ABSTRACT
Two antigenically distinct influenza B lineage viruses (Yamagata/Victoria) have been co-circulating globally since the mid-1980s. The quadrivalent influenza vaccine (QIV) may provide better protection against unpredictable B strains. We conducted a randomized, double-blind, phase III trial to evaluate the immunogenicity and safety of an egg-based inactivated, split-virion QIV (GC3110A). Subjects aged ≥ 19 years were randomized 2:1:1 to be vaccinated with QIV- GC3110A, trivalent influenza vaccine (TIV) containing the Yamagata lineage strain (TIV-Yamagata), or TIV containing the Victoria lineage strain (TIV-Victoria). Hemagglutination inhibition assays were performed 21 days post-vaccination. Solicited/unsolicited adverse events (AEs) were assessed within 21 days after vaccination, while serious AEs were reported up to six months after vaccination. A total of 1,299 were randomized to receive QIV-GC3110A (648 subjects), TIV-Yamagata (325 subjects), or TIV-Victoria (326 subjects). Compared to the TIVs, the QIV-GC3110A met the non-inferiority criteria for all four subtype/lineage strains with respect to the geometric mean titer (GMT) ratio and the difference of seroconversion rate. The safety profiles of QIV-GC3110A were consistent with those of TIV. In conclusion, QIV-GC3110A is safe, immunogenic, and comparable to strain-matched TIV.
Introduction
The World Health Organization (WHO) estimates that 3–5 million cases of severe influenza illness occur around the world annually, resulting in 290,000–600,000 deaths.Citation1 There are four types of influenza viruses, types A, B, C, and D.Citation2 Among them, influenza A and B viruses cause seasonal epidemics in human. Although influenza B has been recognized as being milder than influenza A, many studies have shown that influenza A and B are clinically indistinguishable.Citation3,Citation4 In the epidemiological review of influenza B disease in 15 Asia-Pacific countries, influenza B was identified and associated with 0–92% of laboratory-confirmed influenza cases in any one season/year since 1990.Citation5 Moreover, although hospitalization and death were most common from influenza A/H3N2, the number of hospitalizations and deaths from influenza B was higher than that of seasonal influenza A/H1N1 before the 2009 pandemic.Citation6,Citation7
As for influenza B, two antigenically distinct lineage viruses (B/Victoria-like and B/Yamagata-like) have been co-circulating globally since the mid-1980s.Citation8 Before 2013, seasonal influenza vaccines included two influenza A strains (A/H1N1, A/H3N2), but only one influenza B strain based on the recommendation of WHO. Despite the introduction of quadrivalent influenza vaccine (QIV) in South Korea in 2014, the government still subsidized only the trivalent influenza vaccine (TIV). However, it is difficult to predict the circulating influenza B lineage in the next upcoming season. In fact, 55.6% of circulating influenza B viruses were mismatched during influenza seasons from 2007 to 2014.Citation5 The degree of cross-protection against mismatched influenza B lineages is still undetermined, but it is assumed to be low.
n this Phase III, double-blind, randomized study, we evaluated the immunogenicity and safety of the quadrivalent, inactivated, split-virion influenza vaccine (GC3110A) in healthy Korean adults. The primary objective was to evaluate the non-inferior immunogenicity for the QIV versus the TIV against shared influenza A and B strains. The safety objectives were evaluation of solicited local and systemic adverse events (AEs) and spontaneously reported unsolicited AEs and serious adverse events (SAEs).
Results
Study subjects
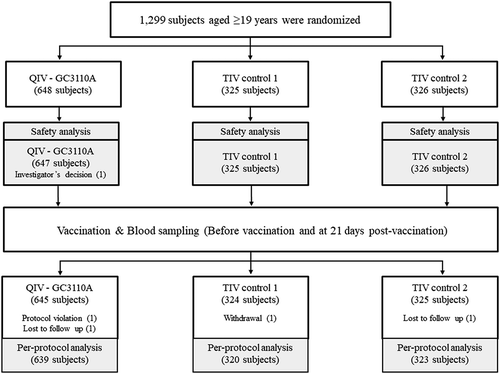
From October 24 to December in 2014, a total of 1,308 healthy subjects aged ≥ 19 years were recruited, and 1,299 were randomized to receive study vaccine (GC3110A [QIV], 648 subjects), control 1 (TIV-Yamagata, 325 subjects), or control 2 (TIV-Victoria, 326 subjects) (). Excluding one subject based on the investigator’s decision, 1,298 subjects received the vaccination and were included in the safety analysis set. The baseline characteristics were well matched among the three groups, as shown in . Among the 1,298 safety analysis set, 1,282 subjects were eligible for per-protocol immunogenicity analysis, excluding 16 subjects because of protocol deviation ().
