ABSTRACT
Comparison of anti-HBs persistence after hepatitis B vaccination on two-dose schedule and three-dose schedule among adults is still controversial. In this study, adults were followed up at 12 years after the primary immunization. Three hundred and forty-one and 288 adults with age 15 through 40 years old were given anti-HBV vaccination on a 0-, 1-, and 6-month schedule or on a 0- and 6-month one, respectively (in 2003). Blood samples of 202 patients on 0-, 1- and 6-month schedule and 194 patients on 0- and 6-month regimen were collected at one month and twelve years (in 2015) after the primary series and anti-HBs levels were measured. The seroprotection rate for 3-dose schedule and 2-dose one was 71.78% (95%CI = 65.04%, 77.87%) and 53.61% (95%CI = 46.07%, 60.49%). The GMC of anti-HBs was 31 mIU/mL (95%CI = 24, 41) and 12 mIU/mL (95%CI = 9, 17), respectively. Participants using three doses had higher seroprotection rate and GMC (P < 0.001). Multivariable analysis showed that subjects with anti-HBs titers ≥100 mIU/ml just after the primary series had a higher probability of anti-HBs levels than <10 mIU/ml and 10–100 mIU/ml at follow-up (OR = 8.36, 95%CI: 3.41–20.49, P< 0.001; OR = 43.28, 95%CI: 11.45–163.51, P< 0.001; β = 0.77, 95%CI: 0.48–1.06, P< 0.001; β = 1.20, 95%CI: 0.86 ~ 1.54, P< 0.001). In conclusions, adults receiving HepB primary immunization on 0-, 1- and 6-month schedule might have more prolonged anti-HBs than those on 0-, 6-month schedule, although good anti-HBs persistence could be achieved after HepB immunization on both schedules.
Introduction
Viral hepatitis B is responsible for approximately 47% of an estimated 1.4 million deaths per year and takes a heavy toll on lives.Citation1 The first global health sector strategies on viral hepatitis (2016–2021) are towards its elimination as a major public health threat by 2030 by achieving a set of ambitious targets. These targets apply to everyone at risk of viral hepatitis B infection: children, adolescents and adults. The Advisoty Committee on Immunization Practices (ACIP) in 2018 recommends hepatitis B vaccine (HepB) of adults at risk for hepatitis B virus (HBV) infection.Citation2 In 2011, Chinese Center for Disease Control and Prevention (CCDC) and Chinese Prevention Medicine Association (CPMA) released the guidelines for HepB vaccination among adults, which also recommended that all unvaccinated adults especially those at high risk for HBV infection should be candidates for vaccination.Citation3 Although HepB is recommended to the adults, 527,566 (27%) men were seroprotective for protective antibody against hepatitis B surface antigen (anti-HBs) and 63% were negative for all HBV markers in men aged 21–49 years in rural China.Citation4 This indicated that they were susceptible to HBV. The reported incidence (per 100 000 population) of acute hepatitis B was at the highest levels of 10.68 among 25–29 year-olds in 2012 in China. This indicated a heavy disease burden of hepatitis B in China among adults.Citation5
The three doses of HepB on 0-1-6 month schedule are recommended to adults by ACIP and CPMA till now, which is believed to a fundamental, classic, complete strategy.Citation2,Citation3 Due to poor compliance with a prescribed 3-dose schedule and the population’s floating, many adults completed only one or two doses of HepB. Previous studies confirmed that HepB have good immunogenicity among adults and the anti-HBs could keep above protective level for many years after primary immunization among adults.Citation6–Citation13 Comparison on anti-HBs persistence of different vaccination schedules can give definite evidence to choose more prolonged ones. The comparison results varied considerably in different populations.Citation14–Citation16 Anti-HBs level was similar between adolescents who receiving a 3-dose schedule and those who receiving 2-dose one after 10 years and 15 years of the primary vaccination.Citation14,Citation15 Another study showed that anti-HBs persistence of 3-dose regimen was superior to alternative 2-dose one among children.Citation16 However, Anti-HBs persistence and factors associated with persistence after hepatitis B vaccination in adults between two schedules are not well investigated.
This study had been performed to find better schedule for HepB primary immunization among adults by comparing anti-HBs persistence between 0-, 1- and 6-month schedule and 0- and 6-month schedule. We also explored factors associated with anti-HBs titers at 12 years after primary immunization.
