ABSTRACT
Immune responses to 13-valent pneumococcal conjugate vaccine (PCV13) and quadrivalent inactivated influenza vaccine (QIV) in older adults may vary with coadministration and previous pneumococcal polysaccharide vaccination. This study assessed safety and noninferiority of immune responses to coadministered PCV13 and QIV compared with each vaccine given alone. Adults ≥50 years old preimmunized with ≥1 dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) ≥1 year before enrollment were randomized 1:1 to receive PCV13+QIV then placebo 1 month later or placebo+QIV then PCV13 1 month later. Administration of PCV13 and placebo was blinded; QIV was administered open-label. Pneumococcal serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) 1 month after PCV13, and influenza hemagglutination inhibition assay GMTs 1 month after QIV were measured. Prespecified noninferiority was demonstrated by a lower bound of the 2-sided 95% CI for geometric mean ratios >0.5. Safety endpoints included proportions of subjects with adverse and serious adverse events. Of 882 randomized subjects, 846 comprised the evaluable immunogenicity population. Immune responses to all 13 pneumococcal serotypes and all 4 influenza strains 1 month after PCV13+QIV were noninferior to responses 1 month after each vaccine given alone. No safety concerns were identified. Immune responses to coadministered PCV13 and QIV were noninferior to responses after each vaccine given alone, although generally lower for coadministered PCV13. PCV13 and QIV can be administered concomitantly to adults ≥50 years of age preimmunized with PPSV23.
Introduction
Disease caused by Streptococcus pneumoniae is responsible for substantial global morbidity and mortality.Citation1 The World Health Organization estimates that 1.6 million people die from pneumococcal disease annually.Citation2 Among adults, the most common clinical manifestation of pneumococcal disease is pneumonia.Citation3 Pneumococcal pneumonia also complicates influenza infection,Citation4,Citation5 another important contributor to adult morbidity and mortality.Citation6 In the United States, seasonal influenza vaccination in adults is the primary means of preventing influenza illness and its complicationsCitation7,Citation8 and offers an important vaccine opportunity for pneumococcal disease as well.
The 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13®, Pfizer Inc, New York, NY) is licensed in the United States for prevention of pneumonia and invasive pneumococcal disease in adults ≥50 years old.Citation9–Citation11 Previous studies evaluating coadministration of PCV13 and trivalent inactivated influenza vaccine (TIV) demonstrated an acceptable safety profile among adults aged 50 to 59 years and ≥65 years, but differences were observed in immune responses to PCV13 coadministered with TIV compared with PCV13 alone. In general, responses to PCV13 measured 1 month after vaccination were lower with cadministered PCV13 and TIV; responses to TIV were not significantly different, with similar findings of reduced OPA titers 1 month after coadaministration compared to PCV13 alone.Citation12,Citation13 The same subjects from one of these studiesCitation12 were evaluated for circulating antibodies annually for 5 years.Citation14 No differences were observed between the coadministration group and the group given PCV13 alone. Responses in both groups to a single PCV13 booster dose given 5 years after initial vaccination were generally the same as – or higher than – responses after the first dose. The differences in responses observed in the coadministration group in the initial study did not translate into differences in circulating antibody levels 5 years later; nor did those differences affect revaccination responses indicative of establishment of immune memory.
Immune responses to PCV13 coadministered with seasonal quadrivalent inactivated influenza vaccine (QIV) have not been evaluated among adults ≥50 years old previously immunized with the 23-valent pneumococcal polysaccharide vaccine (PPSV23). In adult studies, prior PPSV23 receipt decreased responses to subsequent PCV13 immunization.Citation15–Citation17 Given concerns regarding the possible cumulative effect of reduced immune responses in adults preimmunized with PPSV23 and reduced responses to PCV13 when the vaccine is given with influenza vaccine, this study evaluated the immunogenicity of PCV13 coadministered with QIV compared with each vaccine given alone in adults aged ≥50 years who had previously received ≥ 1 dose of PPSV23.