Table 1. Demographic characteristics of the study subjects.
Immunogenicity
Compared to the control TIVs, QIV-GC3110A met the non-inferiority criteria for all four influenza subtype/lineage strains with respect to GMTR and SCR difference (). The GMTR of TIV/GC3110A – QIV was 1.0 (95% CI, 0.9 to 1.2) for A/H1N1 stain, 1.0 (95% CI, 0.9 to 1.1) for A/H3N2 strain, 0.8 (95% CI, 0.7 to 0.9) for B/Yamagata strain, and 1.0 (95% CI, 0.8 to 1.1) for B/Victoria strain. The SCR difference (TIV minus GC3110A -QIV) was 3.4 % (95% CI, −2.0 to 8.8) for A/H1N1, −3.5 % (95% CI, −8.9 to 1.9) for A/H3N2, −16.2 % (95% CI, −22.8 to −9.5) for B/Yamagata strain, and 0.5 % (95% CI, −6.2 to 7.2) for B/Victoria strain. When stratified by age group (18–64 years and ≥ 65 years), non-inferiority criteria for GMTR were met for both young adults (18–64 years) and the elderly (≥ 65 years) (Supplementary Figure 1-A). The non-inferiority criteria of SCR difference were met for all four strains in young adults. In old adults aged ≥ 65 years, however, the upper margin of SCR difference exceeded 10% for influenza A/H1N1 and B/Victoria strain, reflecting rather higher pre-vaccination GMTs in the QIV-GC3110A group compared to TIV groups (Supplementary Figure 1-B and Supplementary Table 1).
Table 2. Post-vaccination non-inferiority analysis, as measured using a hemagglutination-inhibition (HI) assay (per-protocol population): trivalent influenza vaccines (controls) versus quadrivalent influenza vaccine (GC3110A).
Overall, QIV-GC3110A met the CBER criteria in both young adults and the elderly aged ≥ 65 years, except for B/Victoria strain (). As for A/H1N1 strain, both QIV-GC3110A and TIVs did not meet the CBER criteria in old adults aged ≥ 65 years.
Table 3. Post-vaccination immune responses, as measured using a hemagglutination-inhibition (HI) assay (per-protocol population): quadrivalent influenza vaccine (GC3110A) and trivalent influenza vaccines (controls).
Safety
The solicited local and systemic adverse events within 21 days of vaccination are shown in . There were 725 solicited local adverse events in 383 (59.2%) of 647 subjects in the QIV-GC3110A group, 317 events in 169 (52.0%) of 325 subjects in the TIV-Yamagata group, and 284 events in 160 (49.1%) of 326 subjects in the TIV-Victoria group. Although solicited local adverse events were more common in the QIV-GC3110A group compared to controls (p < 0.05), most events (97.1%, 691 among 712 subjects) were grade 1 (noticeable but did not interfere with daily activity) and resolved, including 96.3% (369 among 383 subjects) of the QIV-GC3110A group, 97.6% (165 among 169 subjects) of the TIV-Yamagata group, and 98.1% (157 among 160 subjects) of the TIV-Victoria group. Solicited systemic adverse events were indistinguishable between the QIV-GC3110A group and TIV controls (p = 0.71). There were 622 solicited systemic adverse events in 239 (36.9%) of 647 subjects in the QIV-GC3110A group, 333 events in 131 (40.3%) of 325 subjects in the TIV-Yamagata group, and 259 events in 103 (31.6%) of 326 subjects in the TIV-Victoria group. Most systemic adverse events (95.6%, 452 among 473 subjects) were grade 1, affecting 95.4% (228 among 239 subjects) of the QIV-GC3110A group, 94.7% (124 among 131 subjects) of the TIV-Yamagata group, and 97.1% (100 among 103 subjects) of the TIV-Victoria group. The most common local adverse events were injection site pain and tenderness, which were more common in the QIV-GC3110A recipients compared to the TIV recipients (p < 0.05). Fatigue and myalgia were the most frequently reported solicited systemic adverse events, and those were reported by 24.4%/25.2% of the QIV-GC3110A recipients, 28.3%/25.2% of the TIV-Yamagata recipients, and 20.9%/21.8% of the TIV-Victoria recipients without significant intergroup difference (fatigue, p = 0.95; myalgia, p = 0.48).
