ABSTRACT
Background: Pneumococcal disease remains a public health priority in adults. Previous studies have suggested that administration of pneumococcal polysaccharide vaccine or pneumococcal conjugate vaccine within three years following receipt of PPV23 was associated with increased reactogenicity and reduced antibody titers in comparison to longer intervals. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) was evaluated in adults ≥ 65 years of age with prior history of PPV23 vaccination (V114-007; NCT02573181).
Methods: A total of 250 adults who received PPV23 at least 1 year prior to study entry received a single dose of either PCV15 or PCV13 (125/arm) and were followed for safety for 14 days postvaccination. Serotype-specific Immunoglobulin G (IgG) geometric mean concentrations (GMCs) and opsonophagocytic activity (OPA) geometric mean titers (GMTs) were measured immediately prior and 30 days postvaccination.
Results: Safety profiles were comparable between PCV15 and PCV13 recipients. Following vaccination, serotype-specific antibody responses for the 13 shared serotypes were generally comparable between recipients of PCV15 and PCV13 for IgG GMCs, OPA GMTs, and geometric mean fold rises (GMFRs) and percentages of subjects with ≥ 4-fold-rise from baseline for both IgG and OPA. Recipients of PCV15 had numerically higher antibody responses than PCV13 for two serotypes unique to PCV15 (22F, 33F).
Conclusion: PCV15 was generally well tolerated and induced high levels of IgG and OPA antibodies to all 15 serotypes included in the vaccine when given as a single dose to adults ≥ 65 years of age previously vaccinated with PPV23.
Introduction
Pneumococcal infection is associated with high morbidity and mortality in young children < 5 years of age and adults ≥ 65 years of age, with the greatest burden of mortality occurring in older adultsCitation1,Citation2 Invasive pneumococcal disease (IPD) includes meningitis, sepsis/bacteremia without focus, and bacteremic pneumonia and is associated with a higher case fatality ratio than non-invasive pneumococcal disease (sinusitis, otitis media, nonbacteremic pneumonia)Citation1–Citation4 Incidence of nonbacteremic pneumonia caused by Streptococcus pneumoniae is estimated to be approximately 15 times higher than IPD and represents an important etiology of community-acquired pneumonia (CAP). S. pneumoniae is the most common infection among older adults and approximately 400,000 hospitalizations from pneumococcal pneumonia are estimated to occur annually in the United StatesCitation5
The high incidence of pneumococcal disease in adults 65 years of age and older is mainly due to waning immunity and physiological changes in the respiratory system associated with agingCitation6 In addition, age-related increase in other comorbid medical conditions such as diabetes, stroke, and susceptibility to influenza virus infection have been shown to predispose older adults to pneumoniaCitation7–Citation10
Adult vaccination against pneumococcal disease is recommended in many industrialized countries although vaccine uptake has remained low. Pneumococcal polysaccharide vaccines (PPVs) containing 6–23 serotypes were first developed and were shown to be efficacious against IPD in immunocompetent adults but vaccine effectiveness against nonbacteremic pneumonia varies between studies, depending on the methodology used. PPVs have been shown to be less effective in immunocompromised adults in comparison to immunocompetent adults of the same age range and ineffective in children < 2 years of age due to the immaturity of their immune system. Several pneumococcal conjugate vaccines (PCVs) have been developed in order to overcome the lack of effectiveness of PPVs in children. A 7-valent PCV containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F (PCV7: Prevnar™, Pfizer, Philadelphia, PA) was first licensed in 2000 followed later by the licensure of 10-valent PCV (PCV-10: Synflorix™; GlaxoSmithKline, Rixensart, Belgium), and 13-valent PCV (PCV-13: Prevnar 13™, Pfizer, Philadelphia, PA)Citation11–Citation13 Widespread use of PCVs has been associated with significant reduction in nasopharyngeal carriage and IPD caused by the serotypes included in these vaccines in both vaccinated children and unvaccinated individuals from other age groups (herd protection)Citation14–Citation20
Despite significant advances seen with PCV7 and currently licensed PCV10 and PCV13, serotype replacement remains a concern as new serotypes begin to fill the niche created by the suppression of nasopharyngeal colonization of vaccine serotypes. Notably, serotypes 22F and 33F were shown to be associated with high degree of invasiveness and IPD cases caused by these 2 serotypes have increased in both children and adults in several countriesCitation21–Citation24
The investigational 15-valent pneumococcal conjugate vaccine (PCV15: Merck & Co., Inc., Kenilworth, NJ), contains the 13 serotypes in PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) plus serotypes 22F and 33FCitation25 Previous clinical studies have shown that administration of pneumococcal vaccine within three years following receipt of PPV23 was associated with increased reactogenicity and reduced antibody titers in comparison to longer intervalsCitation26–Citation28 The objective of this study (NCT02573181; V114-007) was to describe the frequency and severity of injection-site and systemic AEs, as well as immune responses (IgG and OPA) following vaccination with PCV15 or PCV13 in subjects who received PPV23 at least 1 year prior to study enrollment.