Results
Baseline characteristics and disposition of subjects
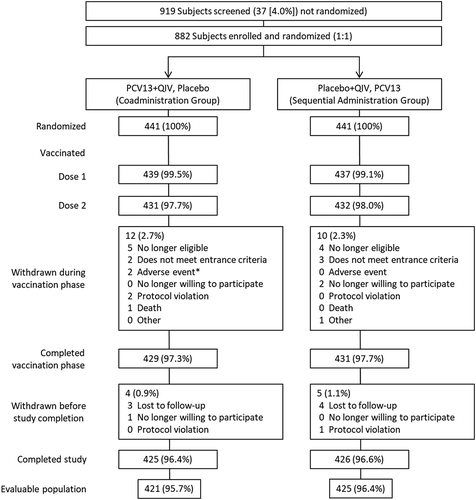
A total of 882 subjects were enrolled and randomized (441 per group; ). The evaluable immunogenicity population consisted of 421 subjects in the PCV13+QIV group and 425 in the QIV- or PCV13-alone group. Among the evaluable immunogenicity population, 55.2% were female, 89.4% were white, and the mean (SD) age was 66.7 (8.96) years at randomization. The majority (93.1%) of subjects had 1 previous dose of PPSV23, and the remainder had ≥2 doses. The mean time from previous PPSV23 receipt was 5.9 years. In all, 97.9% of subjects in the PCV13+QIV group and 99.3% of subjects in the placebo+QIV group reported a medical condition at the first visit. Across both groups, 17.7% of subjects reported cardiac disorders, 6.1% reported chronic obstructive pulmonary disease, 11.9% reported asthma, 0.8% reported type 1 diabetes mellitus, and 25.4% reported type 2 diabetes mellitus. At least one of these conditions was reported by 48.5% and 50.6% of subjects in the PCV13+QIV and PCV13-alone groups, respectively (see Supplementary Table 1).
Figure 1. Subject disposition.
PCV13 = 13-valent pneumococcal conjugate vaccine; QIV = quadrivalent inactivated influenza vaccine. *1 subject reported colitis 19 days after vaccination 1 (not related to study vaccine); 1 subject experienced mild injection-site induration and pain (both vaccines) 1 day after vaccination 1, resolving after 31 days (related to study vaccines).
Immunogenicity
Responses to PCV13
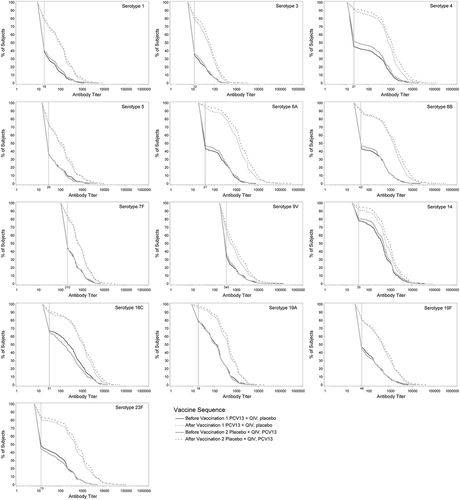
Before PCV13 immunization, pneumococcal OPA GMTs were generally similar between groups (see Supplementary Table 2). In both vaccine groups, OPA titers for all 13 serotypes were several-fold higher after PCV13 than before PCV13 administration. When comparing GMTs between vaccine groups, the noninferiority criterion (lower limit of 95% CI >0.5) was met: pneumococcal OPA GMTs for all 13 serotypes measured 1 month after PCV13+QIV were noninferior to OPA GMTs 1 month after PCV13 alone. Pneumococcal OPA GMTs were generally lower 1 month after PCV13+QIV compared with 1 month after PCV13 given alone. GMTs for serotypes 3, 4, 6A, and 14 were statistically significantly lower after PCV13+QIV than PCV13 alone (). Reverse cumulative distribution curves showed that responses 1 month after PCV13+QIV were generally slightly lower for most serotypes across the range of responses than 1 month after PCV13 alone ().
Table 1. Comparison of Pneumococcal OPA GMTs 1 Month After PCV13+ QIV vs 1 Month After PCV13 Alone (Evaluable Immunogenicity Population).