Results
Study population
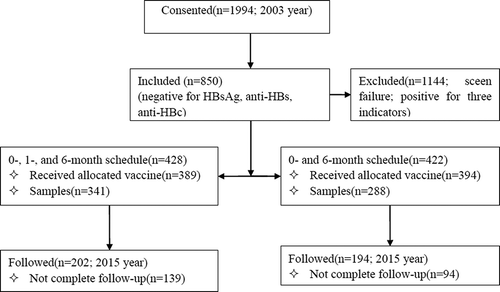
Of the 629 adults who participated the study in 2003 year, 396 subjects finished the follow-up and were included in the final analysis (202 on 0-, 1- and 6-month schedule; 194 on 0- and 6-month regimen), 233 persons were lost follow-up (139 in three-dose group, and 94 in two-dose group). There were no statistically significant differences in gender, anti-HBs titers right after the primary vaccination except for age at the primary series. The demographic characteristics of the subjects are shown in . The average age at primary immunization in 0-, 1- and 6-month schedule and 0- and 6-month schedule was 32.80 [95%CI = 32.00, 33.60] and 32.81 [95%CI = 31.96, 33.48].
Table 1. The characteristic of study population between completed follow-up and not complete follow-up.
Anti-hbs at follow-up
The proportion of subjects with anti-HBs titers ≥10 mIU/mL for 3-dose schedule and 2-dose schedule was 71.78% [95% confidence interval (CI) = 65.04%, 77.87%] and 53.61% [95%CI = 46.07%, 60.49%]. 3-dose schedule resulted in statistically higher anti-HBs seroprotection rate compared with 2-dose one (Pearson χCitation2 = 15.69, P< 0.001). The detailed information for positive rate and geometric mean concentrations (GMC) at 12 years after the primary series stratified by immunization schedule is shown in .
Table 2. Percentage of subjects with anti-HBs titers 0–9, 10–99, 100–999 and ≥1000 mIU/mL and GMC at twelve years after the primary series, stratified by immunization schedule.
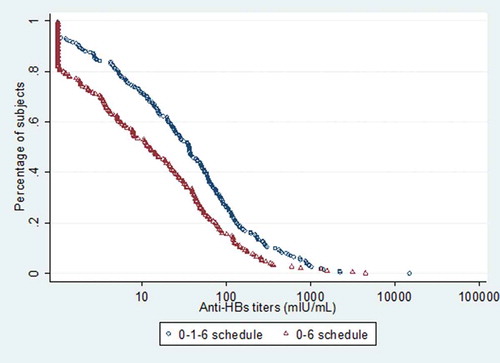
At year 12, The GMC of anti-HBs for 3-dose schedule and 2-dose one was 31 mIU/mL [95%CI = 24, 41] and 12 mIU/mL [95%CI = 9, 17]. Compared with subjects receiving two doses, participants using three doses had higher GMC (F= 20.82, P< 0.001). The overall anti-HepB GMC was 20 mIU/mL [95%CI = 16, 24]. Reverse cumulative distribution (RCD) curve showed that antibody variation among the individual anti-HBs titers. All the individual values in 3-dose schedule were higher than those in 2-dose one and 0-, 1- and 6-month schedule had a higher median anti-HBs and a smaller variance (as shown by the steeper midsection) ().
Factors associated with persistence
Multivariable analysis showed that age at the primary immunization, gender, body mass index (BMI), smoking history, drinking history, hepatitis B among family members, and chronic diseases history were not significantly associated with seroprotection rate and GMC at 12 years, but schedules for the primary immunization were independently associated with seroprotection rate (OR = 0.55, 95%CI: 0.34, 0.90, P= 0.017) and GMC (β = −0.26, 95%CI: −0.42, −0.10, P= 0.002) at 12 years. Moreover, subjects with anti-HBs titers 100–1000 mIU/ml and ≥1000 mIU/ml just after the primary series had a higher probability of anti-HBs levels than <10 mIU/ml and 10–100 mIU/ml at follow-up (OR = 8.36, 95%CI: 3.41, 20.49,P< 0.001; OR = 43.28, 95%CI: 11.45,163.51, P< 0.001; β = 0.77, 95%CI: 0.48,1.06, P< 0.001; β = 1.20, 95%CI: 0.86, 1.54, P< 0.001) ().
Table 3. Multivariable model analysis on seroprotection rate (≥10 mIU/ml) and GMC at twelve years after HepB-SC primary immunization.