Table 4. Solicited local and systemic adverse events within 21 days after vaccination: quadrivalent influenza vaccine (GC3110A) and trivalent influenza vaccines (controls).
During the 21 days after vaccination, unsolicited adverse events were reported by 10.7% (69/647) of the QIV-GC3110A recipients, 12.0% (39/325) of the TIV-Yamagata recipients, and 11.7% (38/326) of the TIV-Victoria recipients without statistically significant difference (p = 0.79). All subjects with unsolicited adverse events recovered without sequelae, and no vaccine-related SAE was reported up to six months post-vaccination.
Discussion
This study showed that a new egg-derived, inactivated, split-virion QIV (GC3110A, Green Cross Corporation) has excellent immunogenicity non-inferior to that of B strain-matched TIVs after vaccination. In addition, QIV-GC3110A was well tolerated, with a similar safety profile to the TIV comparators. These results are consistent with previous large-scale studies of QIVs in adult populations.Citation9–Citation12
In studies with age-stratification, the proportions of influenza B infection were estimated higher among young children compared to old adults during seasonal influenza epidemics.Citation5,Citation13 However, the influenza B epidemic also induced significant public health impacts on adults. In adults and adolescents, the proportion of influenza B reported variably according to geographical region and study period. The proportion ranged from 0% to 40.6% in the US, from 1.6% to 43.2% in Europe, from 0% to 55.8% in South Korea, and from 1.8% to 66.5% in Australia.Citation5,Citation14 In addition, the two influenza B lineages have co-circulated worldwide since the mid-1980, and severe acute respiratory infections have been reported to increase during epidemics in vaccine mismatch seasons.Citation8,Citation15 Actually, it is difficult to predict which influenza B lineage will predominate in any given season. Thus, the mismatch of the dominant circulating B strain against the vaccine strain was reported in 40–60% of the influenza seasons in the US, South Korea, and Finland.Citation5,Citation16,Citation17 Although TIV might provide some cross-reactive immunogenicity against unmatched B-strain, the antibody response was much weaker than that against the matched strain ( and Supplementary Table 1). Thus, QIV-GC3110A may provide better protection against seasonal influenza compared with TIVs, thereby decreasing the real disease burden of influenza.
In this study, both QIV-GC3110A and TIV showed relatively low immunogenicity against B/Victoria strain (B/Brisbane/60/2008). In the courses of egg passages, mutations resulting in the loss of the N-glycosylation site at positions 197–199 of hemagglutinin have been reported in influenza B.Citation18 Such a structural change might influence the immunogenicity against wild-type B virus. Actually in a previous report, egg-adapted B/Brisbane/60/2008 strain was shown to acquire an amino acid substitution, Ser228Thr, in the globular head of the hemagglutinin during egg adaptation, thereby causing structural changes (the loss of an N-glycosylation site) and the low immunogenicity against the original wild-type virus.Citation19 On the other hand in , QIV-GC3110A showed higher SCR against Yamagata-strain than TIV-Yamagata. This seems to be a unique result for this study. The higher SCR for Yamagata strain in QIV-GC3110A might be related to the lower pre-vaccination hemagglutination inhibition (HI) titers compared to TIV-Yamagata in young adults aged 19–64 years (supplementary Table 1). As reported previously, pre-vaccination HI titers for B lineages were rather higher in old adults compared to young adults owing to previous repeated exposure, but SCR was lower.Citation12,Citation20 In young adults with low pre-vaccination HI titers, seroconversion would be more remarkable. In this study, the seroprotection rates were comparable between QIV-GC3110A and TIV-Yamagata at 21 days post-vaccination.
Based on local epidemiology, vaccine effectiveness, and medical costs, the cost-effectiveness of QIV was assessed in US, Canada, UK, Germany, Spain, Finland, Australia and Hong Kong.Citation21 Overall, switching the strategy from TIV to QIV was expected to be cost-effective to further reduce the influenza disease burden. However, the cost-effectiveness of QIV versus TIV might be influenced by several key factors, including vaccine price, level of cross-protection of TIV against the mismatched B strain, proportion of influenza B, and mismatch degree of the circulating B lineages against the vaccine strain. In each country, age-based vaccination policy should be optimized considering the local epidemiology, cost-effectiveness, and budget impact analysis.