Figure 2. Pneumococcal OPA titer reverse cumulative distribution curves before and 1 month after PCV13 administration.
PCV13 = 13-valent pneumococcal conjugate vaccine; QIV = quadrivalent inactivated influenza vaccine. Reverse cumulative distribution curves displaying pneumococcal OPA titers for PCV13 serotypes before (solid lines) and after (dotted lines) PCV13 immunization are shown (evaluable immunogenicity population). Black line, PCV13+QIV/placebo; gray line, placebo+QIV/PCV13. Vertical line, OPA lower limit of quantitation titers for each serotype.
Responses to QIV
Baseline HAI GMTs were similar in both vaccine groups. For both vaccine groups, HAI GMTs for all influenza strains in QIV were statistically significantly higher after vaccination (see Supplementary Table 3).
For each influenza strain in QIV, noninferiority was met for PCV13+QIV compared with QIV alone. Additionally, GMTs for A/H3N2 were statistically significantly higher 1 month after PCV13+QIV compared with 1 month after QIV alone (). Seroconversion rates were low but similar between groups, ranging from 21.3% to 31.6%. The differences in proportions of responders for all influenza strains in QIV between the vaccine groups were small, ranging from −3.8% to 5.1% ().
Table 2. Comparison of Influenza HAI GMTs 1 Month After PCV13+QIV vs Placebo+QIV (Evaluable Immunogenicity Population).
Table 3. Comparison of Seroconversion Rates of Influenza Strains Between PCV13+QIV and Placebo+QIV (Evaluable Immunogenicity Population).
The proportion of subjects with titers ≥40 for influenza strains before and after QIV was assessed in a post hoc analysis. Before QIV immunization, proportions with a titer ≥40 were similar between vaccine groups for all influenza strains in QIV, except for a statistically significant difference for strain A/H3N2 (87.1% before PCV13+QIV vs 80.9% before QIV alone; P = 0.013). The percentage of subjects achieving HAI titers ≥40 1 month after PCV13+QIV and 1 month after QIV alone were similar: 90.6% and 89.3% for A/H1N1, 98.4% and 96.5% for A/H3N2, 49.2% and 46.5% for B/Brisbane, and 65.8% and 63.3% for B/Massachusetts (see Supplementary Figure 2).
Safety
Adverse events were assessed from enrollment through 1 month after vaccination 2. AEs were reported by 15.3% of subjects after PCV13+QIV and by 12.5% of subjects after PCV13; this difference was not statistically significant. The most frequently reported individual AEs after PCV13+QIV were injection-site pain (1.8%), upper respiratory tract infection (1.8%), and urinary tract infection (1.1%). The most frequently reported individual AEs after PCV13 alone were upper respiratory tract infection, urinary tract infection, bronchitis, and sinusitis (all 0.9%). AEs were reported by 11.9% of subjects after QIV alone; the most frequently reported individual AEs in this group were upper respiratory tract infection, pyrexia, and bronchitis (all 0.9%). AEs were reported by 10.4% of subjects after vaccination 2 (placebo) in the PCV13+QIV group; two subjects withdrew from the study due to an AE. One subject reported colitis 19 days after PCV13+QIV, which was not considered related to study vaccine based on the subject’s chronic medical conditions and medication use. Another subject experienced mild AEs of injection-site induration and pain at both vaccination sites 1 day after vaccination. The AEs in the second subject lasted 31 days, resolved, and were considered related to study vaccines.
Severe AEs after PCV13 were reported by a low and similar proportion of subjects in both vaccine groups (0.9%–1.1%). One subject in the PCV13+QIV group died of cardiogenic shock 4 days after placebo receipt (PCV13+QIV were given 35 days before death). Related AEs were reported by a low and similar proportion of subjects in both groups (2.8%–3.0%). Injection-site pain was the most frequently reported individual related AE in both groups (PCV13+QIV, 1.8%; PCV13 alone, 0.7%). No individual SAE was reported by >1 subject (0.2%) in either group, and none was considered related to study vaccines.