Results
Study population
A total of 253 subjects 65 years of age and older were given a single dose of either PCV15 (n = 127) or PCV13 (n = 126). The two vaccination groups were comparable in regard to gender, age, ethnicity/race, pre-existing conditions, prior therapy, and time intervals (1–3 years and > 3 years) since receipt of PPV23. There were fewer subjects 75 years of age or older who reported PPV23 vaccination within 1–3 years prior to study enrollment in both vaccination groups, representing 6.3% (8/127) and 4.8% (6/126) among recipients of PCV15 and PCV13, respectively (). In both vaccination groups, all study subjects completed the protocol-specified study visits.
Table 1. Subject characteristics.
Safety
The safety profile of a single dose of PCV15 was generally comparable to that of PCV13. The most commonly reported injection site and systemic AEs were those solicited on the Electronic Vaccine Report Card (eVRC). Overall, more recipients of PCV15 experienced injection-site AEs than recipients of PCV13 and the most frequent injection site AE in both groups was pain (). Nature and rates of most commonly reported systemic AEs were comparable between the 2 vaccination groups, and included fatigue (tiredness), myalgia (muscle pain), headache, and joint pain. In addition, very few subjects reported an elevated body temperature ≥ 100.4 °F (38.0 °C) (). The majority of these reported injection site and systemic events were transient (lasting 1–3 days) and reported as mild (Grade I) to moderate (Grade II) in intensity. No subject discontinued due to an AE. Two SAEs were reported (both in the active control group): acute myocardial infarction on Day 3 postvaccination and periprosthetic fracture on Day 35 (after the protocol-specified safety follow-up period). Neither of the SAEs was considered to be related to the study vaccine by the investigator and both events resolved.
Table 2. Adverse event summary.
Immunogenicity
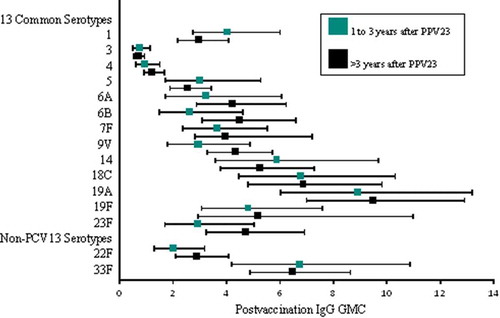
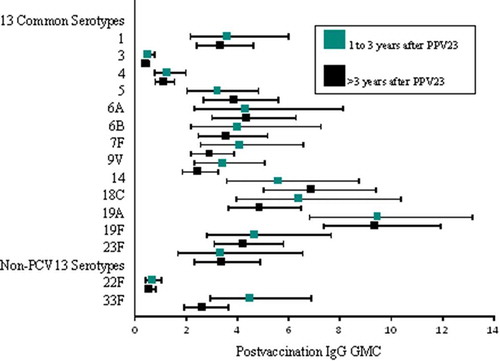
Baseline levels of serotype-specific IgG antibodies varied by serotype and were comparable between the 2 vaccine groups for all 15 serotypes include in PCV15 (). Following vaccination, serotype-specific IgG GMCs increased for all serotypes included in the respective study vaccines. The levels of serotype-specific IgG GMCs measured at 30 days postvaccination and the magnitude of antibody change from baseline to 30 days postvaccination (measured by serotype-specific geometric fold-rise [GMFR] and proportion of individuals with ≥ 4-fold increase) were generally comparable between recipients of PCV15 and PCV13 for the 13 shared serotypes, and higher among recipients of PCV15 than PCV13 for the 2 serotypes unique to PCV15 (22F and 33F) (). Antibody responses measured by OPA assay followed the same trends as those observed with the IgG responses (). Among recipients of PCV15 or PCV13, no notable differences in serotype-specific antibodies were observed when the study vaccine was administered within 1–3 years or more than 3 years after receipt of PPV23 ( and ).
