ABSTRACT
Vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) followed ≥ 1 year by the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended for immunocompetent adults ≥ 65 years of age in the United States. This study assessed antipneumococcal opsonophagocytic activity (OPA) geometric mean titers (GMTs) to PCV13 in PPSV23-naive and PPSV23-preimmunized adults 1 year after a second vaccine dose. Two parent studies were conducted previously: (1) PPSV23 vaccine–naive subjects (60–64 years of age at enrollment) received PCV13 followed by PCV13 or PPSV23 1 year later or PPSV23 followed by PCV13 1 year later; and (2) subjects (≥ 70 years of age at enrollment) vaccinated with PPSV23 ≥ 5 years before study entry received PCV13 or PPSV23 followed by PCV13 1 year later. Overall, 962 subjects (PPSV23-naive, n = 519; PPSV23-preimmunized, n = 443) who received both vaccinations in the parent studies were enrolled. Numerically higher OPA GMTs persisted for at least 1 year after administration of PCV13 as the initial vaccine (PCV13/PPSV23 or PCV13/PCV13) compared with those who received PPSV23 either 1 or 5 years prior (PPSV23/PCV13). This impairment in antibody responses to subsequent PCV13 vaccination produced by initial PPSV23 vaccination persisted for at least 1 year. OPA GMTs were numerically higher for most serotypes 1 year after 2 doses of PCV13 compared with 1 year after the first PCV13 dose. These data suggest PCV13 should be given first if both vaccines are to be administered, higher immune responses were achieved when PCV13 was given first and persisted at least 1 year (ClinicalTrials.gov Identifier: NCT01025336).
Introduction
The incidence of pneumococcal disease in adults is highest in the elderly,Citation1–Citation4 with mortality from infection positively correlated with age.Citation5,Citation6 In addition, older adults often have underlying conditions that increase their susceptibility to pneumococcal diseaseCitation7 and contribute to increased morbidity and mortality.Citation8
Two vaccines to prevent pneumococcal disease are currently approved in the United States for use in older adults: the 23-valent pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).Citation9 The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) recommends vaccination of immunocompetent adults ≥ 65 years of age who have not previously received a pneumococcal vaccine be vaccinated first with PCV13 followed by PPSV23 6 to 12 months later.Citation9 ACIP also recommends that adults ≥ 65 years of age who have already received a dose of PPSV23 receive a dose of PCV13 ≥ 1 year later.
Two earlier studies evaluated the sequential use of PCV13 and PPSV23 as part of the clinical development plan for PCV13 in adults.Citation10,Citation11 Study 1 was conducted in adults 60 to 64 years of age who had not received prior PPSV23 (PPSV23-naive).Citation10 Study 2 was conducted in adults 70 years and older who had received PPSV23 at least 5 years before enrollment (preimmunized).Citation11 In both studies, the 2 pneumococcal vaccines were administered in different sequential order with 1 year between doses. Immunogenicity assessed by opsonophagocytic activity (OPA) approximately 1 year after the second vaccine dose for both studies are reported herein; antibody persistence was assessed when vaccines were administered in different sequential order.
Results
Baseline characteristics and disposition of subjects
A total of 964 subjects from the parent studies participated in this study; 962 were enrolled (n = 519, study 1; n = 443, study 2) and included in the all-available immunogenicity population (Supplementary Figure 2). Two subjects were withdrawn because of a protocol violation. Aside from age, demographic characteristics were similar for all groups (). Mean age at 1 year after vaccination 2 was similar among groups within each study.
Table 1. Baseline characteristics.
Immune responses
OPA antibody levels 1 year after vaccination 2
The primary objective was to describe OPA antibody responses descriptively 1 year after vaccination 2 (). For subjects naive to PPSV23 (study 1), OPA GMTs 1 year after vaccination 2 for PCV13/PCV13 were similar or numerically higher than those for PCV13/PPSV23 for 3 of the 12 common serotypes and serotype 6A (contained in PCV13 but not PPSV23). OPA GMTs 1 year after PCV13/PPSV23 were numerically higher than those after PCV13/PCV13 for 9 of 12 common serotypes. OPA GMTs 1 year after vaccination 2 for PPSV23/PCV13 were lower than those for PCV13/PCV13 and PCV13/PPSV23 for all serotypes except for serotypes 5 and 14, for which OPA GMTs were numerically higher for PPSV23/PCV13 than PCV13/PCV13 ().