Discussion
The risk for contracting invasive pneumococcal disease increases with age and with influenza infection. Annual seasonal influenza vaccination of older adults may present an important opportunity for healthcare providers to also offer pneumococcal vaccination. Prior studies of healthy adults aged 50 to 59 years and ≥65 years naïve to pneumococcal vaccination indicated that concomitant PCV13+TIV induced generally lower pneumococcal OPA GMTs compared with PCV13 given alone, although the antibody responses for the majority of serotypes were noninferior with coadministration.Citation12,Citation13 The clinical significance of these findings is not known; however, a follow-up study found that the differences in response to vaccination did not persist. In the follow-up study, subjects were given a single dose of PCV13 5 years after initial immunization with PCV13+TIV or PCV13 alone. Regardless of whether PCV13 and TIV were given separately or concomitantly, antibody responses 1 month after vaccination were equivalent to – or higher than – responses 1 month after the initial dose, which is indicative of establishment of immunologic memory. These data suggest that concomitant administration of these vaccines does not seem to have a lasting effect on antibody responses or the ability to respond to revaccination with PCV13.Citation14 Based on these data, the lower immune responses observed after coadministration during initial vaccination are not likely to negatively affect protection against vaccine-type pneumococcal disease.
In other studies, prior PPSV23 immunization consistently resulted in lower responses to a subsequent dose of either PCV13 or PPSV23 at an interval of either 1 or 4 years between doses in older adults.Citation15–Citation17 These data have elicited concern that previous PPSV23 administration may lead to an additive negative effect on immune responses to PCV13.
The current study is the first to evaluate the safety and immunogenicity of PCV13 when coadministered with QIV in adults aged ≥50 years who had been vaccinated with PPSV23 at least 1 year before enrollment. Immune responses to coadministered PCV13 and QIV were noninferior to responses elicited by each vaccine administered 1 month apart. The noninferiority criterion was met for OPA GMTs for all 13 pneumococcal serotypes, although similar to observations with PCV13 and TIV, GMTs were generally lower 1 month after PCV13+QIV compared with PCV13 given alone. GMTs after PCV13+QIV were significantly lower – but still noninferior – for serotypes 3, 4, 6A, and 14.
The noninferiority criterion for influenza HAI responses was met for each influenza strain in QIV when comparing PCV13+QIV with QIV given alone, and proportions of subjects achieving titers ≥40 for all 4 influenza strains after vaccination were similar between vaccine groups. High prevaccination QIV titers may be explained by prior exposure to identical antigens during the course of natural disease or in seasonal influenza vaccines produced for the 2013–2014 influenza season, the year before this study.Citation8,Citation18
No notable safety concerns were observed for either study group; AE incidence was similar among PCV13+QIV, PCV13 alone, QIV alone, and placebo groups. No SAEs were considered related to study vaccine, and AEs were generally consistent with conditions common in this age group and with the known safety profile of each vaccine.
The clinical relevance of this study is notable, as recommended seasonal influenza vaccination and pneumococcal vaccination in older adults play essential roles in preventing respiratory infections that constitute substantial clinical and economic burdens of disease. The current study population consisted of older adults with risk factors for pneumococcal disease that prompted their prior immunization with PPSV23. The results presented here demonstrate that immune responses to PCV13 and QIV, when given concomitantly to adults previously vaccinated with PPSV23, are noninferior to immune responses elicited by PCV13 and QIV given separately 1 month apart, despite generally lower immune responses to PCV13 serotypes in the coadministration group. In a follow-up to the study in which PCV13 was coadministered with TIV,Citation14 no differences were observed in circulating antipneumococcal antibody levels tested annually for 5 years after initial PCV13+TIV vaccination compared to when each vaccine was given alone. These results suggested that the small differences observed 1 month after initial coadministration of PCV13 and TIV did not have a long-term impact. It is reasonable to conclude the same is also likely to be true for PCV13 and QIV coadministration.
