ABSTRACT
Objective: To evaluate the efficacy of an intervention utilizing patient navigators (PNs) to 1) educate families on human papillomavirus (HPV) vaccination in a clinic setting and 2) provide personalized reminders for follow-up.
Method: Families with 9–17 year-old children who had no record of completing the HPV vaccination series receiving primary or specialty care in 3 pediatric clinics were approached by PNs between February 1, 2015 and August 31, 2016. Demographic characteristics, visit type, preferred contact method, rates and correlates of completion, and appointments missed were analyzed. In addition, qualitative interviews of 21 providers and PNs assessed their perceptions of the program.
Results: 1,391 adolescents were identified out of 2,162 patients approached as unvaccinated or partially vaccinated prior starters; among the unvaccinated, 930 received the 1st dose after being counseled by the PN (66.9%), either immediately or at a follow-up visit soon thereafter. This included 118 siblings of patients who did not have an appointment that day. Of initiators approached between 2/1/2015 and 8/31/2016, 93% (864/930) completed the series by 8/31/2017. No differences in series completion among initiators were observed by gender or race/ethnicity, but older patients (15–17 years old) were less likely to complete than 11–12 year olds. Of the 688 patients identified as prior starters, 85% completed the series through the program. Qualitative interviews demonstrated that providers felt the program addressed major barriers to HPV vaccination.
Conclusion: Employing PNs dramatically increased HPV vaccine series completion among boys and girls with historically low HPV vaccination rates at pediatric clinics in Texas. Clinic providers felt this program addressed many barriers they observed prior to program implementation. This approach could markedly improve HPV vaccine series completion rates in the US.
Introduction
Despite its proven safety and effectiveness, human papillomavirus (HPV) vaccine initiation and completion rates in the US remain low.Citation1-Citation5 In fact, only 43.4% of all 13–17 year-olds in the United States were up to date with the recommended HPV vaccination series (49.5% for females; 37.5% for males) by 2016, with Texas having even lower rates of 32.9% of adolescents up to date.Citation6 Rates are especially low among children of black caregivers, caregivers unable to get an immunization appointment when needed, those unable to reach their provider, patients of lower socioeconomic status, and children with public insurance.Citation7 Qualitative studies also found that providers expect parents to make follow-up appointments and parents expect to be told when they need to make appointments.Citation8
Few interventions to increase HPV vaccination rates thus far have demonstrated much success.Citation9,Citation10 One reason is that many programs addressed only one issue while barriers exist at multiple levels. To be effective, interventions need to address multiple barriers which may include provider and patient awareness, cost, identification of patients in need of vaccination at all appointments, and missed follow-up appointments.Citation11 We conducted an intervention program using on-site patient navigators (PNs) in 3 pediatric clinics serving low-income patients with low vaccination rates to address these common barriers.Citation12 This program evaluation describes the intervention, HPV vaccine initiation among approached patients, vaccine series completion, and perceptions of PNs and providers who participated in program implementation.
Results
Quantitative program evaluation
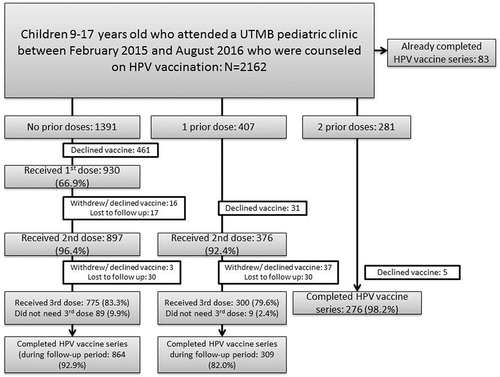
Among the 2,162 children approached, 83 had already completed the HPV vaccine series elsewhere (). Among 1,391 children who had received 0 prior doses, 930 received the 1st dose (66.9%), with a decline rate of 23%. Among those who initiated the 1st dose through this program, 16 declined the 2nd vaccine dose or withdrew from the vaccination program and 17 were lost to follow-up, with 897 receiving a 2nd dose (96%). A total of 775 (83%) received a 3rd dose after an additional 3 declined the last dose and 30 additional patients were lost to follow-up. Due to changes in recommendations that occurred during the time period covered by this project, 89 participants ≤14 years old completed after 2 doses. Thus, the overall completion rate among initiators through this program was 93%.