Table 2. OPA GMTs among the 3 vaccine sequences 1 year after the second vaccination.
For subjects preimmunized with PPSV23 (study 2), OPA GMTs 1 year after vaccination 2 for PCV13/PCV13 were numerically higher than those for PPSV23/PCV13 for 11 of the 12 common serotypes and serotype 6A (unique serotype in PCV13) (). For subjects in both parent studies, OPA GMTs 1 year after the second vaccination were numerically higher for the majority of serotypes when PCV13 was given first before a subsequent vaccination with PCV13 or PPSV23 compared with when PPSV23 was given first ().
Comparison of OPA GMTs 1 year after vaccination 2 among the 3 vaccine sequences in PPSV23-naive adults
PCV13/PPSV23 compared with PPSV23/PCV13
The OPA GMT ratio was > 1 for all serotypes, indicating numerically higher GMTs in the PCV13/PPSV23 group than the PPSV23/PCV13 group. OPA GMTs 1 year after vaccination 2 for PCV13/PPSV23 were statistically significantly higher than those for PPSV23/PCV13 for 10 of the 12 common serotypes and serotype 6A ().
Table 3. Comparison of OPA GMTs 1 year after vaccination 2 in PPSV23-naive and PPSV23-preimmunized subjects.
PCV13/PCV13 compared with PPSV23/PCV13
The OPA GMT ratio was ≥ 1 for 11 of the 12 common serotypes and serotype 6A, indicating similar or numerically higher GMTs in the PCV13/PCV13 group than in the PPSV23/PCV13 group. OPA GMTs 1 year after vaccination 2 for PCV13/PCV13 were statistically significantly higher than those for PPSV23/PCV13 for 5 of the common serotypes and serotype 6A ().
PCV13/PCV13 compared with PCV13/PPSV23
The OPA GMT ratio was ≥ 1 for 4 of the 12 common serotypes and serotype 6A, indicating similar or higher GMTs in the PCV13/PCV13 group than in the PCV13/PPSV23 group. OPA GMTs 1 year after vaccination 2 for the PCV13/PCV13 group were statistically significantly higher than those for the PCV13/PPSV23 group for serotype 23F and statistically significantly lower for 4 serotypes ().
Comparison of OPA GMTs 1 year after vaccination 2 among the 2 vaccine sequences in PPSV23-preimmunized adults
The OPA GMT ratio was > 1 for all serotypes except serotype 14, indicating numerically higher GMTs in the PCV13/PCV13 group than in the PPSV23/PCV13 group. OPA GMTs 1 year after vaccination 2 for the PCV13/PCV13 group were statistically significantly higher than those for the PPSV23/PCV13 group for 5 of the 12 common serotypes and for serotype 6A ().
Comparison of OPA GMTs 1 year after vaccination 2 with 1 year after vaccination 1 in PPSV23-naive adults
PCV13/PPSV23 compared with initial PPSV23
The OPA GMT ratio was ≥ 1 for all serotypes, indicating that OPA GMTs 1 year after vaccination 2 for PCV13/PPSV23 were similar or numerically higher than those at 1 year after the first dose of PPSV23. OPA GMTs for 9 of the 13 serotypes were statistically significantly higher at 1 year after vaccination 2 for PCV13/PPSV23 than 1 year after the first dose of PPSV23 ().
Table 4. Comparison of OPA GMTs 1 year after vaccination 2 with 1 year after vaccination 1 in PPSV23-naive subjects (Study 1).
PCV13/PCV13 compared with initial PCV13
The OPA GMT ratio was ≥ 1 for 10 of 13 serotypes, indicating OPA GMTs were similar or numerically higher 1 year after PCV13/PCV13 than 1 year after the first dose of PCV13. OPA GMTs were statistically significantly higher for 6 serotypes and statistically significantly lower for 3 serotypes at 1 year after vaccination for PCV13/PCV13 than at 1 year after the first dose of PCV13 ().
PPSV23/PCV13 compared with initial PCV13
The OPA GMT ratio was < 1 for 9 of the 13 serotypes, indicating OPA GMTs were numerically lower at 1 year after vaccination 2 for PPSV23/PCV13 than at 1 year after the first dose of PCV13. PPSV23 followed by PCV13 elicited a significantly higher response for 1 serotype and statistically significantly lower responses for 4 serotypes than PCV13 ().