Table 3. Summary of IgG antibody responses (per-protocol population).
Table 4. Summary of OPA antibody responses.
Discussion
Previous studies have suggested that repeated vaccination with PPV23 or initial vaccination with PPV23 followed by PCV13 is associated with blunted immune responses in children and adultsCitation29–Citation32 Older adults 70 years of age with prior history of PPV23 vaccination mounted a significant increase in serotype-specific OPA GMTs following administration of either PCV13 or a second dose of PPV23, but responses were higher in recipients of PCV13. In addition, individuals who received 2 doses of PCV13 had higher OPA GMTs than those who received a dose of PPV23 followed 1 year later by a dose of PCV13Citation32 Our study evaluated the safety and immunogenicity of PCV15 and PCV13 in adults 65 years of age with prior history of PPV23 vaccination and assessed the impact of the time interval between PPV23 and either PCV15 or PCV13. No differences were observed when comparing the tolerability, safety, and antibody responses between recipients of PCV15 and PCV13. The nature, incidence, and intensity of the clinical events reported by recipients of PCV15 were consistent with previous clinical experience with currently licensed pneumococcal conjugate vaccines in the age group evaluated in this clinical trialCitation27,Citation32 Furthermore, time interval between administrations of PPV23 and either PCV15 or PCV13 did not appear to impact the safety profiles of each vaccine and the levels of both IgG GMCs and OPA GMTs measured at 1 month postvaccination within each vaccination group. Such findings are supportive of the policy recommendation for PCV13 administration in adults ≥ 65 years of age with prior history of PPV23 vaccination, requiring that such individuals should receive PCV13 at least one year after PPV23.Citation33 Following vaccination, similar trends in the increases of IgG GMCs and OPA GMTs were observed, and antibody titers were comparable across the 2 vaccination groups for the shared serotypes. As expected, PCV15 induced higher IgG GMCs and OPA GMTs to serotypes 22F and 33F than PCV13.
Our study has several limitations. It was a descriptive study and was not powered to statistically compare the safety and immunogenicity of PCV15 and PCV13. Given the small number of subjects within each time interval between receipt of PPV23 and administration of either PCV15 or PCV13, no statistical analysis was performed to compare the impact of time interval (1–3 years versus > 3 years) on the safety and immunogenicity of PCV15; furthermore, no formal comparison was made to analyze the impact of each time interval (1–3 years and > 3 years) on the safety and immune responses between recipients of PCV15 and PCV13
Overall, PCV15 is highly immunogenic and induces both IgG and OPA to all 15 serotypes included in the vaccine at levels comparable to PCV13 for shared serotypes. PCV15 also induced high levels of antibodies to serotypes 22F and 33F, which are not included in PCV13 and have emerged as leading causes of IPD in both children and older adults following widespread use of PCVs in many countries worldwide.
Methods
Study design
This was a randomized, multi-site, double-blind study comparing the safety, tolerability, and immunogenicity profiles of a single dose of either PCV15 or PCV13 in adults ≥ 65 years of age in good health who were vaccinated with PPV23 at least 1 year prior to study entry. It was conducted from November 2015 through January 2016 at 17 sites in the United States. Approximately 250 subjects were randomly (1:1 ratio) assigned to either PCV15 or PCV13 vaccination group. Randomization was stratified according to subject age (65–74 years of age, ≥ 75 years of age [~ 30% of subjects]) and time since PPV23 vaccination (1–3 years, > 3 years).