Among 407 prior starters, 376 received their 2nd dose (92%) after 31 patients declined the vaccine dose. This completed the series for 9. Three hundred received a final 3rd dose after 37 declined the final dose and 30 patients were lost to follow-up. Finally, 281 had previously received 2 doses of the vaccine. Of these, 276 received the 3rd dose. Overall, the completion rate among prior starters was 85.0%. Among all patients who participated in the program (initiators and prior starters), 91.6% completed the series.
Slightly over half of program-eligible children were male (). Patients were approached in clinics staffed by both primary care providers and pediatric specialists, who share clinic common areas. The same resources were available in both areas. Of the 1,117 unvaccinated patients visiting a generalist, 74.6% received their first dose through this program compared to 33% of 264 unvaccinated patients who had an appointment with a specialist (results not shown). Siblings comprised 118 of initiators who did not have a scheduled appointment on the day they were approached. Most vaccination fees (72.6%) were covered by Medicaid (supplemental Table 1).
Table 1. Characteristics for patients approached about the pediatric HPV vaccination program (N = 2,162).
The parents of 204 children (9.4% of all children approached) had declined HPV vaccination at least once before when approached by PNs (data not shown in table). Of those, 21 (10.3%) were 9–10 years, 119 (58.3%) were 11–12 years, 32 (15.7%) were 13–14 years, and 32 (15.7%) were 15–17 years old when first approached about HPV vaccination by this program’s PNs.
Vaccine initiation varied by race/ethnicity (p < 0.001) and age (p < 0.001) but not gender (Supplemental Table 2). Vaccine initiation was more than twice as likely among previously unvaccinated Hispanic and black children as compared to whites. Children 9–10 years, 13–14 years, and 15–17 years were less likely to initiate HPV vaccination through this program compared to 11–12 year olds.
Table 2. Determinants of completion among pediatric HPV vaccination program, vaccine initiators (N = 910).
Subsequent vaccination (2nd or 3rd dose) among prior starters did not differ by gender or age group (data not shown, p > 0.05), but decliners in this group were more frequently white (p < 0.01). None of the patients included in the initiators group were counted in the prior starters group. Completion among initiators was not associated with gender or race/ethnicity, but adolescents 15–17 years of age were less likely to complete the series than those 11–12 years (). Among prior starters, completion of the series was associated with race/ethnicity and age but not gender (). In this group, Hispanic patients were more than twice as likely and black patients more than three times as likely to complete as whites. Patients (initiators and prior starters) 9–10 years old and those 15–17 years old were less likely to complete compared to 11–12 year olds during the time period examined.
Table 3. Determinants of vaccine completion among entire pediatric HPV vaccination program (N = 2079).
About half (53%) of those who opted into the reminder program requested both voicemail and text follow-up reminders, but voice mail was more popular than text among those who preferred a single method. Out of the 1,582 vaccine-eligible patients who agreed to vaccination, 44.2% (n = 699) missed at least one follow-up vaccine appointment without cancellation. Typically, no-show rates in these clinics for scheduled nurse visits are about 17% and are about 29% for well-child visits. No-shows did not vary by gender () or race/ethnicity. Older patients were less likely to miss previously scheduled HPV vaccine appointments, while 9–10 year olds were almost twice as likely to miss appointments compared to 11–12 year olds.
Table 4. Characteristics associated with not keeping an appointment in the pediatric HPV vaccination program (N = 1582).
Qualitative program evaluation
During qualitative interviews, both health care providers and PNs were positive about the program. Although providers still administered the injections and answered any additional questions parents had about the vaccine, they felt the program addressed major barriers including cost, scheduling follow-up appointments, education for patients and providers, patient recall, and increase in provider recommendation (). Providers also felt that the program was valuable because it reduced their clinic workload, allowing them time to address other important topics.
Table 5. Pediatric HPV vaccination program benefits reported by patient navigators and clinic health care providers.
Three dominant themes emerged when providers and PNs were asked what they thought kept their patients from getting vaccinated: parents were still worried about side effects, the vaccine was not mandatory, and lack of information on why parents refuse. One medical resident noted, “…some parents are concerned about side effects from the vaccines, which is a reasonable concern.” One nurse (LVN) said that the parents often felt that the HPV vaccine was not mandatory for school attendance, and so did not feel that it was needed. She said, “I think it’s more of the reaction of the child because it hurts compared to some of the other vaccines and so I think they’re like oh well you know it’s not really needed for school, why do I get it, you know we don’t really need it.” When discussing vaccine refusal, we found that sometimes the providers did not know the reason. For example, one physician (MD) stated, “I never know if it’s because adolescents are in the room and [parents] don’t want to express their fears about that child having sexual relations or…I never understand exactly why.”