Comparison of OPA GMTs 1 year after vaccination 2 with 1 year after vaccination 1 in PPSV23-preimmunized adults
PCV13/PCV13 compared with initial PCV13
The OPA GMFR was > 1 for 12 of the 13 serotypes, indicating OPA GMTs were numerically higher at 1 year after vaccination 2 for PCV13/PCV13 than at 1 year after the first dose of PCV13. OPA GMTs for 12 serotypes (except serotype 6A) were statistically significantly higher at 1 year after vaccination 2 for PCV13/PCV13 than at 1 year after the first dose of PCV13 ().
Table 5. Comparison of OPA GMTs 1 year after vaccination 2 with 1 year after vaccination 1 in PPSV23-preimmunized subjects (Study 2).
PPSV23/PCV13 compared with initial PCV13
The OPA GMT ratio was ≥ 1 for 9 of 13 serotypes, indicating that OPA GMTs were similar or numerically higher at 1 year after vaccination 2 for PPSV23/PCV13 than at 1 year after the first dose of PCV13. OPA GMTs were statistically significantly higher for 3 serotypes and statistically significantly lower for 2 serotypes at 1 year after vaccination 2 for PPSV23/PCV13 than at 1 year after the first dose of PCV13 ().
Comparison of OPA GMTs 1 year after vaccination 2 with OPA GMTs measured at each time point in the parent studies
OPA GMTs 1 year after vaccination 2 versus 1 month after vaccination 2
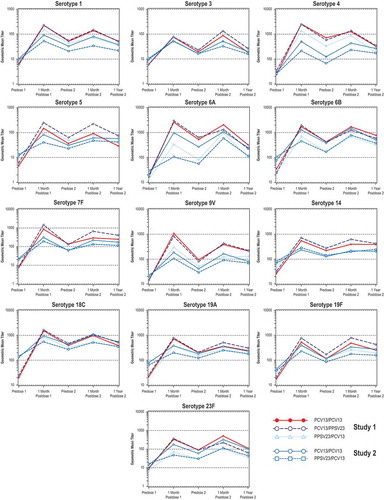
Except for serotypes 7F and 9V for the PPSV23/PCV13 group in study 1 and for serotype 14 for the PCV13/PCV13 and PPSV23/PCV13 groups in study 2, all GMFRs were < 1, indicating numerically lower OPA GMTs at 1 year after vaccination 2 compared with those at 1 month after vaccination 2. This is expected due to decline in antibodies over time. GMFRs were similar for all serotypes across the PCV13/PCV13 and PPSV23/PCV13 groups in both studies (10,11; Supplementary Tables 1–2)
OPA GMTs 1 year after vaccination 2 versus before vaccination 2
For most serotypes across the PCV13/PCV13 and PPSV23/PCV13 groups from both studies, GMFRs were ≥ 1, indicating that OPA GMTs at 1 year after vaccination 2 were similar or numerically higher than those before vaccination 2 (1 year after vaccination 1) (; Supplementary Tables 3–4).
Figure 1. Serotype-specific OPA GMTs from all study time points from before the first vaccination through 1 year after the second vaccination. The first 3 points for each serotype correspond with data from study 1Citation10 and study 2.Citation11 Abbreviations: GMT = geometric mean titer; OPA = opsonophagocytic activity.
OPA GMTs 1 year after vaccination 2 versus 1 month after vaccination 1
The GMFRs in both studies were < 1 for all serotypes across the PCV13/PCV13 and PPSV23/PCV13 groups, except for serotype 6A for the PPSV23/PCV13 group in study 2, indicating numerically lower OPA GMTs at 1 year after vaccination 2 than at 1 month after vaccination 1 (; Supplementary Tables 5–6).
OPA GMTs 1 year after vaccination 2 versus before vaccination 1
The GMFRs in both studies were > 1 for all serotypes across the PCV13/PCV13 and PPSV23/PCV13 groups, indicating numerically higher OPA GMTs at 1 year after vaccination 2 than before vaccination 1. For each serotype except serotype 5, the highest GMFRs were in the PCV13/PCV13 and PCV13/PPSV23 groups among the PPSV23-naive subjects (; Supplementary Tables 7–8).