Serum samples collected on Day 1 prior to vaccination and on Day 30 postvaccination were assayed for vaccine-induced immune responses to the 15 serotypes contained in PCV15. Primary immunogenicity endpoint was measurement of serotype-specific Immunoglobulin G (IgG) responses using pneumococcal electrochemiluminescence (Pn ECL) assay. Serotype-specific opsonophagocytic activity (OPA) using the multiplexed OPA (MOPA) assay was tested as a secondary endpoint. The Per-protocol (PP) population served as the primary population for the analysis of immunogenicity data in this study. The PP population consisted of those subjects who were not considered protocol violators.
Injection-site and systemic adverse events (AEs) were collected for 14 days postvaccination. Solicited AEs were recorded by subjects on a validated hand-held eVRC and included injection site AEs (i.e., redness, swelling, and pain/tenderness) occurring on Days 1 through 5, and systemic AEs (muscle pain, joint pain, headache, and tiredness) occurring on Days 1 through 14. Body temperature was measured orally and collected Days 1 through 5 postvaccination. Serious AEs were collected from the time the consent form was signed through 30 days postvaccination or over the duration of the subject’s participation in the study.
Study vaccines
Subjects were randomly assigned to 1 of 2 vaccination groups: PCV15 (Lot WL00061714) or PCV13 (Lot L87117/WL00062729). The dose of PCV15 used in this trial is similar to that evaluated in the proof-of-concept clinical trial conducted in adults ≥ 50 years of ageCitation34 PCV15 contains 2μg of 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F and 4μg of 6B; 30μg of CRM197 and 125μg of aluminum phosphate adjuvant per 0.5 mL dose. PCV13 contains 2.2μg of 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, and 23F and 4.4μg of 6B; 34μg of CRM197 and 125μg of aluminum per 0.5mL dose. The dose of PCV13 is consistent with the prescribing recommendation of the vaccine for adults. The timing for the administration of PCV15 or PCV13 in this study is consistent with recommendation from the Advisory Committee on Immunization Practices (ACIP) from US Centers for Disease Control and Prevention (CDC) for adults who have previously received ≥ 1 doses of PPV23 but have not yet received a dose of licensed PCV13.
Study objectives
The primary study objectives were: (1) to describe the safety and tolerability profiles of PCV15 and PCV13 when administered as a single dose in adults ≥ 65 years of age with a prior history of PPV23, and (2) to summarize the serotype-specific IgG responses measured at Day 1 and Day 30 postvaccination in recipients of PCV15 and PCV13 for the 13 shared pneumococcal serotypes contained in both vaccines (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) and the 2 serotypes unique to PCV15 (22F and 33F). The secondary study objective was to summarize the serotype-specific OPA responses measured immediately prior to vaccination at Day 1 and at Day 30 postvaccination in recipients of PCV15 and PCV13 for the 13 shared pneumococcal serotypes contained in both vaccines and the 2 serotypes unique to PCV15. The exploratory study objectives were: (1) to summarize the safety and immunogenicity (as measured by the Pn ECL assay and the MOPA assay) of PCV15 and PCV13 by time since receipt of PPV23 (1–3 years versus > 3 years) for each age cohort (65–74 years of age versus ≥ 75 years of age); and (2) to compare the immunogenicity (as measured by the MOPA and Pn ECL assays) at Day 30 postvaccination in recipients of PCV15 and PCV13 for the 13 shared pneumococcal serotypes contained in both vaccines and the 2 serotypes unique to PCV15.