HBV breakthrough infection
Out of 20 subjects were antibody against hepatitis B core antigen (anti-HBc) positive during follow-up. The proportion of breakthrough infection was found not statistically different in schedules, age at the primary series, gender, BMI, smoking history, drinking history, chronic diseases history. However, those subjects who had family members with chronic hepatitis B infection had higher breakthrough infection rate (28.57% vs. 4.63%; P= 0.044).
Discussion
Anti-HBs persistence is a more important indicator to evaluate seroprotective response after HepB vaccination, which is also the main factor to determine the necessity of booster dose.
According to the present study, 71.78% and 53.61% adult vaccinees still had seroprotective antibodies 12 years after HepB primary immunization on 0-1-6 month schedule and 0–6 month schedules respectively. The result is similar to that in the study among children, where there were 73.00% and 54.30% children keeping protective anti-HBs 15 years after the primary vaccination on 0-1-6 month schedule and 0–6 month schedule respectively.Citation15 Our study proved that the anti-HBs persistence was better after completing on 0-1-6 month schedule than after 0–6 month schedule with the difference of 20% in anti-HBs protective rate. There are 245 million migrant populations with an average age of 29.8 years in China.Citation17 Owing to the convenience of the migrant population, the 0–6 month schedule might be an alternative selection if vaccination compliance wasn’t high, which could also achieved good anti-HBs persistence.Citation18
Among participants on 0-, 1- and 6-month schedule and 0- and 6-month one with an anti-HBs level ≥ 100 mIU/mL at initial series, those with a higher primary anti-HBs level and seroprotection rate were more likely to demonstrate a higher follow-up antibody levels and seroprotection rate than those with lower anti-HBs titers according to the multivariable analysis. This suggests that those with anti-HBs levels ≥100 mIU/mL 12 years after the primary immunization haven’t fall below <10 mIU/mL, the farther the level is to 100 mIU/mL, the higher the probability that the persistence will acquire. The findings on no association between the proportion of subjects with seroprotective antibody levels at 12 years and BMI, age at the primary vaccination coincide with a recent study and our previous study.Citation8,Citation9 However, many studies have drawn firm conclusions that increasing age is associated with a decline in anti-HBs titers.Citation7,Citation19,Citation20 This isn’t consistent with our finding and may be largely attributed to the smaller proportions in younger adults. We haven’t observed clear significant difference between other factors and anti-HBs persistence among adults. The results of our study and other studies revealed no significance regarding to gender.Citation19,Citation21–Citation24 More studies are needed to identify the effect.
Those who had contact history with their family members have higher breakthrough infection rate. It shows breakthrough infection among adults may be due to horizontal transmission in the household.Citation24 This reminds us of importance to give HepB to the population at high risk.
Compared with the complete three-dose vaccination schedule, a two-dose one reduces a dose and facilitates better compliance of vaccinee, in addition to offering benefits associated costs. So, the greatest advantage in our study is verified that the anti-HBs persistence of a 2-dose schedule to adults is a viable alternative to the conventional three-dose schedule owing to the convenience to the migrant population.
The main limitation is the high loss to follow-up of the participants. This study was conducted in the rural areas in China, where many people leave their hometown and work in the cities for the higher salary and better work environment. Although age of the participants was different between the two schedule-groups, given the fact that we did not find age at the primary immunization was associated with the anti-HBs persistence, we could conclude that the difference in age at the primary immunization might have little impact on the main results.
In conclusion, a 0-, 1-, and 6-month schedule in adults provides a better anti-HBs persistence when compared with 0–6 month schedule. Also, owing to the convenience to the migrant population in China, a vaccination schedule of 0- and 6-month schedule may be suitable. Anti-HBs titers after primary immunization are the independent predictive factors of anti-HBs persistence.
Materials and methods
Subjects
1,994 healthy adults who were aged 15–40 years from 20 villages of a town, Jiyang County, Shandong province, China were used the cluster sampling according to villages and enrolled and screened HBV serological markers including hepatitis B surface antigen (HBsAg), anti-HBs and anti-HBc in 2003. A total of 850 were seronegative for all three indicators and randomly allocated to 0, 1, and 6 months schedule or a 0, 6 months schedule. 428 and 422 subjects received 10μg HepB (batch number 20021159–1) derived in Saccharomyces cerevisiae (HepB-SC) according to a 0, 1, and 6 months schedule or a 0, 6 months schedule, respectively. Among vaccine recipients, 629 (341 vs. 288) subjects completed the three doses of HepB and the anti-HBs test one month after vaccination and were included in this study attending follow-up. 396 subjects (202 vs. 194) attended the 12-year long-term follow-up conducted in April 2015 (). Written informed consent was signed from all subjects and the study was approved by the Ethics Committees of Shandong CDC.