Safety
No AEs or SAEs were reported during the period between signing of the informed consent for the blood draw and when the blood draw was taken.
Discussion
ACIP recommends PCV13 and PPSV23 be administered in a series to immunocompetent adults ≥65 years of age who had not previously received a pneumococcal vaccine, with PCV13 administered first followed by PPSV23 ≥1 year later.Citation9 This recommended vaccine order is based on studies demonstrating a better response to serotypes shared between both vaccines when PCV7 and PCV13 were given first;Citation9 both parent studies used for the current analysis support this recommendation.Citation10,Citation11
In parent study 1, PPSV23-naive adults 60 to 64 years old received PCV13 and PPSV23 in different sequential order at an interval of 1 year between doses.Citation10 An initial PCV13 dose increased the response to subsequent PPSV23 administration for many shared serotypes. In contrast, an initial PPSV23 dose diminished antibody responses to subsequent PCV13 administration for all serotypes.Citation10 With a relatively short 1-year interval between doses, antibody responses after a second vaccination with PCV13 (PCV13/PCV13) or PPSV23 (PCV13/PPSV23) were noninferior for the majority of serotypes compared with the initial PCV13 dose, but were not improved with a subsequent dose. However, a longer interval of 3.5 to 4 years between vaccine administrations demonstrated significantly higher antibody responses after PCV13/PPSV23 for most of the common serotypes than those observed after an initial dose of PPSV23 or PCV13.Citation12 Antibody responses to a second dose of PCV13 were comparable to initial PCV13 responses and significantly higher for many of the serotypes. These observations are reflected in the ACIP recommendation that longer intervals (ie, ≥ 1 year) may lead to improved immune responses against serotypes in both vaccines compared with an initial dose of PCV13 or PPSV23.Citation9
In parent study 2, adults ≥ 70 years who received PPSV23 ≥ 5 years before study entry were vaccinated with PCV13 or PPSV23.Citation11 Subjects received an additional PCV13 dose 1 year later. Antibody responses were significantly higher after PCV13 than PPSV23 administration for most serotypes. Additionally, antibody responses to a second dose of PCV13 resulted in responses similar to those after the first dose, indicating that receipt of a first PCV13 dose does not negatively influence the ability to respond to a subsequent dose. In contrast, responses to PCV13 administered after PPSV23 were significantly lower for all 13 serotypes compared with the initial PCV13 dose, indicating that PPSV23 diminished the response to subsequent PCV13 vaccination.Citation11
In the current analyses, antibody levels 1 year after the second vaccine administration declined from the peak levels observed 1 month after the first and 1 month after the second vaccine administration, as expected. Antibody levels were similar or numerically higher 1 year after vaccination 2 compared to 1 year after vaccination 1 for most serotypes across the vaccine sequences in both studies. For all serotypes and vaccine sequences, antibody levels remained numerically higher 1 year after vaccination 2 than at baseline (before vaccination 1). Comparisons of antibody levels among the 3 treatment sequences showed functional antibody for almost all serotypes at 1 year after vaccination 2 in both studies were numerically higher in adults who received PCV13 first (PCV13/PCV13 and PCV13/PPSV23) than in adults who received PPSV23 first (PPSV23/PCV13). These results were consistent with the results seen 1 month after vaccination 2 in the parent studies.
The current analysis demonstrates the persistence of effects of the first vaccine on the responses to the second vaccine in the sequence. The parent study in PPSV23-naive adults demonstrated that PCV13 does not impair the antibody responses to subsequent PPSV23 vaccination but rather improves the antibody response to PPSV23;Citation10 the current study indicates these improvements persist for at least 1 year. In contrast, the negative effect of PPSV23 on subsequent PCV13 in the parent study also persisted for at least 1 year. The enhanced antibody response to a second dose of PCV13 was not generally observed in PPSV23-naive adults 1 month after the second vaccination in the parent study.Citation10 The short interval of 1 year between dose administrations may be a reason for this observation. A lack of a consistent booster response when using intervals of 3 months to 1 year between pneumococcal conjugate vaccine and PPSV23 vaccine doses has been described earlier.Citation13–Citation15 Responses to a second dose of PCV13 with a longer interval of 3.5 to 4 years between vaccine administrations have shown generally at least comparable responses to initial PCV13 responses and statistically significantly higher responses for many serotypes,Citation12 indicating that the interval between vaccine administrations may be critical to obtaining an optimal immunological effect of the conjugated vaccine. The PCV13 OPA responses 1 year later in this study suggest that the second dose induced a durable response. Similarly, in PPSV23-preimmunized adults, OPA responses showed durability of the boosted response observed 1 month after the second vaccination.