This study has several limitations. First, long-term immunogenicity (≥ 6 months) was not evaluated. Second, data on influenza vaccination in the previous season was not available. In addition, gender difference was not considered in the assessment of immunogenicity and safety.
In summary, QIV-GC3110A is safe and immunogenic in both young adults and the elderly. Vaccination with this QIV would be useful to decrease the influenza B disease burden during epidemics of mismatched B strains.
Materials and methods
Study design
A randomized, double-blind, active-controlled, phase Ⅲ trial was conducted to evaluate the immunogenicity and safety of an egg-based inactivated, split-virion, quadrivalent influenza vaccine (GC3110A, Green Cross Corporation) among healthy adults aged 19 years or older at nine centers in South Korea from the 2014–15 influenza season (Clinical Trial Number – NCT02352584). The exclusion criteria were a history of hypersensitivity to any component of the vaccines, including eggs and chicken meat; receipt of an influenza vaccine within the last six months; history of Guillain-Barré syndrome; any immune deficiency; receipt of immunosuppressive agents, immunomodulating agents, or cytotoxic radiation therapy within 3 months prior to screening; administration of high dose (≥ 20 mg prednisolone) steroid within the last three months; receipt of immunoglobulin or blood derived products within the 3 months prior to screening; a history of known drug or alcohol abuse (> 21 units/week, 1 unit = 10g of pure alcohol); anticipated receipt of immunoglobulin or blood-derived products during the trial period; any coagulation disorder contraindicating intramuscular injection or receipt of anticoagulants; pregnant or breast-feeding; acute febrile illness (temperature > 38.0°C) within 72 hours prior to scheduled receipt of vaccine in the trial; moderate to severe chronic illness potentially interfering with full participation in the trial as determined by the researcher; receipt of vaccine (of any kind) within 30 days prior to screening; participation in a clinical trial within 30 days prior to screening; and other significant medical or psychiatric conditions deemed inappropriate for trial entry by the researcher.
After obtaining written informed consent, participants who met the entry criteria for the study were screened for eligibility. Then, the participants were stratified by age (19– 64 years or ≥ 65 years) and were randomly assigned in a 2:1:1 ratio to receive experimental QIV (GC3110A), control 1 (seasonal TIV; GC Flu pre-filed syringe®, Green Cross Corporation), or control 2 (experimental TIV with alternate influenza B virus strain) (). Using a randomization table provided by the study sponsor, each participant was randomly assigned to a study group using the block randomization method. During study periods, the investigators, participants, and all study personnel were blinded.
After a baseline blood sample was collected, the vaccine was administered as a single intramuscular injection into the deltoid muscle, and the subjects were observed for 30 minutes. On days 5–8, a phone interview was conducted to assess the safety after vaccination. A second blood sample was obtained at day 21 ± 3 after vaccination.
The study was conducted in accordance with the Declaration of Helsinki and the standards of Good Clinical Practice defined by the International Conference on Harmonization. The protocol and consent forms were approved by the institutional review board of each participating study site. Informed written consent was obtained from all participants following a detailed explanation of schedules and study contents.
Vaccines
The study vaccine (GC3110A) was an egg-derived inactivated split-virion QIV developed by Green Cross Corporation. Each 0.5 mL dose of the vaccine contained 15 µg of each of the four purified hemagglutinin antigens (60 µg total): A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Massachusetts/02/2012, and B/Brisbane/60/2008. Two egg-derived control TIVs were also developed to assess comparatively the immunogenicity against alternate B lineage antigens by Green Cross Corporation. Control 1 (GC Flu pre-filed syringe®, Green Cross Corporation; TIV-Yamagata) was an inactivated split-virion TIV containing Yamagata lineage influenza B strain. A 0.5 mL dose of the vaccine contained 15 µg of each of three purified hemagglutinin antigens (45 µg total): A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), and B/Massachusetts/02/2012. Control 2 (TIV-Victoria) was an inactivated split-virion TIV containing Victoria lineage influenza B strain. A 0.5 mL dose of the vaccine contained 15 µg of each of three purified hemagglutinin antigens (45 µg total): A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), and B/Brisbane/60/2008.