Study strengths include a large sample size powered for establishing noninferiority and a double-blind, placebo-controlled study design. A study limitation includes the lack of a direct comparator group of PPSV23-naïve subjects, although not including such a group does not diminish the study findings.
Conclusion
Immune responses to PCV13 and QIV after concomitant administration were noninferior to responses after each vaccine was given alone, with safety findings consistent with prior experience with PCV13 in adults. Compared with separate immunizations, PCV13 and QIV demonstrated satisfactory safety and immunogenicity profiles when administered concomitantly to adults ≥50 years of age preimmunized with PPSV23.
Patients and methods
Study design
This phase 4, randomized, placebo-controlled, double-blind, parallel-group study (ClinicalTrials.gov identifier, NCT02124161) was an FDA postmarketing commitment conducted between September 2014 and May 2015 at 42 sites in the United States. The protocol was reviewed and approved by the institutional review board(s) for each participating investigational site, and the study was conducted according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All participants provided written informed consent before enrollment and before performance of study-related procedures. Medical history was obtained from all subjects at study visit 1, and subjects’ race and ethnicity were self-identified. PCV13 and placebo were administered in a double-blind fashion; QIV administration was open-label.
Participants
Eligible subjects were ≥50 years old and had received ≥1 dose of PPSV23 at least 1 year before enrollment. Subjects with chronic, nonserious, stable disease not requiring substantial change in therapy or hospitalization 6 weeks before vaccination were eligible. Exclusion criteria included previous vaccination with a pneumococcal conjugate vaccine, history of severe adverse reactions to any vaccine or vaccine-related component, vaccination with any influenza vaccine within 6 months before study vaccination 1, documented S. pneumoniae infection within the past 5 years, serious chronic disorders (including metastatic malignancy, severe chronic obstructive pulmonary disease requiring supplemental oxygen, end-stage renal disease, or clinically unstable cardiac disease), known or suspected immunodeficiency, and permanent residence in a nursing home.
Interventions
Subjects were randomized 1:1 to receive PCV13+QIV concomitantly followed by placebo 1 month later (concomitant administration group) or placebo+QIV concomitantly followed by PCV13 1 month later (QIV-alone or PCV13-alone group). PCV13 and placebo were administered intramuscularly in the left deltoid and QIV in the right deltoid. Subjects were stratified by age (50–64 years and ≥65 years) and by years since last PPSV23 dose (1–5 years and >5 years) to maintain balance within vaccine and age groups. Within each stratum, subjects were randomized 1:1 to vaccine groups. Blood samples were taken immediately before vaccination 1 and 1 month after vaccinations 1 and 2 (see Supplementary Figure 1).
Vaccines administered
PCV13 (lot #H32094, Pfizer Inc) contains polysaccharides from pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F individually conjugated to nontoxic diphtheria toxin cross-reactive material 197 (CRM197). Each 0.5-mL dose contains 2.2 µg of each polysaccharide, except for 4.4 µg for 6B, 5 mM succinate buffer, 0.85% sodium chloride, 0.02% polysorbate 80, and 0.125 mg aluminum as aluminum phosphate. Placebo (lot #J13932) matched the PCV13 formulation for aluminum phosphate, succinate buffer, sodium chloride, and polysorbate 80. QIV (Fluzone Quadrivalent; lot #UI158AA) was manufactured by Sanofi Pasteur MSD (Swiftwater, PA, USA) and contained hemagglutinin antigens from 4 influenza strains: 2 A strains(A/H1N1/California/7/2009 and A/H3N2/Texas/50/2012) and 2 B strains (B/Brisbane/60/2008 and B/Massachusetts/2/2012).
Study objectives
The coprimary objectives were to demonstrate that immune responses induced 1 month after concomitant vaccination with PCV13 and QIV were noninferior compared to immune responses induced by either vaccine given alone and to assess the safety profile of PCV13 given concomitantly with QIV and alone.