Serum samples collection and laboratory testing
There were three collection. The first collection was used for screening. The second one was after three doses of HepB. We made the third colletion during follow-up. Blood samples of 3–5 ml were collected and screened before HepB vaccination. Moreover, blood samples were collected and tested one month after the third post 6-month vaccination and at the follow-up visit. Serological markers including HBsAg, anti-HBs and anti-HBc were screened by Solid-Phase Radioimmunoassay (SPRIA) using reagents kit (batch number 200311) produced by National Vaccine & Serum Institute before HepB vaccinatio.Citation25,Citation26 Anti-HBs after the primary series were detected by SPRIA using reagents kit (batch number 040720) produced by Beijing North Institute of Biological and Technology after the third post 6-month vaccination in 2004. We measured quantitatively anti-HBs, anti-HBc and HBsAg when anti-HBs was less than 10 mIU/ml for follow-up through Chemiluminescence Microparticle Immunoassay (CMIA) (Abbott reagent, Abbott ARCHITECT-i2000 Immuno-luminescence detector, the same below) at the follow-up visit in 2015.
Statistical analyses
Demographic and epidemiological data included: socio-demographics (e.g. gender, age), lifestyle habits (e.g. smoking history, drinking history), health status (chronic diseases history). An anti-HBs level≥10 mIU/mL was considered seroprotective. Data are expressed as number and proportion of persons with seroprotection as categorical variables, geometric mean concentrations (GMC) and 95% CI as quantitative variables where appropriate. Quantitative anti-HBs titers were log-transformed to calculate GMC and 95% CI. Statistical software R 3.0.2 for Windows was utilized to analyze differences between groups: oneway analysis of variance was applied to quantitative variables, Pearson chi-square tests to categorical variables, multivariable linear regression model to factors associated with persistence for quantitative variables, nonconditional logistic regression model to the factors for categorical variables. A P-value of <0.05 (two tails) was chosen to indicate statistical significance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank the Jiyang Center for Disease Control and Prevention and relevant personnel for their contribution to this study.
Additional information
Funding
References
- WHO. [accessed 2016 06] http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- Blumberg EA. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices: a summary of the MMWR report. Am J Transplant. 2018;18(5):1285–1286. doi:10.1111/ajt.14763.
- Cui FQ, Chinese Prevention Medicine Association; National Immunization Program, Chinese Center for Disease Control and Prevention. Technical guide for adult hepatitis B immunization in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32(12):1199–1203.
- Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, Yan D, Liu M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016;16(1):80–86. doi:10.1016/S1473-3099(15)00218-2.
- Zhang GM, Sun XJ, Wang FZ, Zheng H, Gong XH, Miao N, Jia YX, Wu ZH, Cui FQ. Analysis of epidemiological characteristics of hepatitis B among the population of 18-59 year-old and HepB immunization strategies. Zhongguo Yi Miao He Mian Yi. 2013;19(3):266–270.
- Chlibek R, Von Sonnenburg F, Van Damme P, Smetana J, Tichy P, Gunapalaiah B, Leyssen M, Jacquet JM. Antibody persistence and immune memory 4 years post-vaccination with combined hepatitis A and B vaccine in adults aged over 40 years. J Travel Med. 2011;18(2):145–148. doi:10.1111/j.1708-8305.2010.00499.x.
- Van Damme P, Leroux-Roels G, Crasta P, Messier M, Jacquet JM, Van Herck K. Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined hepatitis A and B vaccine. J Med Virol. 2012;84(1):11–17. doi:10.1002/jmv.22264.
- Höhler T, Groeger-Bicanic G, Hoet B, Stoffel M. Antibody persistence and immune memory elicited by combined hepatitis A and B vaccination in older adults. Vaccine. 2007;25(8):1503–1508. doi:10.1016/j.vaccine.2006.10.024.
- Wu WL, Yan BY, Lyu JJ, Liu JY, Feng Y, Chen SY, Zhou LB, Liang XF, Cui FQ, Wang FZ, et al. Antibody persistence following primary vaccination with hepatitis B vaccine among normal and high-responder adults: 5-year follow-up study. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50(6):484–490. doi:10.3760/cma.j.issn.0253-9624.2016.06.003.
- Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-Year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi:10.1093/infdis/jiv748.