This is the first study to assess the persistence of antibodies 1 year after a second pneumococcal vaccine administration. Potential limitations of this study include the period of evaluation, which was not continued beyond 1 year after the second vaccine administration, and the descriptive nature of the study as there were too many comparisons to meaningfully calculate p-values. A notable strength of this study was the large number of subjects in each group returning for evaluation at 1 year.
The data from this study confirm the conclusion from the parent studies that in PPSV23-naive and PPSV23-preimmunized adults, PCV13 should be given first if both vaccines are to be given; higher immune response were achieved when PCV13 was given first and immunity persisted at least 1 year after administration of vaccine sequences.
Patients and methods
Study design and population
This phase 3 study evaluated antibody persistence after PCV13 vaccination in 962 healthy adults who completed 2 parent studiesCitation10,Citation11; subjects received either 2 doses of PCV13 or PCV13 and PPSV23 in different sequential order. Subjects in study 1 were 60 to 64 years of age at enrollment, naive to pneumococcal vaccine, and received either PCV13 at year 0 and 1 (PCV13/PCV13), PCV13 at year 0 and PPSV23 at year 1 (PCV13/PPSV23), or PPSV23 at year 0 and PCV13 at year 1 (PPSV23/PCV13; Supplementary Figure 1).Citation10 Subjects in study 2 were ≥ 70 years of age at enrollment and received 1 dose of PPSV23 ≥ 5 years before enrollment [ie, PCV13 at year 0 and 1 (PCV13/PCV13) or PPSV23 at year 0 and PCV13 at year 1 (PPSV23/PCV13; Supplementary Figure 1).Citation11 Blood samples were collected 351 to 420 days after the last vaccination in studies 1 and 2. No vaccine was administered in the current study. Vaccine administration and key inclusion and exclusion criteria have been previously described.Citation10,Citation11
Participants provided written informed consent. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki; the protocol was approved by the institutional review board or independent ethics committee at each site.
Study objectives
The primary objective was to evaluate antibody levels 1 year after vaccination 2 in the parent studies as measured by serotype-specific OPA geometric mean titers (GMTs) using descriptive statistics only. Secondary objectives included comparison of OPA GMTs 1 year after vaccination 2 among the 3 vaccine sequences in PPSV23-naive adults and the evaluation of antibody persistence 1 year after vaccination 2 compared with all prior antibody responses 1 year after vaccination 1.
Analysis populations, immunogenicity assessments, safety evaluations
The all-available immunogenicity population consisted of subjects with valid and determinate assay results related to the proposed analysis who received both vaccinations in the parent studies. The methodology for determination of OPA titers was previously described.Citation10,Citation11 Adverse events (AEs) and serious AEs (SAEs) were recorded from the signing of the informed consent for the blood draw (1 year after the second vaccination) to the completion of the blood draw.
Statistical analysis
There was no formal sample size calculation performed. The sample size of this study was based on the number of potentially eligible subjects from the 2 parent studies who were willing to participate in the follow on study. Approximately 1000 subjects who had completed either of the 2 studies were enrolled in the study and had a blood sample obtained. All immunogenicity data were summarized descriptively.
Serotype-specific OPA titers were logarithmically transformed for analysis. Two-sided 95% CIs for the OPA GMTs were constructed by back transforming the two-sided 95% CIs for the mean logarithm of the titers.
OPA GMTs and two-sided 95% CIs at 1 year after the second vaccination were constructed for each vaccine sequence group in the parent studies. Comparison of OPA GMTs between the vaccine sequence groups at 1 year after the second vaccination were based on the GMT ratios and the corresponding two-sided 95% CIs that were constructed using similar methods. Additionally, similar comparisons of OPA GMTs between a vaccine sequence group at 1 year after the second vaccination and either the same or a different vaccine sequence group at 1 year after the first vaccination were made based on the GMT ratio and corresponding two-sided 95% CIs.
