperature (25 °C) before injecting animals.
Immunization schedule
BALB/c mice were randomly divided into three experimental groups (20 mice in each group), namely: original vaccine, Vaccine-MF59, and Vaccine-Golden03. Mice were immunized three times, on the 1st, 3rd, and 7th day. Fractional doses were diluted in PBs, and administered as follows: full-dose, 1/2 dose, 1/4 dose, 1/6 dose, and 1/8 dose. All experimental mice had identical injection volumes (0.2 mL) administered by intramuscular injection (i.m.) on the right forearm. Tail vein blood was collected before each vaccine injection. Serum was separated by centrifugation at 3500 × g for 20 min and stored at −20 °C until tested.
Virus titration
Infectious rabies virus particles were tested by endpoint dilution assay in BHK-21 cells. The measure of infectious virus titer was expressed as 50% tissue culture infective dose (TCID50)/mL, which represents the amount of virus per mL that gives rise to infection in 50% of inoculated tissue culture cells. Virus titration was performed according to the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Office International des Epizooties, 2008).
ELISA for igg
Golden03 stimulation of a high seroconversion rate of IgG in BALB/c mice was tested using the ELISA method. Tail vein blood was collected at from experimental mice 14 DPI and the serum separated by centrifugation. In order to measure mouse IgG levels, we used the Mouse Anti-Rabies Virus IgG ELISA Kit (Alpha Diagnostic Intl. Inc. San Antonio, USA. Cat. No. 600–030-MRG).
Rapid fluorescent focus inhibition test (RFFIT)
The rapid fluorescent focus inhibition test (RFFIT) was used to detect mouse effective neutralizing antibodies after immunization at 5 DPI. The rabies immunoglobulin titer determination method was followed according to the Pharmacopoeia (2015 Edition) formulated by the China National Pharmacopoeia Committee (Reference:3512). Rabies virus nuclear protein antibody (FITC) was purchased from Biorbyt Ltd., (California, US; Cat. No. Orb241176).
The number of fluorescent foci (NFF) in the 96-well plate were counted, and used to calculate the number of focus-forming units (FFU) according to the formula:
As the standard serum titer from NICPBP was 200 IU/mL, it was used to calculate the experimental serum titer, using the following formula:
Immunospot (ELISPOT) assay
Mouse IFN-γ and IL-4 ELISPOT kits (Mabtech, Sweden) were used for this assay. Single cell suspensions of spleen cells with a density of 106/mL were prepared. 105 cells (100 μL) and 10 μL rabies vaccine were added to each well, following which plates were incubated for 36 hour in a humidified incubator at 37 °C and 5% CO2. Plates were then washed and processed according to the manufacturer’s protocol. Finally, spots were scanned and enumerated using a Spot Reader B1 (SLT Instruments, UK).
Rt-qpcr assay for inflammatory gene expression
RNA extraction and real time quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) assay were performed for quantification of inflammatory gene expression. Six mice per group were euthanized at two time points (5 DPI and 10 DPI). RNA extraction was performed using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentration was calculated using Nano Vue Spectrophotometry (GE Healthcare, Bucks, UK), and 100 ng was used for reverse transcription. Reverse transcription and q-PCR were performed as described by Kip,Citation31 including the accurate calculation formulas and methods. All primer sequences are listed in . using the following program: 2 min at 95 °C, followed by 45 cycles of: 20 sec at 95 °C and 30 sec at 62 °C. A melting curve analysis was performed in order to verify the specificity of amplification. It used the GAPDH as internal reference gene for quality control.
Table 4. Primers for inflammatory genes used in real-time PCR.
Detection of SDH activity
The succinate dehydrogenase (SDH) activity was measured using an SDH Activity Colorimetric Assay Kit (Catalog Number MAK197) purchased from Sigma-Aldrich Co. LLC (Saint Louis, US). After correcting for the background, SDH activity in muscle tissue of experimental mice was determined following adjuvant and vaccine stimulation.
Statistical analysis
Statistical analyses were performed using IBM® SPSS® statistics, version 21.0. The seroconversion rates were compared using the χ2 test (Fisher’s exact test). Differences in the distribution of antibody neutralization titers were tested for by one-way analysis of variance. SDH activity units were represented as the mean ± SD of three or more independent experiments. If the data were homogenous, analysis of variance, Student-Newman-Keulsa, and Pearson’s correlation were used. If the data were not homogenous, Kruskal-Wallis and Games-Howell tests, and a Spearman’s correlation analysis, were used. Survival curves for the immunized groups were determined using a Kaplan-Meier test. Statistical significance was defined as a P-value < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Competing interests
There were no competing interests in this study.