Discussion
Our program achieved good initiation rates and excellent completion rates in the clinics where it was implemented. We achieved an uptake rate of 67% among 9–17 year olds approached, which is higher than the 2016 reported rate of 60% among US adolescents 13–17 years old.Citation6 Further, we observed no differences in initiation or completion between boys and girls in contrast to an intervention reported by Farmar et. al using medical assistants.Citation13 Farmar did observe a higher initiation rate than we did, but this is most likely due to the inclusion of 9–12 year olds in our program while theirs was limited to 13–17 year olds.Citation13 In addition, we did not collect data on patients whose records indicated they were already vaccinated as our focus was on those who needed vaccination. Therefore, patients who previously completed the series were not included in our analyses which lowered our overall rates.
Our completion rate of 93% among those who initiated through this program greatly surpasses other programs.Citation14 This is especially noteworthy given the high rate of no shows in this clinic population. It is likely that automatic scheduling of follow-up appointments by PNs in addition to frequent reminders for subsequent doses through multiple formats increased parents’ awareness that additional doses were needed and reduced the workload on the parents to complete the series. This is important, as parents often do not understand how many doses are required.Citation8 Furthermore, providers often expect parents to schedule their own follow-up visits after telling them when to come back and clinics often lack systems that remind patients when follow up doses are due.Citation8 These results highlight the importance of multiple reminders and of providing ample opportunities for patients to reschedule appointments, especially as the no-show rates were higher than those observed for other common visits, such as well-child and nurse visits. Implementing a system of text reminders combined with automatic call reminders is easy, as there are commercial companies available that can provide these services. Personal phone calls are more resource intensive, but also can be useful to reinforce the importance of follow-up doses.
The success of our approach confirms Jarrett’s observation that multi-component and dialogue-based interventions are most effective in increasing overall vaccination rates.Citation11 It is also in agreement with prior reports showing that one of the best ways to increase HPV vaccine uptake is to ensure that providers recommend it at every eligible opportunity.Citation15,Citation16 However, there are barriers to relying on this strategy alone. Some providers overestimate parental hesitancy toward the HPV vaccine and do not bring up the topic at all.Citation17,Citation18 Others worry they will not have enough time to address all concerns or that bringing up the topic will offend parents. Moreover, nearly half do not recommend same-day vaccinationCitation18 even though requiring a visit on another day creates another barrier. We found that having PNs involved decreased the risk that providers would miss patients and ensured that all families with an eligible child were offered an opportunity to vaccinate them the same day.
Another reason for our program’s success may be that parents who declined vaccination for their children were offered another opportunity to reconsider when they returned to the clinic. Almost 10% of parents who agreed to vaccination had declined at least once. This is consistent with prior surveys on all vaccines in which pediatricians reported they were able to convince about 30% of parents to vaccinate their children who initially refused.Citation19-Citation21 These parents were most likely not against vaccination but rather needed more time to think about it. If not asked when they returned to the clinic, providers may have been reluctant to broach the subject again. Our results confirm the importance of offering parents multiple opportunities to vaccinate their children.
A unique aspect of this program was that parents were given the opportunity to vaccinate siblings that same day, even if they did not have an appointment. This resulted in the vaccination of an additional 118 adolescents who were eligible but would not normally have been vaccinated that day. The adoption of a system which asks about other members of the household and allows scheduling of same-day appointments by more providers could help increase HPV vaccination rates.
Hispanic and black patients were more likely to both accept the vaccine and complete the series than whites. A prior study found that parents of Hispanic and black adolescents were more likely than whites to intend to vaccinate their children against HPV.Citation22,Citation23 It has been speculated that this may be due to a greater perceived need for vaccination due to higher rates of cervical cancer among these groups, which is especially an issue in Texas.Citation24-Citation26 By offering free vaccines, education, and informed staff to answer questions for all patients, this project eliminated cost and improved trust – issues both of which have been cited by black patients as barriers to vaccination.Citation27
Qualitative interviews demonstrated positive views among providers who stated the program complemented their efforts. They felt that they and their patients were better educated and that the program decreased cost barriers. They also observed that more patients returned to get follow-up doses. Furthermore, providers noted that the program reduced their workload. This is important as nationally only 22 minutes are available to discuss all issues in most adolescent-care practices.Citation28-Citation30 However, it was noted that there were still some concerns among parents, according to the providers interviewed. These comments from providers demonstrated that more work needs to be done to assure parents of the vaccine’s safety.