- Van Damme P, Leroux-Roels G, Suryakiran P, Folschweiller N, Van Der Meeren O. Persistence of antibodies 20 y after vaccination with a combined hepatitis A and B vaccine. Hum Vaccin Immunother. 2017;13(5):972–980. doi:10.1080/21645515.2016.1274473.
- Wang ZZ, Gao YH, Wang P, Wei L, Xie CP, Yang ZX, Lan J, Fang ZL, Zeng Y, Yan L, et al. Comparison of immunogenicity between hepatitis B vaccines with different dosages and schedules among healthy young adults in China: A 2-year follow-up study. Hum Vaccin Immunother. 2018;14(6):1475–1482. doi:10.1080/21645515.2018.1438090.
- Yao J, Li J, Chen Y, Shan H, Dai XW, Yang LN, Jiang ZG, Ren JJ, Xu KJ, Ruan B, et al. The response of hepatitis B vaccination on seronegative adults with different vaccination schedules. Hum Vaccin Immunother. 2015;11(5):1102–1107. doi:10.4161/21645515.2014.985500.
- Beran J, Kervyn D, Wertzova V, Hobzova L, Tichy P, Kuriyakose S, Leyssen M, Jacquet JM. Comparison of long-term (10 years) immunogenicity of two- and three-dose regimens of a combined hepatitis A and B vaccine in adolescents. Vaccine. 2010;28(37):5993–5997. doi:10.1016/j.vaccine.2010.06.104.
- Beran J, Van Der Meeren O, Leyssen M, D’silva P. Immunity to hepatitis A and B persists for at least 15 years after immunisation of adolescents with a combined hepatitis A and B vaccine. Vaccine. 2016;34(24):2686–2691. doi:10.1016/j.vaccine.2016.04.033.
- But DY, Lai CL, Lim WL, Fung J, Wong DK, Yuen MF. Twenty-two years follow-up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: final report. Vaccine. 2008;26(51):6587–6591. doi:10.1016/j.vaccine.2008.09.034.
- Department of Mobile Population of National Health Commission of the People’s Republic of China. Report of China’s migrant population development 2017. Hai Dian, Beijing, China: China Population Publishing House; 2017.
- Greengold B, Nyamathi A, Kominski G, Wiley D, Lewis MA, Hodge F, Singer M, Spiegel B. Cost-effectiveness analysis of behavioral interventions to improve vaccination compliance in homeless adults[J]. Vaccine. 2009;27(5):718–725. doi:10.1016/j.vaccine.2008.11.031.
- Norouzirad R, Shakurnia AH, Assarehzadegan MA, Serajian A, Khabazkhoob M. Serum levels of anti-hepatitis B surface antibody among vaccinated population aged 1 to 18 years in ahvaz city southwest of iran. Hepat Mon. 2014;14(1):e13625. doi:10.5812/hepatmon.13625.
- Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32(4):307–313. doi:10.1097/INF.0b013e31827bd1b0.
- Baghianimoghadam MH, Shadkam MN, Hadinedoushan H. Immunity to hepatitis B vaccine among health care workers. Vaccine. 2011;29(15):2727–2729. doi:10.1016/j.vaccine.2011.01.086.
- Coppola N, Corvino AR, De Pascalis S, Signoriello G, Di Fiore E, Nienhaus A, Sagnelli E, Lamberti M. Impact and long-term protection of hepatitis B vaccination:17 years after universal hepatitis B vaccination in Tunisia. Epidemiol Infect. 2016;144(16):3365–3375. doi:10.1017/S0950268816001849.
- Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. The long-term immunogenicity ofhepatitis B virus (HBV) vaccine: contributionuniversal HBV vaccination in Italy. BMC Infect Dis. 2015;15(1):1–7. doi:10.1186/s12879-014-0722-x.
- Zhang L, Yan BY, Li MS, Song LZ, Lü JJ, Xu Q, Xu AQ. Preliminary analysis on the prevalence and causes of breakthrough hepatitis B virus infection among children in Shandong province, China. Zhonghua Yu Fang Yi Xue Za Zhi. 2013;47(10):933–939.
- Gong XH, Li YH, Liu LR, Jia L, Xing YL, Wang YQ. Study on the afficacy of hepatitis B immunization among youngsters in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(5):388–390.
- Wang DH, Hu GL, Tu QF, Yu ZY, Guo SC. Study on hepatitis B virus infection and influence among children in Jiangxi Province. Zhongguo Ji Hua Mian Yi. 2004;10(10):267–270.