The primary immunogenicity endpoints were the serotype-specific IgG geometric mean concentrations (GMCs) prior to vaccination at Day 1 and at Day 30 postvaccination, geometric mean fold rise (GMFR) from baseline and proportion of subjects with ≥ 4-fold rise from baseline for the 15 serotypes in PCV15 based on the serotype-specific IgG responses as measured by the Pn ECL assay. The secondary immunogenicity endpoints were the OPA geometric mean titers (GMTs) prior to vaccination at Day 1 and at Day 30 postvaccination, GMFR from baseline and proportion of subjects with ≥ 4-fold rise from baseline for the 15 serotypes in PCV15 based on the OPA responses as measured by the MOPA assay. For IgG GMCs/OPA GMTs and GMFR, the point estimates were calculated by exponentiating the estimates of the mean of the natural log values and the within-group confidence intervals (CIs) were derived by exponentiating the CIs of the mean of the natural log values based on the t-distribution. For the proportion of subjects with ≥ 4-fold rise, the within-group CIs were calculated using the exact method for a single binomial proportion.Citation35 Additionally, estimated Day 30 IgG GMCs and 95% CIs were calculated using a constrained longitudinal data analysis (cLDA) method.Citation36
Key safety measures for an overall assessment of safety included proportions of subjects with: (1) any adverse event (AE) through 14 days postvaccination, (2) any injection-site AE through 14 days postvaccination, (3) any systemic AE through 14 days postvaccination, (4) any SAEs through Visit 2 (~ 30 days postvaccination), (5) any vaccine-related SAEs and any deaths through Visit 2 (~ 30 days postvaccination), and (6) any discontinuation due to an AE. Other key safety parameters included proportions of subjects reporting the following solicited AEs: elevated body temperature as well as injection site swelling, redness, and pain/tenderness occurring Days 1 through 5 postvaccination, and systemic AEs of muscle pain, joint pain, headache, and tiredness occurring Days 1 through 14 postvaccination.
There were no safety hypotheses in this study. The analysis of safety results followed a tiered approach. For Tier 1 safety endpoints, point estimates, risk differences with 95% CIs and corresponding p-values are provided. Tier 2 parameters were assessed via point estimates and risk differences with 95% CIs; only point estimates by group are provided for Tier 3 safety parameters.
Tier 1 safety endpoints included solicited injection-site AEs (redness, swelling, and pain/tenderness) during Day 1 to Day 5 postvaccination, and solicited systemic AEs (muscle pain, joint pain, headache, and tiredness) during Day 1 to Day 14 postvaccination. Temperatures collected from Day 1 through Day 5 were treated as Tier 2 events. These analyses were performed using the stratified Miettinen and Nurminen method, an unconditional, asymptotic methodCitation37 The All Subjects as Treated (ASaT) population was used in analyzing the safety endpoints. The ASaT population consisted of all randomized subjects who received at least 1 dose of study treatment.
Author contributors
JT Peterson and HL Stacey: enrollment of subjects and/or data collection, review of the manuscript.
JE MacNair, J Li, and P Benner: analysis and interpretation of data, and preparation of manuscript.
JS Hartzel, TM Sterling, GM Tamms, and LK Musey: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Financial Disclosures
No author was paid for their work on this manuscript.
Acknowledgments
Jon E. Stek (Merck & Co., Inc.) assisted the authors with various administrative activities related to the submission of the manuscript and provided editorial assistance.
Additional information
Funding
References
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al.. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi:10.1086/648593.
- Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) report: emerging infections program network streptococcus pneumoniate, provisional-2009 [web-based report]. [accessed 2018 Mar 29] http://www.cdc.gov/abc.
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al.. Epidemiology of invasive streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735.
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Report emerging infections program network streptococcus pneumoniae, 1997 [web-based report]. [accessed 2018 Mar 29]. http://www.cdc.gov/ncidod/dbmd/abcs.
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi:10.1016/j.vaccine.2011.02.088.
- Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi:10.1016/S1473-3099(04)00931-4.
- Kyaw MH, Rose CE Jr, Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi:10.1086/431521.
- Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–35. doi:10.1016/j.ijid.2015.05.003.
- Baxter R, Yee A, Aukes L, Snow V, Fireman B, Atkinson B, Klein NP. Risk of underlying chronic medical conditions for invasive pneumococcal disease in adults. Vaccine. 2016;34:4293–4297. doi:10.1016/j.vaccine.2016.07.003.
- Morton JB, Morrill HJ, LaPlante KL, Caffrey AR. Risk stacking of pneumococcal vaccination indications increases mortality in unvaccinated adults with Streptococcus pneumoniae infections. Vaccine. 2017;35:1692–1697. doi:10.1016/j.vaccine.2017.02.026.
- Prevnar [package insert]. Philadelphia (PA): Wyeth Pharmaceuticals Inc. [accessed 2018 Mar 29]. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm137038.pdf.