Although our program used PNs dedicated solely to implementing the HPV vaccination strategies, which is staffing and cost-intensive, many of the duties performed by our PNs could be adopted by clinics using current staff. This would reduce the amount of resources needed to develop and maintain a similar program. For example, licensed vocational nurses or desk clerks could be trained to carry out some of the same activities as the PNs we employed. After patients receive a dose of the HPV vaccine, designated staff can schedule the follow-up appointments automatically at check-out instead of waiting for patients to call for one after their visit. Automated reminders can be supplemented with personal follow-up calls from these same personnel. Educational sessions for providers could be integrated into standard faculty and staff meetings, or offered for continuing medical education credits. These strategies can also be applied to all other vaccine series by the same personnel, including the meningococcal conjugate booster and Hepatitis B vaccine series to help mitigate the utilization of resources for one type of vaccine.
This report has some limitations. First, the program was limited to southeast Texas and may not be generalizable to other parts of the country. Also, this was a service project so we were unable to study the effectiveness of individual components of the intervention or include a control group. Thus, we do not know which components of the intervention were essential to achieve these large increases in uptake and completion rates. For example, it is possible that the providers had a stronger influence on the parents’ decision for vaccination than the PNs. However, the PNs reminded providers to address the HPV vaccine with their patients who had not been fully vaccinated, which would have increased provider recommendation to patients.
Overall, the results from our program evaluation support the use of multi-faceted interventions to increase HPV vaccination rates. This is needed to overcome the many complex barriers encountered by families in getting their children vaccinated. PNs can assist providers by helping with education, scheduling, and reminders. Implementation of multicomponent interventions across the US could dramatically increase vaccination rates and subsequently decrease the morbidity and mortality resulting from HPV-related cancers.
Patients and methods
A baseline survey of 239 mothers with a 9–17 year old child receiving care at The University of Texas Medical Branch (UTMB) conducted between October 2011 and June 2013 demonstrated that HPV vaccine completion rates were approximately 16% among daughters and 7% among sons. These data were collected to better understand HPV vaccination rates in the clinic population (unpublished data) prior to initiation of this project. At that time, HPV vaccination relied solely on provider recommendation. The providers in these clinics consisted of residents, faculty physicians, and nurse practitioners who may not have received much education about the HPV vaccine. Since it is not mandated for school entry in Texas, some may not have been providing strong recommendations for the vaccine to parents before this program was initiated. Moreover, a prior study at our institution demonstrated that our patients are often unaware of the vaccine and do not know to ask for it.Citation31 To address the low rates our survey found in these clinics, UTMB established a multi-component program, consisting of provider education and patient navigation in two large multi-specialty pediatric clinics and one adolescent gynecology clinic. These clinics are staffed by healthcare providers who provide both primary and specialty care, and serve a diverse patient population of which approximately 30% are Hispanic, 33% black, and 33% white. A large percentage of the patients from these clinics (61.3% during the period studied) receive Medicaid benefits. Data for patients approached between February 1, 2015 and August 31, 2016 are included in this program evaluation, which allowed for 12–30 months of follow-up (until August 31, 2017) for all patients.
Provider education
To increase awareness about HPV vaccination among providers, the program director (ABB) presented 45 minute lectures to faculty, residents, medical students, and staff working in the clinics. These sessions aimed to increase knowledge about HPV and the vaccine, as well as teach providers how to discuss the vaccine with their patients. It included information about the new program and explained how PNs would assist vaccination efforts. Overall, there were 16 presentations with 884 attendees. Similar education efforts have been previously published.Citation32,Citation33
Patient navigators
On-site PNs, funded through a prevention grant from the Cancer Prevention Research Institute of Texas (CPRIT), identified patients 9–17 years of age who were scheduled for well-child visits, acute care, or specialty visits and were incompletely vaccinated or unvaccinated. At least one PN was assigned every day to each of the 3 clinics. These clinics served a total of 7,260 9–17 year old patients eligible for assessment regarding their HPV vaccination status during the 18 month intervention period. Parents of unvaccinated or incompletely vaccinated children were informed about HPV vaccination while they waited for their children’s health providers in private clinic rooms to confirm the need for additional HPV vaccine doses. Parents of children who needed ≥ 1 dose were offered personal counseling and given handouts from the Centers for Disease Control and Prevention (CDC) in English or Spanish.Citation34-Citation36 Parents also were asked about the vaccination status of their other children. Those who agreed to vaccinate their child(ren) were asked to sign a State of Texas consent form documenting permission to administer the vaccine. Those with additional questions about the vaccine were advised to speak with their child’s pediatric health care provider. Providers were informed by PNs about parents who declined the vaccine or requested more information. This strategy was developed to allow the health provider an opportunity to discuss HPV vaccination with these parents and allow parents to ask any questions about the vaccine.