Geometric mean fold rises (GMFRs) and corresponding two-sided 95% CIs were constructed to assess the change in antibody levels 1 year after the second vaccination relative to 4 time points (1 month after the second vaccination, before the second vaccination, 1 month after the first vaccination, and before the first vaccination) using the ratio of OPA titers between the 2 time points of interest for each subject based on logarithmically transformed assay results.
GMTs of one vaccine sequence group were considered statistically significantly higher than another group if the lower limit of the 2-sided 95% CI for the GMT ratio (or GMFR) was > 1. GMTs of one vaccine sequence were considered statistically significantly lower than another if the upper limit of the 2-sided 95% CI for the GMT ratio (or GMFR) was < 1.
Disclosure of potential conflicts of interest
BS-T, AG, TRJ, WCG, and DAS are current employees of Pfizer and may hold stock and/or stock options. RNG received support from Pfizer for attendance at a scientific meeting. The University of Kentucky was supported in part by a National Institutes of Health research grant to their aging center (P30AG028383) and has received research grants from Viropharma, T2, PaxVax, and Bavarian-Nordic. LAJ received support from Pfizer for attendance at a scientific meeting to present study findings. MvC has no conflicts to disclose. The Washington Health Research Institute of Kaiser Permanente has received grants from Pfizer, Novartis, Novavax, Takeda, Inviragen, Sanofi-Pasteur, the National Institutes of Health, and the Centers for Disease Control and Prevention. VS is an employee of inVentiv Health Clinical, LLC, a company contracted by Pfizer Inc. All authors approved the final article.
Supplemental Material
Download Zip (191.6 KB)Acknowledgments
Editorial support was provided by Daniel E. McCallus, PhD, and Susan DeRocco, PhD, of Complete Healthcare Communications, LLC (West Chester, PA), a CHC Group company, and was funded by Pfizer Inc. The University of Kentucky ID Clinical Research Team was Elizabeth Plummer, Dana Hargis, Heather Flynn, Debra Plummer, Malissia VanHook, Connie Geradot, and the UK-CCTS Clinic.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Butler JC, Schuchat A. Epidemiology of pneumococcal infections in the elderly. Drugs Aging. 1999;15(suppl 1):11–19.
- Jansen AG, Rodenburg GD, de Greeff SC, Hak E, Veenhoven RH, Spanjaard L, Schouls LM, Sanders EA, van der Ende A. Invasive pneumococcal disease in the Netherlands: syndromes, outcome and potential vaccine benefits. Vaccine. 2009;27(17):2394–2401. doi:10.1016/j.vaccine.2009.01.127.
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285(13):1729–1735.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. [ accessed 2017 Sept 18]. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html.
- Ricketson LJ, Nettel-Aguirre A, Vanderkooi OG, Laupland KB, Kellner JD. Factors influencing early and late mortality in adults with invasive pneumococcal disease in Calgary, Canada: a prospective surveillance study. PLoS ONE. 2013;8(10):e71924. doi:10.1371/journal.pone.0071924.
- Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K; Competence Network for Community-Acquired Pneumonia Study Group. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–146. doi:10.1183/09031936.00092507.
- Kyaw MH, Rose CE Jr., Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192(3):377–386. JID34127 [pii]. doi:10.1086/431521..
- Verhaegen J, Flamaing J, De Backer W, Delaere B, Van Herck K, Surmont F, Van Laethem Y, Van Damme P, Peetermans W. Epidemiology and outcome of invasive pneumococcal disease among adults in Belgium, 2009-2011. Euro Surveill. 2014;19(31):14–22.
- Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, Pilishvili T. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944–947. doi:10.15585/mmwr.mm6434a4.
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine. 2014;32(20):2364–2374. doi:10.1016/j.vaccine.2014.02.002.
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31(35):3585–3593. doi:10.1016/j.vaccine.2013.05.010.
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–3602. doi:10.1016/j.vaccine.2013.04.084.
- Shelly MA, Jacoby H, Riley GJ, Graves BT, Pichichero M, Treanor JJ. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65(1):242–247.
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis. 2009;49(9):1318–1325. doi:10.1086/606046.
- Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55-70 years of age. Clin Infect Dis. 2009;49(2):241–248. doi:10.1086/599824.