- Synflorix [package insert]. Rixensart (Belgium): GlaxoSmithKline Biologicals s.a. [accessed 2018 Mar 29]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf.
- Prevnar 13 [package insert]. Philadelphia (PA): Wyeth Pharmaceuticals Inc. [accessed 2018 Mar 29]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM574852.pdf.
- Kaplan SL, Center KJ, Barson WJ, Ling-Lin P, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Peters TR, Gurtman A, et al.. Multicenter surveillance of Streptococcus pneumoniae isolates from middle ear and mastoid cultures in the 13-valent pneumococcal conjugate vaccine era. Clin Infect Dis. 2015;60:1339–1345. doi:10.1093/cid/civ067.
- Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother. 2014;58:6484–6489. doi:10.1128/AAC.03344-14.
- Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola SW, et al.. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J. 2015;34:1168–1174. doi:10.1097/INF.0000000000000849.
- Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, et al.. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58:918–924. doi:10.1093/cid/ciu006.
- Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. doi:10.1056/NEJMoa1209165.
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014;2:387–394. doi:10.1016/S2213-2600(14)70032-3.
- Rodrigo C, Bewick T, Sheppard C, Greenwood S, Mckeever TM, Trotter CL, Slack M, George R, Lim WS. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J. 2015;45:1632–1641. doi:10.1183/09031936.00183614.
- Yildirim I, Hanage WP, Lipsitch M, Shea KM, Stevenson A, Finkelstein J, Huang SS, Lee GM, Kleinman K, Pelton SI. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–288. doi:10.1016/j.vaccine.2010.10.032.
- Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, Tyrrell GJ, Desai S, Sherrard L, Adam H, et al.. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol. 2013;59:778–788. doi:10.1139/cjm-2013-0614.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al.. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–309. doi:10.1016/S1473-3099(14)71081-3.
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. doi:10.1016/S1473-3099(15)70044-7.
- Sobanjo-Ter Meulen A, Vesikari T, Malacaman EA, Shapiro SA, Dallas MJ, Hoover PA, McFetridge R, Stek JE, Marchese RD, Hartzel J, et al.. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2015;34:186–194. doi:10.1097/INF.0000000000000516.
- Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55-70 years of age. Clin Infect Dis. 2009;49:241–248. doi:10.1086/599824.
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–3602. doi:10.1016/j.vaccine.2013.04.084.
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32:2364–2374. doi:10.1016/j.vaccine.2014.02.002.
- Sigurdardottir ST, Center KJ, Davidsdottir K, Arason VA, Hjalmarsson B, Elisdottir R, Ingolfsdottir G, Northington R, Scott DA, Jonsdottir I. Decreased immune response to pneumococcal conjugate vaccine after 23-valent pneumococcal polysaccharide vaccine in children. Vaccine. 2014;32:417–424. doi:10.1016/j.vaccine.2013.11.029.
- Moberley S, Licciardi PV, Balloch A, Andrews R, Leach AJ, Kirkwood M, Binks P, Mulholland K, Carapetis J, Tang MLK, et al. Repeat pneumococcal polysaccharide vaccine in Indigenous Australian adults is associated with decreased immune responsiveness. Vaccine. 2017;35:2908–2915. doi:10.1016/j.vaccine.2017.04.040.
- Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, Waqatakirewa L, Pryor J, Nelson J, Byrnes GB, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine. 2010;28:3341–3349. doi:10.1016/j.vaccine.2010.02.087.
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31:3585–3593. doi:10.1016/j.vaccine.2013.05.010.
- Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, Pilishvili T. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). Mmwr. 2015;64:944–947. doi:10.15585/mmwr.mm6434a4.
- Ermlich SJ, Andrews CP, Folkerth S, Rupp R, Greenberg D, McFetridge RD, Hartzel J, Marchese RD, Stek JE, Abeygunawardana C, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adults ≥50 years of age. Vaccine. 2018;36(45):6875–6882. doi:10.1016/j.vaccine.2018.03.012.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. doi:10.1093/biomet/26.4.404.
- Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankyha: Indian J Stat. 2000;62(Series B, Part 1):134–148.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226.