If parents agreed, the vaccine was administered that day, unless there was a contraindication or time constraint. In these cases, a return appointment was scheduled by PNs. Appointments were also scheduled by PNs for unvaccinated siblings, either on the same day or in the future. This was possible because the participating pediatric clinics offer walk-in vaccine-only appointments for established patients. Vaccine costs were paid by Vaccines for children (VFC) or private insurance and administrative costs were paid by Medicaid, Children’s Health Insurance Program (CHIP), or private insurance. If no other source of funding was available, CPRIT paid for the vaccine and/or administrative charge.
Follow-up doses were scheduled by PNs before patients left the clinic. To increase series completion, PNs tracked appointments and multiple reminder methods were utilized. Parents chose to get reminders by text and/or automated phone calls, which are services routinely provided by the pediatric clinics to all patients. PNs also called parents to remind them the day before their appointment and to reschedule missed appointments. Patients who missed ≥ 5 appointments or could no longer be reached were considered lost to follow-up. Parents who no longer wanted to participate were not contacted again. Parents who declined vaccination at the initial visit were approached up to 3 additional times, as many parents were unsure whether they wanted to vaccinate their children and requested additional time to seek information. PNs did not re-approach or contact parents a second time if they declined vaccination and requested not to be approached again.
Vaccines were administered by licensed clinic staff according to the most current CDC guidelines. After guidelines changed on December 15, 2016 from 3 to 2 doses for 9–14 year olds, the program changed its definition of completion accordingly, and patients with scheduled appointments were called and informed of the new vaccination schedule. Adolescents who began the series after age 15 were considered completers after 3 doses. In this report, initiators are defined as those who had not received any prior doses. Those who had received 1–2 doses but not completed the series are referred to as prior starters.
Statistical analysis
Demographic characteristics, visit type, preferred method of contact, insurance type, and whether parents were counseled for > 1 child were examined. Bivariate analyses compared characteristics between unvaccinated children who received an initial dose with those whose parents declined vaccination. Adjusted multivariable binary logistic regression analyses, which included gender, race/ethnicity, and age as potential confounding variables, were conducted to examine factors associated with HPV vaccine initiation (1 = initiated, 0 = declined) among those approached and completion (1 = completed according to current guidelines, 0 = did not complete) among initiators. Moreover, we examined characteristics associated with not keeping appointments (no shows without cancellation). Data analyses were conducted using SAS® statistical software, version 9.3 (Cary, NC).
Qualitative evaluation
To evaluate the perceptions of PNs and health providers, in-person interviews were conducted during December 2016 (22 months after implementation). We sampled 14 nurses, residents, and faculty pediatricians working in the clinics where the program was implemented and 7 PNs employed by this program. After collecting demographic information, audiotaped semi-structured interviews were conducted in a private area. Providers and PNs were asked to comment on program benefits they perceived and barriers to vaccination that persisted. Interview participants received a $20 gift. All interviews were transcribed and cross-checked by a second person for accuracy.
Interviews were analyzed using thematic analysis techniques to examine program fit and perceptions of the HPV vaccine program in the clinics. Co-investigators (JH, LC, and ED) conducted interviews and reviewed transcripts to develop preliminary inductive codes based on emerging themes and develop a codebook. The codes were applied to all transcripts, and verified by a second co-investigator. All coding was conducting using the NVivo qualitative data analysis software (QSR International Pty Ltd. version 10, 2012). Coders met regularly to discuss comparisons of coding using NVivo generated node reports and reached consensus about the categories. Both the quantitative and qualitative evaluations were approved by the UTMB Institutional Review Board.