Consent for publication
All authors provide consent for this publication.
Additional information
Funding
References
- Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013;12(5):498–513. doi:10.1016/S1474-4422(13)70038-3.
- Li YR, Zhu LL, Zhu WY, Tao XY. Epidemiology of human rabies in China, 2016. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(1):40–43.
- Raux H, Flamand A, Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol. 2000;74(21):10212–10216.
- Morimoto K, Shoji Y, Inoue S. Characterization of P gene-deficient rabies virus: propagation, pathogenicity and antigenicity. Virus Res. 2005;111(1):61–67. doi:10.1016/j.virusres.2005.03.011.
- cited as. https://www.cdc.gov/rabies/symptoms/.
- Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. Current status of rabies and prospects for elimination. Lancet. 2014;384(9951):1389–1399. doi:10.1016/S0140-6736(13)62707-5.
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1459–1544.
- cited as. http://www.who.int/rabies/rabies_Infographic_updated_Global_International_meeting.pdf?ua=1.
- cited as. http://www.who.int/wer/2010/wer8532.pdf?ua=1.
- cited as. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1.
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709.
- cited as. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/perspectives-intradermal-rabies-preexposure-immunization.
- Hu X, Liu R, Zhu N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology. 2013;218(12):1524–1528. doi:10.1016/j.imbio.2013.05.006.
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatorydendritic cells. J Exp Med. 2008;205(4):869–882.
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi:10.1111/j.0818-9641.2004.01272.x.
- Haixiang L, Perrin P. Influence of aluminum adjuvant to experimental rabies vaccine. Chinese J Exp Clin Virol. 1999;13(2):133–135.
- Iho S, Maeyama J, Suzuki F. CpG oligodeoxynucleotides as mucosal adjuvants. Hum Vaccin Immunother. 2015;11(3):755–760. doi:10.1080/21645515.2014.1004033.
- Liu L, Jianjun L, Hua J, Jianhua M, Yanwei Y, Fang L, Dongsheng P, Yu Z, Yan S, Jilin R, et al. Safety evaluation on repeated dose of CpG 684-rabies vaccine in mice. Chin Pharm J. 2016;51(19):1657–1665.
- Kalimuddin S, Wijaya L, Chan YFZ, Wong AWL, Oh HML, Wang LF, Kassim JA, Zhao J, Shi Z, Low JG. A phase II randomized study to determine the safety and immunogenicity of the novel PIKArabies vaccine containing the PIKA adjuvant using an accelerated regimen. Vaccine. 2017;35(51):7127–7132.
- cited as. http://www.who.int/en/news-room/fact-sheets/detail/rabies.
- Yelei Z, Peilu S, Xuejie Y, Xuexing Z. Advances in the study of rabies vaccine adjuvant. J Pathog Biol. 2017;12(3):278–282.
- Schijns VE, Claassen IJ, Vermeulen AA, Horzinek MC, Osterhaus AD. Modulation of antiviral immune responses by exogenous cytokines: effects of tumour necrosis factor-alpha, interleukin-1 alpha, interleukin-2 and interferon-gamma on the immunogenicity of an inactivated rabies vaccine. J Gen Virol. 1994;75(Pt1):55–63.
- Ryan DG, O’Neill LAJ. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017;591(19):2992–3006.
- Williams NC, O’Neill LAJ. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. 2018;9:141.
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017t;390(10105):1833–1842.
- Dianqing Z, Qiqi W, Chenghua G, Hongchang Y, Yi L, Chunming Z, LIying M, Songshan C. Effects of compound radix salviae miltiorrhizae and ubiquinone-10 on the succinodehydrogenase activity in myocardial mitochondria. J Harbin Med Univ. 1991;25(6):413–415.
- Brauner H, Lüthje P, Grünler J, Ekberg NR, Dallner G, Brismar K, Brauner A. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin Exp Immunol. 2014;177(2):478–482. doi:10.1111/cei.12316.
- Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40(4):413–418. doi:10.1111/jcpt.12280.
- Zhonghai W. Adjuvant effect research of interferon in rabies vaccine. China Pharmaceuticals. 2009;18(24):17–19.
- Jinrong Z, Xiaojun L, Yu Z, Yongge X, Shuqiao W. Efficacy of interferon-αin protecting against rabies in mice. J Per Med Inf. 1999;15(3):129–130.
- Kip E, Staal J, Verstrepen L, Tima HG, Terryn S, Romano M, Lemeire K, Suin V, Hamouda A, Kalai M, et al. MALT1 controls attenuated rabies virus by inducing early inflammation and T cell activation in the brain. J Virol. 2018;92(8):e02029–17. doi:10.1128/JVI.02029-17.