Immunogenicity assessments
The primary immunogenicity endpoints were pneumococcal serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) 1 month after PCV13 administration (1 month after vaccination 1 in the PCV13+QIV group and 1 month after vaccination 2 in the PCV13-alone group). Coprimary endpoints were influenza hemagglutination inhibition assay (HAI) GMTs for each strain in QIV 1 month after QIV receipt (1 month after vaccination 1 in the PCV13+QIV and QIV-alone groups). Secondary endpoints were pneumococcal serotype-specific OPA geometric mean fold rises (GMFRs), the proportion of subjects achieving seroconversion in HAI titers, and HAI GMFRs for each influenza virus strain.
The evaluable immunogenicity population was the primary analysis population and included eligible subjects who were randomized, had received all vaccinations specified per vaccination sequence, had at least 1 valid and determinate assay result for pneumococcal serotype or QIV antigen, had blood draws within the specified time frame, and had no major protocol violations. A post hoc analysis was conducted to determine the proportion of subjects with a titer ≥40 for each influenza strain before and after QIV administration.
Safety assessments
Safety endpoints were the proportions of subjects with adverse events (AEs) and serious AEs (SAEs), which were recorded from enrollment to 1 month after vaccination 2. The safety population included those who received ≥ 1 dose of investigational product. Solicited reactogenicity information was not collected because such data have been collected in previous studies.Citation12,Citation16
Sample size
Sample sizes were based on OPA and HAI assay variability and were estimated from previous studies.Citation16,Citation19 Based on a dropout rate of 7%, 410 evaluable subjects per group were needed to ensure a power of ≥90% across all endpoints when comparing results between study arms. It was assumed that time-related and sequence effects were negligible relative to the effects of vaccination and covaccination.
Statistical analyses
This study was powered to show noninferiority of PCV13+QIV relative to PCV13 alone for pneumococcal OPA GMTs and noninferiority of PCV13+QIV relative to QIV alone for HAI GMTs. The noninferiority criterion was set at 2-fold (lower bound of 2-sided 95% CI for geometric mean ratios >0.5) for PCV13+QIV relative to PCV13 alone; the same criterion was used to assess PCV13+QIV relative to QIV alone. The pneumococcal immunogenicity criteria are the same as those used in phase 3 PCV13 immunogenicity studies.Citation20
The OPA GMTs for each serotype were determined before any vaccination and 1 month after vaccinations 1 and 2. Differences in OPA GMTs were considered significantly lower if the upper limit of the 95% CI for GMT ratios was <1 and significantly higher if the lower limit of the 95% CI for GMT ratios was >1.
The HAI GMTs and the 2-sided 95% CIs were determined similarly to OPA GMTs. The proportion of subjects achieving seroconversion in HAI titers depended on baseline titers. Seroconversion for influenza strains was defined as a prevaccination 1 (baseline) HAI titer <1:10 and a postvaccination 1 HAI titer ≥1:40, or a prevaccination 1 HAI titer ≥1:10 and a minimum 4-fold rise in postvaccination 1 HAI titer compared to the baseline titer.Citation21
Disclosure of potential conflicts of interest
NPK reports research grants from Pfizer, Merck & Co., GlaxoSmithKline, Sanofi Pasteur, Protein Science, and MedImmune for other vaccine research. HJD reports research grants from GlaxoSmithKline and Pfizer for vaccine research. AT, WW, KC, KUJ, WCG, DAS, and BS-T are employees of Pfizer Inc and may hold stock. SP and SS were employees of Pfizer Inc at the time of writing. SP is currently an employee of Sanofi Pasteur, Swiftwater, PA. VS, an employee of inVentiv Health Clinical, LLC, a company contracted by Pfizer Inc, was a paid consultant to Pfizer for the statistical analysis and support for this study.
Supplemental Material
Download MS Word (96.2 KB)Acknowledgments
To our sorrow, Roger Baxter, MD, passed away before study results could be submitted for publication. We would like to formally acknowledge him as an essential contributor to this study and corresponding manuscript and are grateful for his substantial contributions to all aspects of this work. We also thank the study participants, clinical staff, other investigators, and team members who contributed to the study. We thank James Trammel, MS, and the programming staff at inVentiv Health Clinical for support with data analysis. Editorial/medical writing support was provided by Jill E. Kolesar, PhD, of Complete Healthcare Communications, LLC, and was funded by Pfizer Inc. Additional editorial support was provided by Ann L. Davis, MPH, CMPP, an employee of Pfizer Inc.
SP, WCG, DAS, AT, and BS-T designed the study; RB, HJD, NPK, WW, KUJ, SS, AT, and BS-T acquired data; all authors contributed to data analysis and interpretation, and the drafting, review, and approval of the manuscript. Roger Baxter acquired data and contributed to data analysis and interpretation, and the drafting, review, and approval of the penultimate draft of the manuscript. SP and VS also contributed to statistical analyses. AT declares that she had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data reported in this manuscript were presented previously as an oral presentation at the 4th Annual Meeting of the Infectious Diseases Society of America (IDWeek) in San Diego, CA, USA, on October 10, 2015.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;(1):CD000422. doi:10.1002/14651858.CD000422.pub2.
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine: WHO position paper. Wkly Epidemiol Rec. 2008;83(42):373–384.
- Centers for Disease Control and Prevention. Pneumococcal Disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington (DC): Public Health Foundation; 2015. p. 279–296.
- Walter ND, Taylor TH, Shay DK, Thompson WW, Brammer L, Dowell SF, Moore MR; Active Bacterial Core Surveillance Team. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50(2):175–183. doi:10.1086/649208.
- Boyd M, Clezy K, Lindley R, Pearce R. Pandemic influenza: clinical issues. Med J Aust. 2006;185(10 Suppl):S44–7.
- Appiah GD, Blanton L, D’Mello T, Kniss K, Smith S, Mustaquim D, Steffens C, Dhara R, Cohen J, Chaves SS, et al. Influenza activity - United States, 2014–15 season and composition of the 2015–16 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2015;64(21):583–590.
- Fluzone®. influenza vaccine. Swiftwater (PA): Sanofi Pasteur Inc; 2015.
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB; Influenza Division NCfI, Respiratory Diseases C. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697.
- PREVNAR. 13® Pneumococcal 13-Valent Conjugate Vaccine [Diphtheria CRM197 Protein]. Collegeville (PA): Pfizer Inc; 2014.
- Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T; Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–825.
- Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, Pilishvili T. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944–947. doi:10.15585/mmwr.mm6434a4.
- Frenck RW Jr., Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D, Giardina PC, Emini EA, Gruber WC, Scott DA, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19(8):1296–1303. doi:10.1128/CVI.00176-12.
- Schwarz TF, Schmoele-Thoma B. Assessment of functional antibacterial opsonophagocytic antibodies elicited by 13-valent pneumococcal conjugate vaccine administered concomitantly with trivalent influenza vaccine in a randomized clinical trial in adults aged ≥65 years. Vaccine. 2013;31(2):291–294. doi:10.1016/j.vaccine.2012.10.077.
- Frenck RW Jr., Fiquet A, Gurtman A, van Cleeff M, Davis M, Rubino J, Smith W, Sundaraiyer V, Sidhu M, Emini EA, et al. Immunogenicity and safety of a second administration of 13-valent pneumococcal conjugate vaccine 5 years after initial vaccination in adults 50 years and older. Vaccine. 2016;34(30):3454–3462. doi:10.1016/j.vaccine.2016.04.093.
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60–64 years of age. Vaccine. 2014;32(20):2364–2374. doi:10.1016/j.vaccine.2014.02.002.
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31(35):3585–3593. doi:10.1016/j.vaccine.2013.05.010.
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–3602. doi:10.1016/j.vaccine.2013.04.084.
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the advisory committee on immunization practices–United States, 2013–2014. MMWR Recomm Rep. 2013;62(RR–07):1–43.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewe W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged >/=18 years: a phase III, randomized trial. Vaccine. 2014;32(13):1480–1487. doi:10.1016/j.vaccine.2014.01.022.
- deVore N Summary basis for regulatory action BLA/STN 125324/262; 2011. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM287412.pdf.
- US Food and Drug Administration. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines; 2007. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm.