Abbreviations
| HPV | = | human papillomavirus |
| PNs | = | patient navigators |
| US | = | United States |
| UTMB | = | University of Texas Medical Branch |
| CPRIT | = | Cancer Prevention Research Institute of Texas |
| EMRs | = | electronic medical records |
| CHIP | = | Children’s Health Insurance Program |
| VFC | = | Vaccines for Children |
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download Zip (20.7 KB)Acknowledgments
We would like to acknowledge the efforts of the patient navigators who approached parents of pediatric patients and informed them about the HPV vaccine.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Castellsagué X, Giuliano AR, Goldstone S, Guevara A, Mogensen O, Palefsky JM, Group T, Shields C, Liu K, Maansson R, et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33(48):6892–6901. doi:10.1016/j.vaccine.2015.06.088.
- Stillo M, Carrillo Santisteve P, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015;14(5):697–712. doi:10.1517/14740338.2015.1013532.
- Gonçalves AK, Cobucci RN, Rodrigues HM, de Melo AG, Giraldo PC. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;18(6):651–659. doi:10.1016/j.bjid.2014.02.005.
- Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–e39. doi:10.1542/peds.2014-3745.
- Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial Neoplasia in women. New England J Med. 2015;372(8):711–723. doi:10.1056/NEJMoa1405044.
- Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi:10.15585/mmwr.mm6633a2.
- Widdice LE, Hoagland R, Callahan ST, Kahn JA, Harrison CJ, Pahud BA, Frey SE, Berry AA, Kotloff KL, Edwards KM, et al. Caregiver and adolescent factors associated with delayed completion of the three-dose human papillomavirus vaccination series. Vaccine. 2018;36(11):1491–1499. doi:10.1016/j.vaccine.2017.12.060.
- Perkins RB, Chigurupati NL, Apte G, Vercruysse J, Wall-Haas C, Rosenquist A, Lee L, Clark JA, Pierre-Joseph N. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12(6):1528–1535. doi:10.1080/21645515.2015.1118594.
- Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31(40):4293–4304. doi:10.1016/j.vaccine.2013.07.013.
- Fu LY, Bonhomme L-A, Cooper SC, Joseph JG, Zimet GD. Educational interventions to increase HPV vaccination acceptance: A systematic review. Vaccine. 2014;32(17):1901–1920. doi:10.1016/j.vaccine.2014.01.091.
- Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ. Strategies for addressing vaccine hesitancy – A systematic review. Vaccine. 2015;33(34):4180–4190. doi:10.1016/j.vaccine.2015.04.040.
- Ali-Faisal SF, Colella TJ, Medina-Jaudes N, Benz Scott L. The effectiveness of patient navigation to improve healthcare utilization outcomes: A meta-analysis of randomized controlled trials. Patient Educ Couns. 2017;100(3):436–448. doi:10.1016/j.pec.2016.10.014.
- Farmar A-LM, Love-Osborne K, Chichester K, Breslin K, Bronkan K, Hambidge SJ. Achieving high adolescent HPV vaccination coverage. Pediatrics. 2016;138:5. doi:10.1542/peds.2015-2653.
- Krantz L, Ollberding NJ, Beck AF, Burkhardt MC. Increasing HPV vaccination coverage through provider-based interventions. Clin Pediatr. 2018 Mar;57(3):319–326. doi: 0009922817722014.
- Krakow M, Beavis A, Cosides O, Rositch AF. Characteristics of adolescents lacking provider-recommended human papillomavirus vaccination. J Adolesc Health. 2017;60(5):619–622. doi:10.1016/j.jadohealth.2016.11.028.
- Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: A systematic review. Prev Med. 2017;95:26–37. doi:10.1016/j.ypmed.2016.11.019.
- Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: how is human papillomavirus vaccine different? Prev Med. 2015;77:181–185. doi:10.1016/j.ypmed.2015.05.024.
- Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1673–1679. doi:10.1158/1055-9965.EPI-15-0326.
- American Academy of Pediatrics. Periodic survey #66: pediatricians’ attitudes and practices surrounding the delivery of immunizations. Communication and Documentation of Vaccine Risks/Benefits 2006; https://www.aap.org/en-us/professional-resources/Research/Pages/PS66_Executive_Summary_PediatriciansAttitudesandPracticesSurroundingtheDeliveryofImmunizationsPart2.aspx. Accessed 2018 Jan 9
- Hough-Telford C, Kimberlin DW, Aban I, Hitchcock WP, Almquist J, Kratz R, OConnor KG. Vaccine delays, refusals, and patient dismissals: a survey of pediatricians. Pediatrics. 2016;138:3. doi:10.1542/peds.2016-2127.
- Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, Devere V, Zhou C, Robinson JD. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037–1046. doi:10.1542/peds.2013-2037.
- Cheruvu VK, Bhatta MP, Drinkard LN. Factors associated with parental reasons for “no-intent” to vaccinate female adolescents with human papillomavirus vaccine: national Immunization Survey - Teen 2008–2012. BMC Pediatr. 2017;17:52. doi:10.1186/s12887-017-0969-7.
- Mohammed KA, Vivian E, Loux TM, Arnold LD. Factors Associated With Parents’ Intent to Vaccinate Adolescents for Human Papillomavirus: findings from the 2014 national immunization survey–teen. Prev Chronic Dis. 2017;14:E45. doi:10.5888/pcd14.160314.
- Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi:10.1093/jnci/djs491.
- American Cancer Society. Key statistics for cervical cancer. 2018; https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed 2018 Jan 8
- Zhan FB, Lin Y. Racial/ethnic socioeconomic, and geographic disparities of cervical cancer advanced-stage diagnosis in Texas. Women’s Health Issues. 2014;24(5):519–527. doi:10.1016/j.whi.2014.06.009.
- Quinn S, Jamison A, Musa D, Hilyard K, Freimuth V. Exploring the continuum of vaccine hesitancy between african american and white adults: results of a qualitative study. PLoS Currents. 2016;8:ecurrents.outbreaks.3e4a5ea39d8620494e8620492a8620492c8620874a8620493c8624201.
- Katz IT, Bogart LM, Fu CM, Liu Y, Cox JE, Samuels RC, Chase T, Schubert P, Schuster MA. Barriers to HPV immunization among blacks and latinos: a qualitative analysis of caregivers, adolescents, and providers. BMC Public Health. 2016;16(1):874. doi:10.1186/s12889-016-3529-4.
- Rutten LJ, St Sauver JL, Beebe TJ, Wilson PM, Jacobson DJ, Fan C, Breitkopf CR, Vadaparampil ST, Jacobson RM. Clinician knowledge, clinician barriers, and perceived parental barriers regarding human papillomavirus vaccination: association with initiation and completion rates. Vaccine. 2017;35(1):164–169. doi:10.1016/j.vaccine.2016.11.012.
- Alexander SC, Fortenberry JD, Pollak KI, Bravender T, Davis JK, Ostbye T, Tulsky JA, Dolor RJ, Shields CG. Sexuality talk during adolescent health maintenance visits. JAMA Pediatr. 2014;168(2):163–169.
- Berenson AB, Brown VG, Fuchs EL, Hirth JM, Chang M. Relationship between maternal experiences and adolescent HPV vaccination. Hum Vaccin Immunother. 2017;13(9):2150–2154. doi:10.1080/21645515.2017.1332551.
- Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A brief educational intervention increases providers’ human papillomavirus vaccine knowledge. Hum Vaccin Immunother. 2015;11(6):1331–1336. doi:10.1080/21645515.2015.1022691.
- Groom HC, Irving SA, Caldwell J, Larsen R, Beaudrault S, Luther LM, Naleway AL. Implementing a multipartner HPV vaccination assessment and feedback intervention in an integrated health system. J Public Health Manag Pract. 2017;23(6):589–592. doi:10.1097/PHH.0000000000000562.
- Centers for Disease Control and Prevention (CDC). HPV (Human papillomavirus) vaccine: what you need to know. http://www.immunize.org/vis/hpv.pdf. Accessed 2018 Apr 20
- Centers for Disease Control and Prevention (CDC). Diseases and the vaccines that prevent them: HPV also known as human papillomavirus. https://www.cdc.gov/vaccines/parents/diseases/teen/hpv-indepth-color.pdf. Accessed 2018 Apr 20
- Centers for Disease Control and Prevention (CDC). What parents should know about HPV vaccine safety and effectiveness. https://www.cdc.gov/vaccines/partners/downloads/teens/vaccine-safety.pdf https://www.cdc.gov/vaccines/parents/diseases/teen/hpv-indepth-color.pdf. Accessed 2018 Apr 20