Immunogenicity assessment
Serum samples were assessed for HI antibodies to each hemagglutinin of the H1N1, H3N2, and B strains contained in the vaccine using a standard assay with use of chicken erythrocytes at GreenCross LabCell.Citation22 HI antibody titers that were below the detection limit (i.e., < 1:10) were assigned a value of 1:5. Immunogenicity was assessed according to the licensure criteria of US Center for Biologics Evaluation and Research (CBER).Citation23
The primary immunogenicity end points after vaccination were (a) geometric mean titer (GMT) ratio, defined as the geometric mean ratio of the post-vaccination HI titers for comparator TIVs to GC3110A-QIV and (b) differences in seroconversion rates (SCRs) between the comparator TIVs and GC3110A-QIV. For influenza B, the immunogenicity against shared lineage strain was compared: QIV versus TIV-Yamagata for B/Yamagata and QIV versus TIV-Victoria for B/Victoria.
The secondary immunogenicity end points were the proportion of subjects with either seroconversion or a four-fold or greater increase in antibody titer (SCR) and the proportion of subjects with antibody titers of 1:40 or more on HI assay (seroprotection rate, SPR). According to the criteria set by the CBER, the criteria were met if the lower limit of the 2-sided 95% CI on the SCR was ≥ 40% (aged 18–64 years) or ≥ 30% (aged ≥ 65 years), and the lower limit of the 2-sided 95% CI on the seroprotection rate (SPR) was ≥ 70% (aged 18–64 years) and ≥ 60% (aged ≥ 65 years).Citation23
Safety assessment
Both a digital thermometer and a diary card were given to the enrolled subjects at the first visit. For seven days after vaccination, the subjects were educated to record the severity of solicited local adverse events and solicited systemic adverse events daily in the diary card, using a standard scale.Citation24
Solicited local adverse events were pain, tenderness, redness/erythema, and induration/swelling, and solicited systemic adverse events were fever, sweating, chills, nausea/vomiting, diarrhea, fatigue/malaise, headache, myalgia and arthralgia. We collected both solicited and unsolicited reports during the first 21-day study period. Any SAEs were required to be reported within 24 hours after the awareness of the investigator for up to six months after vaccination.
Statistical analysis
For the sample size of the immunogenicity subset, assuming a 10% dropout rate and a 2:1:1 randomization schedule, 1,299 participants were planned (643 in the QIV group, 328 in each control TIV group) to achieve at least 80% overall power to demonstrate non-inferiority with respect to two co-primary endpoints (GMT ratio [GMTR] and the SCR difference for all four strains).
Immunogenicity analyses were run on the per-protocol set, which included all enrolled subjects who received the vaccine correctly, provided evaluable serum samples at relevant time points, and had no major protocol deviations. Safety was analyzed for all subjects exposed to study vaccines. All statistical analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC, USA). Immunogenicity data were expressed in terms of GMT and CBER criteria with a two-sided 95% confidence interval (CI). Two-sided 95% CIs for GMT, GMT-fold increase, and GMTR were calculated using the normal approximation of log-transformed titers, and percentages were calculated with approximate or exact 95% CIs. Safety data were described as the proportion of study subjects reporting local and systemic adverse reactions. Results were considered statistically significant if the p-value was less than 0.05.
For non-inferiority, the following criteria should be met: (1) the upper bound of the two-sided 95% confidence interval (CI) of the GMTRs (TIV/QIV-GC3110A) for all four vaccine strains should not exceed 1.5, and (2) the upper bound of the two-sided 95% CI for the SCR difference (TIV minus QIV-GC3110A) for all four vaccine strains should not exceed 10%.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download Zip (123.7 KB)Supplemental Material
Supplemental data for this article can be accessed at tandfonline.com/khvi.
Additional information
Funding
References
- Wilschut J, McElhaney JE, Palache AM. Influenza. Philadelphia, USA: Mosby Elsevier; 2006.
- Ducatez MF, Pelletier C, Meyer G, Influenza D. virus in cattle, France, 2011-2014. Emerg Infect Dis. 2015;21(2):368–371. doi:10.3201/eid2102.141449. PMID:25628038.
- Mosnier A, Caini S, Daviaud I, Nauleau E, Bui TT, Debost E, Bedouret B, Agius G, van der Werf S, Lina B, et al. Clinical characteristics are similar across type A and B Influenza virus infections. PLoS One. 2015;10(9):e0136186. doi:10.1371/journal.pone.0136186. PMID:26325069.
- Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, Belongia EA. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respir Viruses. 2012;6(1):37–43. doi:10.1111/j.1750-2659.2011.00263.x. PMID:21668663.
- Jennings L, Huang QS, Barr I, Lee PI, Kim WJ, Buchy P, Sanicas M, Mungall BA, Chen J. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Other Respir Viruses. 2018;12(3):383–411. doi:10.1111/irv.12522. PMID:29127742.
- Wu P, Presanis AM, Bond HS, Lau EHY, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998-2013. Sci Rep. 2017;7(1):929. doi:10.1038/s41598-017-01021-x. PMID:28428558.
- Wu S, Wei Z, Greene CM, Yang P, Su J, Song Y, Iuliano AD, Wang Q. Mortality burden from seasonal influenza and 2009 H1N1 pandemic influenza in Beijing, China, 2007-2013. Influenza Other Respir Viruses. 2018;12(1):88–97. doi:10.1111/irv.12515. PMID:29054110.
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175(1):59–68. PMID:2309452.
- van de Witte S, Nauta J, Montomoli E, Weckx JA. Phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine. 2018;36:6030–6038. doi:10.1016/j.vaccine.2018.04.043. PMID:29709447.
- Treanor JT, Albano FR, Sawlwin DC, Graves Jones A, Airey J, Formica N, Matassa V, Leong J. Immunogenicity and safety of a quadrivalent inactivated influenza vaccine compared with two trivalent inactivated influenza vaccines containing alternate B strains in adults: A phase 3, randomized noninferiority study. Vaccine. 2017;35(15):1856–1864. doi:10.1016/j.vaccine.2017.02.066. PMID:28302411.
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–5578. doi:10.1016/j.vaccine.2013.08.069. PMID:24016810.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewe W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged >/=18 years: a phase III, randomized trial. Vaccine. 2014;32(13):1480–1487. doi:10.1016/j.vaccine.2014.01.022. PMID:24486352.
- Panatto D, Signori A, Lai PL, Gasparini R, Amicizia D. Heterogeneous estimates of influenza virus types A and B in the elderly: results of a meta-regression analysis. Influenza Other Respir Viruses. 2018;12(4):533–543. doi:10.1111/irv.12550. PMID:29498477.
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43–51. doi:10.2105/AJPH.2012.301137. PMID:23327249.
- Lapinscki B, Pereira LA, Nogueira MB, Vidal LR, Riediger I, Debur MC, Presibella M, Raboni SM. Molecular epidemiology of influenza B virus and implications in immunization strategy, Southern Brazil. Vaccine. 2018;36(1):107–113. doi:10.1016/j.vaccine.2017.11.033. PMID:29174679.
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin Infect Dis. 2014;59(11):1519–1524. doi:10.1093/cid/ciu664. PMID:25139969.
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28(9):2149–2156. doi:10.1016/j.vaccine.2009.11.068. PMID:20003926.
- Gatherer D. Passage in egg culture is a major cause of apparent positive selection in influenza B hemagglutinin. J Med Virol. 2010;82(1):123–127. doi:10.1002/jmv.21648. PMID:19950248.
- Kishida N, Fujisaki S, Yokoyama M, Sato H, Saito R, Ikematsu H, Xu H, Takashita E, Tashiro M, Takao S, et al. Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clin Vaccine Immunol. 2012;19(6):897–908. doi:10.1128/cvi.05726-11. PMID:22492743.
- Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged >/=18 years. BMC Infect Dis. 2013;13:343. doi:10.1186/1471-2334-13-343. PMID:23883186.
- de Boer PT, van Maanen BM, Damm O, Ultsch B, Dolk FCK, Crepey P, Pitman R, Wilschut JC, Postma MJ. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):249–265. doi:10.1080/14737167.2017.1343145. PMID:28613092.
- Cox NJ, Ziegler T. Influenza viruses. In: Murray PR, Barron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology. 8th ed. Washington (DC): ASM Press; 2003. p. 1360–1365.
- U.S. Department of Health and Human Services FaDA, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of trivalent inactivated influenza vaccines; 2007 [accessed 2010 Aug 17. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091985.pdf
- U.S. Department of Health and Human Services FaDA, Center for Biologics Evaluation and Research. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials; [accessed 2010 Aug 17]. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRe