ABSTRACT
Human papillomavirus (HPV) is one of the main causes of infection-related cancer. The bivalent vaccine (2vHPV) (16/18) and quadrivalent (6/11/16/18) HPV vaccine (4vHPV) have been included in the Spanish vaccination calendar since 2007. The new nonavalent HPV vaccine (9vHPV), approved in Europe in 2015, includes nine HPV types 6/11/16/18/31/33/45/52/58 and has been available in Spain since May 2017. Our study aims to estimate the epidemiological impact and the cost-effectiveness of a girls-only and a gender-neutral vaccination program with 9vHPV compared to the current vaccination program in Spain. A dynamic transmission model simulating the natural history of HPV infections was calibrated to the Spanish setting and applied to estimate costs and quality-adjusted life years (QALYs) associated with vaccination strategies using a payer perspective and a 100-year time horizon.
A girls-only vaccination strategy at age 12 years with 9vHPV was found to be a cost-effective strategy compared with 4vHPV (incremental cost-effectiveness ratio (ICER) of €7,718 per QALY). Compared with girls-only vaccination with 4vHPV, gender-neutral vaccination with 9vHPV was associated with further reductions of up to 28.5% in the incidence of cervical intraepithelial neoplasia (CIN) 2/3 and 17.1% in the incidence of cervical cancer, as well as with a 14.0% reduction in cervical cancer mortality. Furthermore, a gender-neutral vaccination program with 9vHPV could potentially be cost-effective considering some parameters as head and neck protection or discount rates, leading to a reduction in the burden of HPV-related diseases in both sexes in the Spanish population.
Introduction
Human papillomavirus (HPV) is one of the most frequent sexually transmitted infections and one of the main causes of infection-related cancer, accounting for 4.8% of the total cancer burden worldwide.Citation1 To date, more than 150 HPV types have been completely sequenced.Citation2 High-risk HPV types are related to almost all cases of high-grade cervical intraepithelial neoplasia (CIN) and invasive cervical cancers. In Europe, between 86.9% and 100% of precancerous anogenital lesions in men and women are attributable to HPV, with 47% of cases related to HPV types 6/11/16/18 and 82% related to HPV types 6/11/16/18/31/33/45/52/58. Moreover, an average of 83% of anogenital cancers in men and women are related to HPV, with 72.8%-87.1% related to HPV types 16/18 and 89% related to HPV types 16/18/31/33/45/52/58.Citation3 In addition, low-risk HPV types 6/11 cause approximately 90% of cases of genital warts.Citation4 The three currently available HPV vaccines are the bivalent vaccine (2vHPV), the quadrivalent vaccine (4vHPV), and, more recently, the nonavalent vaccine (9vHPV). 2vHPV includes only the HPV types 16/18Citation5 and 4vHPV includes the HPV types 6/11/16/18.Citation6 Both vaccines are indicated for use in individuals from nine years of age onward for preventing precancerous anogenital lesions, cervical and anal cancer associated with certain types of HPV,Citation5,Citation6 and, in the case of 4vHPV, also for preventing genital warts.Citation6 Both vaccines induce a strong immune response.Citation7 They were initially approved in a three-dose schedule, but an alternative two-dose schedule was subsequently approved for 2vHPV in children aged 9 to 14 yearsCitation8 and for 4vHPV for children aged 9 to 13 years.Citation9 Both vaccines were licensed in 2006–2007 and were included in the Spanish vaccination program for 2007–2008. The new 9vHPV was developed to protect against most oncogenic HPV genotypes and relevant low-risk types and includes HPV types 6/11/16/18/31/33/45/52/58Citation10: four of these types were already covered by 4vHPV, and five other oncogenic HPV types. In the pivotal trialCitation11,Citation12, 9vHPV was compared with 4vHPV in women aged 16–26 years. The results in the per protocol population (completely vaccinated, seronegative on day 1, and PCR-negative from day 1 to month 7 for the HPV types analyzed) showed that 9vHPV reduced by 96.7% the risk of the combined incidence of CIN2/3, adenocarcinoma in situ, cervical cancer, high-grade vulvar intraepithelial neoplasia (VIN2/3), high-grade vaginal intraepithelial neoplasia (VaIN2/3), vulvar cancer, and vaginal cancer caused by the five additional HPV types (31/33/45/52/58). This study also showed that the immune response for the four common HPV types was non-inferior and that geometric mean titers were 50-fold higher for each of the five new HPV types.Citation11,Citation12
9vHPV was approved by the United States Food and Drug Administration (FDA) in December 2014Citation13 and received a positive opinion from the European Medicine Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in 2015Citation14. Since 2016, it has been available in some European countries, such as Austria and Germany. The two-dose schedule was also approved that year.Citation15 In Spain, this vaccine has been available since May 2017.
An HPV vaccination program with a three-dose schedule was introduced into the Spanish vaccination calendar in 2007 for girls aged 11 to 14 years. At that moment, only 2vHPV and 4vHPV were available. Nowadays, HPV vaccination targets 12-year-old girls based on a two-dose schedule,Citation16 with an estimated national vaccine coverage of 77.8% in 2016.Citation17 9vHPV is already included in the vaccination calendar of some regions in Spain. The Spanish HPV vaccination program is mainly tender-based, and these tenders and the vaccine delivery system are managed directly by each of the 19 Spanish regions. Ten of the 19 regions have a school-based delivery program; in the remaining nine, HPV vaccination is managed in primary care centers. As no catch-up for women older than age 14 years was implemented at the beginning of the HPV vaccination program, an individualized recommendation to vaccinate women not targeted by the national vaccination program has been in place since 2007. However, the coverage reached in this group of women until age 45 years does not exceed 1%.Citation18
In Spain, different cervical screening strategies are applied in different regions. Most are opportunistic and heterogeneous in their characteristics and application criteria. It is estimated that around 71% of women aged 25 to 65 have been screened at least once during the last three years under these opportunistic screening programs.Citation19
The present analysis aimed to evaluate the cost-effectiveness of implementing a girls-only or a gender-neutral (girls and boys) vaccination program with 9vHPV in Spain compared with the current vaccination strategy (girls only) with 4vHPV.
Results
Model calibration
The model outcomes were consistent with the targets in overall incidence and mortality rates. However, the incidence of CIN and the incidence and mortality of vaginal and vulvar cancers were significantly underestimated, with a > 15% difference between model outcomes and targets (see Tables A6 and A7).
Epidemiological results
The adoption of 9vHPV would add benefits over 4vHPV. The model shows further reductions in the number of cases of disease and deaths, as well as in the incidence and mortality rates of diseases related to HPV types 16/18/31/33/45/52/58 over the analyzed time horizon with 9vHPV compared with 4vHPV in the girls-only vaccination scenario ( and ).
Table 1. Disease events and deaths related to HPV types 6/11/16,718/31/33/45/52/58 prevented over a time horizon of 100 years.
Table 2. Additional reductions in the incidence and mortality rates of diseases related to HPV types 6/11/16/18/31/33/45/52/58 over a time horizon of 100 years.
shows the epidemiological impact of the vaccination strategies over a time horizon of 100 years. A significant decrease in the incidence and mortality rates of diseases related to HPV types 6/11/16/18/31/33/45/52/58 becomes noticeable after 25 years of simulation. Moreover, considerable reductions in the number of cases of cervical and anal cancers begin approximately 20 years after the start of the vaccination program. The same trend is seen for genital warts and CIN, whose reductions begin 10 years earlier.
Figure 1. Epidemiological impact of GNV vaccination strategies over a 100-year time horizon.
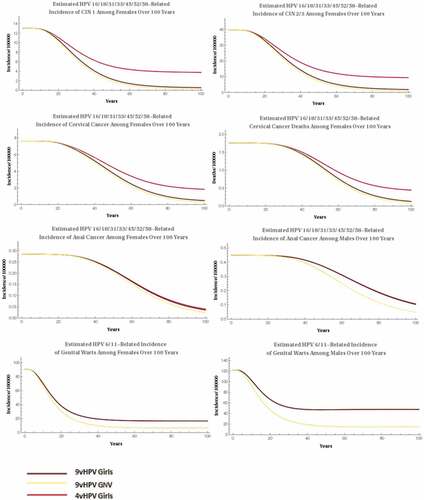
The additional benefits observed with 9vHPV were greater when the gender-neutral vaccination with 9vHPV was compared with the girls-only vaccination with 4vHPV. Furthermore, reductions in incidence were recorded, as follows: 35% in CIN1, 28.5% in CIN2/3, 17.1% in cervical cancer, and 14% in cervical cancer mortality ( and ).
The incidence of genital warts related to HPV types 6/11 further decreases with gender-neutral vaccination with 9vHPV compared with girls-only vaccination with 4vHPV.
By using only 9vHPV, the inclusion of boys in the national immunization program could also positively impact the epidemiology of HPV, with reductions of 29.2% and 44.3% in the incidence of genital warts in females and males, respectively, and 10.8% in the incidence of anal cancer in males and 3.6–6.4% in the incidence of cervical pathology in females ( and ).
Cost-effectiveness results
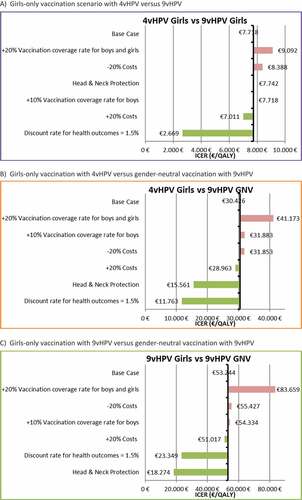
Our analysis shows that switching to 9vHPV compared with 4vHPV is highly cost-effective in Spain. In a girls-only vaccination scenario, 9vHPV is considered cost-effective vs. 4vHPV while maintaining a stable VCR (77.8%), with an incremental cost-effectiveness ratio (ICER) of €7,718 per quality-adjusted life year (QALY), which is below the threshold commonly used in Spain (€30,000/QALY)Citation20. Compared with a girls-only vaccination scenario with 4vHPV, the cost-effectiveness result for the gender-neutral vaccination scenario with 9vHPV (€30,426/QALY) falls very close to the reference threshold used in Spain (), considering a VCR of 77.8% for girls and 55% for boys.
Table 3. Cost-effectiveness results in the base case analysis.
Compared with girls only vaccination scenario with 9vHPV, the cost-effectiveness results for the gender-neutral vaccination scenario with 9vHPV exceeds the Spanish cost-effectiveness threshold in the base case analysis (€53,244/QALY).
Sensitivity analyses
Sensitivity analyses were performed deterministically to test for uncertainty. The impact on the ICER result of changing inputs such as VCR in boys and girls, VCR in boys only, discount rate, variations in costs, and considering head and neck protection conferred by vaccines was assessed ().
The results of the sensitivity analyses for VCR in the girls-only and gender neutral scenarios suggested that increasing the coverage rate by 20% in both females and males and by 10% in males yielded higher ICERs.
Variations in the discount rates can also modify the results significantly. Assuming a discount rate for health outcomes of 1.5% resulted in lower ICERs (€2,669/QALY for the girls-only vaccination with 9vHPV vs 4vHPV and €11,763/QALY for the gender neutral vaccination with 9vHPV vs 4vHPV girls-only vaccination). In this scenario, a switch to a gender-neutral vaccination with 9vHPV vs girls-only vaccination with 9vHPV would be also considered cost-effective, with an ICER of €23,349/QALY.
Finally, the base case analysis considered only the diseases included in the summary of product characteristics of the vaccines. Owing to increasing evidence for the role of HPV infections in head and neck cancer, we included this variable in the sensitivity analysis. The health and economic benefits of a gender-neutral vaccination program with 9vHPV increased if head and neck cancer was considered in the analysis. In this case, the ICER of gender-neutral vaccination with an 9vHPV scenario compared with girls-only vaccination with 4vHPV decreased below the €30,000/QALY threshold (€15,561/QALY), thus indicating that gender neutral vaccination with 9vHPV is a cost-effective strategy in Spain. When gender-neutral vaccination was compared with girls-only vaccination, both with 9vHPV and considering protection against head and neck cancer, the ICER for gender-neutral vaccination with 9vHPV was €18,274/QALY. Therefore, in this scenario, gender-neutral vaccination with 9vHPV would also be considered cost-effective.
Discussion
This is the first cost-effectiveness analysis of a vaccination program with 9vHPV in Spain.
A dynamic model was adapted to Spain in order to compare this new HPV vaccine with 4vHPV, which is already included in the national vaccination program. The model was applied in two vaccination scenarios, girls-only and gender-neutral. The results show that replacing 4vHPV with 9vHPV could reduce the incidence and mortality rates of HPV-related diseases in Spain and would be cost-effective.
The inclusion of 9vHPV in the Spanish HPV vaccination program could decrease the frequency of diseases related to HPV types 6/11/16/18/31/33/45/52/58 compared with 4vHPV. This clinical benefit is greater when the gender-neutral vaccination scenario with 9vHPV is compared with girls-only vaccination with 4vHPV, resulting in an additional 18,665 prevented cases of cervical cancer, up to 804,853 prevented cases of genital warts (607,659 in men and 197,194 in women), and reductions in the incidence rate of anal cancer of up to 4% in women and 11% in men.
In economically developed countries such as those in Western Europe, the implementation of cervical screening programs has significantly reduced the incidence of cervical cancer in women. However, other diseases associated with HPV, such as anal cancer, squamous cell cancers of the oral cavity, and oropharyngeal cancer, are not amenable to screening, and their incidence is rising in both sexes. This situation has resulted in a considerable increase in the burden of HPV-associated cancers in men.Citation21 In Spain, a recent study found that hospitalization rate due to malignant neoplasm and in situ carcinoma of the anus in males increased significantly between 2009 and 2013. During this period, 2,060 hospitalizations due to malignant neoplasm and in situ carcinoma were registered in males.Citation22 Additionally, the crude incidence rate of head and neck cancer in Spain is five-fold higher in men than in women.Citation23
As mentioned, our study shows that in the current Spanish HPV vaccination scenario, where only girls are included, 9vHPV is a cost-effective strategy compared with 4vHPV. In comparison with the girls-only vaccination with 4vHPV, gender-neutral vaccination with 9vHPV is at the limit of cost-effectiveness, based on list prices. After including head and neck cancers in the analysis, gender-neutral vaccination with 9vHPV is cost-effective vs. girls-only vaccination with 4vHPV with an ICER of €15,561/QALY. Moreover, again based on list prices and compared with girls-only vaccination with 9vHPV, gender-neutral vaccination with 9vHPV would be cost-effective, with an ICER of €18,274/QALY.
Gender-neutral vaccination could substantially reduce the burden of HPV-associated disease in men, irrespective of their sexual orientation, with a rapid decline in the prevalence of HPV in the population. In this context, implementing vaccination programs for both boys and girls seems to be an efficient public health strategy.
As stated previously, the price considered for this analysis is the list price, which is higher than that included in Spanish tenders, thus making the ICER value even lower than that obtained and confirming that gender-neutral vaccination with 9vHPV is a cost-effective strategy.
When lower discount rates are considered in the sensitivity analysis (1.5%), the incremental cost-effectiveness ratio of gender-neutral vaccination decreases substantially, and gender-neutral vaccination strategies based on 9vHPV would be cost-effective. Recently, the United Kingdom Joint Committee on Vaccination and Immunisation (JCVI) highlighted that a discount rate of 1.5% can be considered when the impact of a lifesaving intervention is sustained over a period of at least 30 years. Such is the case of HPV, given that HPV-related cancer can appear several decades after initial infection and more than 30 life years would be lost in some cases.Citation24
Our results are in accordance with those of three studies on the cost-effectiveness of 9vHPV in the US. These studies showed that, in gender-neutral vaccination scenarios, 9vHPV was likely to be cost-effective and even cost-saving compared with 4vHPV.Citation25-Citation27 A later study confirmed the cost-effectiveness of 9vHPV in the United States.Citation28 In Europe, recent studies in Austria,Citation29 GermanyCitation30, ItalyCitation31 and, more recently, in the UKCitation24, have also shown the cost-effectiveness of 9vHPV in gender-neutral vaccination scenarios.
Our study is subject to a series of limitations. The model did not consider cross-protection against types not included in the vaccines. Therefore, we may have underestimated the impact of current vaccines, although the evidence suggests that the cross-protection effect is limited.Citation32 In addition, we did not compare gender-neutral vaccination with 9vHPV vs. gender-neutral vaccination with 4vHPV, because it was expected that 9vHPV would already have replaced 4vHPV by the time gender neutral vaccination is introduced in Spain.
The results of the model could be highly conservative as a consequence of the limited data on sexual behavior in Spain during recent years. As already observed in other countries, it is reasonable to expect that from 2003 until now, a larger number of sexual partners and more flexible patterns of sexual mixing could have increased the risk of HPV infection, leading to a potentially higher impact of HPV vaccines.
This study may also underestimate the societal benefits of HPV vaccination. We did not analyze the effect of cervical lesions on neonatal morbidity and mortality or the impact of conizations.Citation33 Similarly, we did not consider losses in productivity, although HPV-related lesions impair work productivity and functionality in the workplace.Citation34 Moreover, in the case of direct medical costs of penile, anal, and head and neck cancers, we only considered hospital costs, as no other information was available.Citation35,Citation36
Another important limitation of our study is the underestimation of the additional benefits of vaccination with 9vHPV for prevention of CIN, which is a key aspect of the value of 9vHPV.Citation3,Citation37 This limitation results from the structure of our model and cannot be improved upon without compromising the calibration of cervical cancer estimates. Therefore, the results can be reasonably considered conservative estimates.
Finally, our model does not allow running a probabilistic sensitivity analysis; so, as the previously published adaptationsCitation29-Citation31, only deterministic sensitivity analysis was carried out.
Conclusions
Our study shows the potential cost-effectiveness of vaccination with 9vHPV in Spain. The implementation of vaccination with 9vHPV can provide significant incremental public health benefits and is cost-effective when compared with the current vaccination program with 4vHPV. Moreover, implementation of a gender-neutral vaccination program with 9vHPV could potentially be cost-effective considering head and neck protection or similar discount rates, which are already used in international independent studies. In addition, such a vaccination program could further reduce the burden of HPV-related diseases in both sexes in the Spanish population.
Methods
Model description
We used a dynamic HPV disease transmission model based on a deterministic susceptible-infected-recovered-susceptible model originally developed by Merck & Co., Inc., Kenilworth, NJ, USA. This model simulated the natural history of HPV infections and assessed the epidemiologic consequences of administering 4vHPV in the US in terms of cervical diseases and genital warts, as well as the cost-effectiveness. It accounted for the herd protection effect and had a 100-year time horizon.Citation38 The length of the horizon was chosen because this was consistent with the time frame from which the system approached a steady state and the majority of benefits and costs of vaccination could be realized, as recently recommended by a European Vaccine Economics Community.Citation39 In 2010, the model was updated to include vaginal, vulvar, anal, head and neck, and penile cancers, as well as recurrent respiratory papillomatosis (RRP).Citation40 Finally, in 2014 it was extended to evaluate 9vHPV and to include diseases related to HPV types 31/33/45/52/58.Citation41 However, in this model, these additional genotypes were considered responsible only for cervical diseases and anal cancer. The present analysis was carried out as an adaptation of this latest version of the model to the Spanish setting.
The dynamic model structure consisted of three connected modules.
A demographic module that defined the demographic characteristics of the population being simulated. This was divided into 19 age groups and classified according to sexual activity. Individuals move across successive age groups until death, with an additional age- and stage-dependent death rate for cancer patients.
An epidemiologic module that simulated HPV transmission and the occurrence of HPV-related diseases. Individuals were categorized according to their status regarding infection, disease, screening, and treatment. This module included:
one HPV6-specific model (CIN1, genital warts, and RRP);
one HPV11-specific model (genital warts and RRP);
one model for each HPV16- or HPV18-related disease (CINs, cervical cancer, VINs, vulvar cancer, VaINs, vaginal cancer, anal intraepithelial neoplasias, anal cancer, penile intraepithelial neoplasias, penile cancer, and head and neck cancer);
two models for the HPV types 31/33/45/52/58 (one for cervical diseases and the other for anal diseases).Citation29
An economic module that estimated costs and quality of life associated with the different vaccination and screening strategies.
The analysis evaluated different strategies (girls-only and gender-neutral vaccination) with 9vHPV compared to the current vaccination strategy with 4vHPV (girls only). All strategies considered a two-dose schedule for girls and boys at 12 years of age. No vaccination of adult men or women was considered.
Input parameters
Demographics
All demographic data were retrieved from the Spanish National Statistics Institute (INE).Citation41 According to this source, the total population in Spain in January 2018 was estimated to be 46,659,302 people.Citation42
Sexual behavior
Data on sexual behavior specific to Spain were collected from the Health and Sexual Habits Survey 2003Citation43 and complemented with data from the National Survey of Sexual Attitudes and Lifestyles (NATSAL)-3 study in the UK.Citation44
Screening
The percentage of females undergoing gynecological cancer screening at least once every three years was 72.7% according to the European Health Survey in Spain (EESE).Citation45 The percentage of women undergoing clinical follow-up after an abnormal Papanicolau test (Pap test or Pap smear) result was estimated at 90.8% based on previous studies,Citation46,Citation47 as no Spanish source was found. Since vulvar and vaginal cancer are not screened for in Spain, the percentage of females receiving regular vaginal cancer screening was 0%.
In terms of diagnostic performance, the sensitivity and specificity of colposcopy were 96% and 48% respectively, whereas the specificity of the Pap test was 94%.Citation40
Natural history of the disease
Parameters related to the natural history of the disease, such as probability of transmitting genital HPV infection, rate of recurrence of treated CINs, and cancer progression rate, were taken into account (See Table A1). It was assumed that the progression from infection to disease follows a natural history structure similar to that of the initial US model.Citation40
Treatment patterns
Age-specific hysterectomy rates were retrieved from a Spanish study.Citation48 The parameters related to the percentage of treated CIN, VaIN, VIN, and carcinoma in situ (CIS) were estimated through a calibration process, as was the percentage of females with cancer who seek treatment after recognizing their symptoms.
Mortality
Survival data from the European Cancer Registry Based Study on Survival and Care of Cancer Patients 5 (EUROCARE-5) was used to estimate the mortality associated with HPV-related cancers.Citation49 Since specific Spanish survival data by age group and cancer stage were not available, data from Cancer Research UK were usedCitation50 (See Table A2). The correspondence between stages was based on expert opinion.
Vaccine properties
The prophylactic efficacy of the vaccine or the degree of protection conferred by the vaccine was retrieved from clinical trialsCitation11,Citation12,Citation51-Citation54 (). We assumed that the efficacy of the vaccine against HPV types 16/18 was the same for all three vaccines, that 2vHPV was not efficacious against infections caused by HPV types 6/11, and that 2vHPV and 4vHPV was not efficacious against infections caused by HPV types 31/33/45/52/58. In the base case, lifelong duration of protection was assumed, as was the need to administer two doses of the vaccine to consider efficacy complete, as previously reported.Citation29-Citation31
Table 4. Vaccine efficacy assumptions (International).
Vaccination strategy
The current HPV vaccination program in Spain targets mainly girls in their 12th year of life.Citation16
Consequently, we chose 11–12 years as the only age group to receive the vaccine. In accordance with the latest vaccination coverage report published by the Spanish Ministry of Health,Citation56 a vaccination coverage rate (VCR) of 77.8% was set for this age group in girls. There were no data on VCR for boys aged between 11 and 12. Based on expert assumptions, we assumed that the VCR for boys would be 55% in a gender neutral vaccination program setting. Moreover, as the report of the Spanish Ministry of Health only includes one- and three-dose compliance rates, we projected a two-dose compliance rate of 89.5% by assuming a linear relationship between the two doses.
Costs and discounting
Costs were retrieved from the literature and inflated to 2017 euro values using the Spanish Harmonized Index of Consumer Prices (HICP) for Health.Citation57 A discount rate of 3% was considered for costs and for health outcomes.
For vaccines, official list prices per dose for 2vHPV and 4vHPV were used: €78.03 for 2vHPV, €104.00 for 4vHPV, and €120.00 for 9vHPV.Citation58 In all cases, an administration charge of €5.38 per dose was applied, as reported in previous publications.Citation59
Costs per episode of care of HPV-related diseases are provided by Spanish studies,Citation35,Citation36,Citation59-Citation64 with the exception of costs per episode of RRP, where we used the UK estimateCitation65 (). Costs of screening and diagnostic tests were also provided by Spanish sources.Citation59,Citation60,Citation66-Citation68
Table 5. Costs of diagnosing and treating diseases caused by HPV infection.
All analyses were performed from the Spanish payer’s perspective.
Health-related quality of life
Health utility values for cancer patients were derived from several sources, as no Spanish specific utilities for health states were found (Table A3-A5).Citation70 In the absence of UK-specific stage-stratified data in the population with HPV-related disease, a combination of best available UK and US data were used to calculate the required utilities.Citation71-Citation73
Model calibration and validation
The model was calibrated according to Spanish data on incidence and mortality rates and the proportions of diseases attributable to HPV infection (), as well as according to the adjusted HPV-related incidence and mortality rates for cancers and incidence rates for genital warts (see Table A6 and A7).
Table 6. Model calibration.*
The calibration process involved iterative modification of the model inputs to obtain model outcomes closer to the validation targets. We prioritized the targets with the greatest impact on overall cost-effectiveness and those with the highest-quality data. We did not modify the natural history parameters, because they were already extensively calibrated in the original model.Citation40 Additionally, we adjusted local variables such as mortality rates and the proportion of individuals seeking treatment in order to refine the results to match each target (see Table A7).
Model analysis
We used the set of inputs described above to estimate the total number of events, incidence, and mortality rates of HPV-related cervical cancer, CIN, anal cancer, and genital warts. We also estimated QALYs per person over a time horizon of 100 years. We then calculated the ICERs as the quotient of incremental costs divided by incremental QALYs. Two base case scenarios were defined, thus reflecting the spectrum of current and potential practice:
9vHPV girls-only vaccination vs. 4vHPV girls-only vaccination
9vHPV gender-neutral vaccination vs. 4vHPV girls-only vaccination
In addition, given that 9vHPV is already included in some regional vaccination calendars in Spain, a third scenario comparing 9vHPV girls-only vaccination with 9vHPV gender-neutral vaccination was also tested. Scenarios with 2vHPV were also assessed, and further details are provided in Tables B1 and B2.
Finally, a deterministic sensitivity analysis was performed to assess the robustness of the results. The following key parameters were tested: VCR, time horizon, discount rates, and the inclusion of head and neck cancer, penile cancer, and RRP. The inclusion of those parameters was based on the previously published adaptations of the modelCitation29-Citation31. Price was not considered a parameter in our deterministic sensitivity analysis as both vaccines (9vHPV and 4vHPV) are already in the market in Spain and have official list prices.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All study data are presented either in the article or in the additional files.
Competing interests
Jesús de la Fuente received honoraria for acting as an advisor and/or speaker, as well as travel support for attending at events or meetings, from Sanofi Pasteur MSD, and MSD. Juan J. Hernández Aguado received honoraria for acting as an advisor and/or speaker, as well as travel support for attending at events or meetings, from Sanofi Pasteur MSD, MSD, Bial, Pfizer, GSK, and Roche Diagnostics. Paula Ramirez, Sergio Cedillo, Noelia López, and María San Martín are employees of MSD Spain
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
Authors’ contributions
All authors contributed equally to the bibliographic research and drafting and review of the manuscript.
Abbreviations
| AIN | = | Anal Intraepithelial Neoplasia |
| CHMP | = | Committee for Medicinal Products for Human Use |
| CIN | = | Cervical Intraepithelial Neoplasia |
| CIS | = | Carcinoma in Situ |
| EESE | = | European Health Survey in Spain |
| EMA | = | European Medicine Agency |
| EUROCARE | = | European Cancer Registry Based Study on Survival and Care of Cancer Patients |
| FIGO | = | International Federation of Gynecology and Obstetrics |
| HICP | = | Harmonized Index of Consumer Prices |
| HPV | = | Human Papillomavirus |
| ICER | = | Incremental Cost-Effectiveness Ratio |
| INE | = | Spanish National Statistics Institute |
| JCVI | = | Joint Committee on Vaccination and Immunisation |
| NATSAL | = | National Survey of Sexual Attitudes and Lifestyles |
| PIN | = | Penile Intraepithelial Neoplasia |
| QALY | = | Quality-Adjusted Life Year |
| RRP | = | Recurrent Respiratory Papillomatosis |
| UK | = | United Kingdom |
| US | = | United States |
| ValN | = | Vaginal Intraepithelial Neoplasia |
| VCR | = | Vaccination Coverage Rate |
| VIN | = | Vulvar Intraepithelial Neoplasia |
Supplemental Material
Download Zip (49.2 KB)Acknowledgments
Editorial assistance was provided by Content Ed Net, Madrid, Spain.
Additional information
Funding
References
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–23. doi:10.1016/j.vaccine.2012.07.055.
- Doorbar J, Quint W, Banks L, Ig B, Stoler M, Tr B, Ma S. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–70. doi:10.1016/j.vaccine.2012.06.083.
- Hartwig S, Baldauf J, Dominiak-Felden G, Simondon F, Alemany L, de Sanjosé S, Castellsagué X. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in Europe: potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res. 2015;1:90–100. doi:10.1016/j.pvr.2015.06.003.
- European Centre for Disease Prevention and Control. Introduction of HPV vaccines in EU countries – an update. Stockholm: ECDC; 2012 [ accessed 2018 Jun 26]. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/20120905_GUI_HPV_vaccine_update.pdf.
- Cervarix. Summary of Product Characteristics. European Medicines Agency. London, UK: European Medicines Agency; 2015 [ Accessed 8 Mar 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000721/WC500024632.pdf.
- Gardasil. Summary of Product Characteristics. European Medicines Agency. London, UK: European Medicines Agency; 2017 [ Accessed 8 Mar 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000703/WC500021142.pdf.
- Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7:161–169. doi:10.4161/hv.7.2.13690.
- GSK Cervarix® two-dose schedule receives European marketing authorisation: glaxoSmithKline. 2013 [ accessed 2017 Mar 9]. http://us.gsk.com/en-us/media/press-releases/2013/gsk-cervarix-two-dose-schedule-receives-european-marketing-authorisation/.
- Gardasil EPAR Summary Updated Final. [ accessed 2017 Mar 9]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000703/WC500021146.pdf.
- Gardasil 9. Summary of Product Characteristics. European Medicines Agency. London, UK: European Medicines Agency; 2016 [ Accessed 8 Mar 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003852/WC500189111.pdf.
- Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, J M, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-Valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi:10.1056/NEJMoa1405044.
- Huh WK, Joura EA, Giuliano AR, Iversen O-E, Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi E, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomized, double-blind trial. Lancet. 2017;390:2143–2159. doi:10.1016/S0140-6736(17)31821-4.
- Food and Drug Administration. Summary Basis of Regulatory Action (SBRA). 2014 [ accessed 2017 Mar 10]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM428239.pdf.
- Summary of opinion Gardasil 9 human papillomavirus 9-valent vaccine (recombinant, adsorbed). European Medicine Agency, Committee for Medicinal Products for Human Use. London, UK: Committee for Medicinal Products for Human Use, European Medicines Agency; 2015 [ Accessed 9 Mar 2017]. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003852/WC500184904.pdf.
- Gardasil 9. EPAR - Procedural steps taken and scientific information after the authorisation. 2016 [ accessed 2017 Mar 9]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Procedural_steps_taken_and_scientific_information_after_authorisation/human/003852/WC500202890.pdf.
- [Immunization schedule. spanish pediatric association and vaccine advisory committee]. [ accessed 2017 Mar 10]. http://vacunasaep.org/profesionales/calendario-de-vacunaciones-de-la-aep-2016.
- Spanish Ministry of Health, Social Services and Equality. HPV vaccination coverage. 2015 [ accessed 2017 Mar 10]. https://www.msssi.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/CoberturasVacunacion/Tabla3.pdf.
- Fiol G, Hernández-Aguado J, Torné A [Estimation of HPV vaccination coverage in women 15–45 years old in Spain]. XXVII Meeting of the Spanish Association of Cervical Pathology and Colposcopy (AEPCC); 2015 Nov 26–28; Córdoba, Spain.
- Torné Bladé A, Del Pino Saladrigues M, Cusidó Gimferrer M, Alameda Quitllet F, Andia Ortiz D, Castellsagué Piqué X, Cortés Bordoy J, Granados Carreño R, Guarch Troyas RM, LLoveras Rubio B, et al. [Guidelines for cervical cancer screening in Spain, 2014]. Prog Obs Ginecol. 2014;57(Suppl 1):1–53.
- Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. [What is an efficient health technology in Spain?]. Gac Sanit. 2002;16:334–343. doi:10.1016/S0213-9111(02)71933-X.
- Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10:2109–2111. doi:10.4161/hv.29137.
- López N, Á G-D-M, Pascual-García R, Gil-Prieto R. Hospitalizations associated with malignant neoplasia and in situ carcinoma in the anus and penis in men and women during a 5-year period (2009–2013) in Spain: an epidemiological study. Hum Vaccin Immunother. 2017;13:2292–2299. doi:10.1080/21645515.2017.1348443.
- ICO Information Centre on HPV and Cancer. Human papillomavirus and related diseases report: Spain. 2016 [ accessed 2017 Mar 15]. http://www.hpvcentre.net/statistics/reports/ESP.pdf.
- Statement on HPV vaccination – joint committee on vaccination and immunisation. [ accessed 2018 Jul]. https://www.gov.uk/government/publications/jcvi-statement-extending-the-hpv-vaccination-programme-conclusions
- Weiss T, Pillsbury M, Dasbach E Potential health and economic impact of the investigational 9-valent HPV vaccine in the United States. 29th Int Papillomavirus Conf Clin Work; 2014 Aug 21–25; Seattle, Washington.
- Brisson M, Laprise J-F, Chesson HW, Drolet M, Malagón T, Boily M-C, Markowitz LE. Health and Economic Impact of Switching from a 4-Valent to a 9-Valent HPV Vaccination Program in the United States. J Natl Cancer Inst. 2016; 4;108(1). doi:10.1093/jnci/djv282.
- Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: estimates from a simplified transmission model. Hum Vaccin Immunother. 2016;12:1363–1372. doi:10.1080/21645515.2016.
- Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A. 2016;113:5107–5112. doi:10.1073/pnas.1515528113.
- Boiron L, Joura E, Largeron N, Prager B, Uhart M. Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria. BMC Infect Dis. 2016;16:153. doi:10.1186/s12879-016-1483-5.
- Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res. 2016:1–14. doi:10.1080/14737167.2016.1208087.
- Mennini FS, Bonanni P, Bianic F, de Waure C, Baio G, Plazzotta G, Uhart M, Rinaldi A, Largeron N. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff Resour Alloc. 2017;15:11. doi:10.1186/s12962-017-0073-8.
- Malagón T, Drolet M, Boily M-C, Franco EL, Jit M, Brisson J. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–789. doi:10.1016/S1473-3099(12)70187-1.
- Soergel P, Makowski L, Schippert C, Staboulidou I, Hille U, Hillemanns P. The cost efficiency of HPV vaccines is significantly underestimated due to omission of conisation-associated prematurity with neonatal mortality and morbidity. Hum Vaccin Immunother. 2012;8:243–251. doi:10.4161/hv.18519.
- Lerner D, Parsons SK, Justicia-Linde F, Chelmow D, Chang H, Rogers WH, Greenhill AM, Perch K, Kruzikas D. The impact of precancerous cervical lesions on functioning at work and work productivity. J Occup Environ Med. 2010;52:926–933. doi:10.1097/JOM.0b013e3181f12fb0.
- Gil-Prieto R, Ester PV, Álvaro-Meca A, Msm R, De Miguel ÁG. The burden of hospitalizations for anus and penis neoplasm in Spain (1997–2008). Hum Vaccin Immunother. 2012;8:201–207. doi:10.4161/hv.18388.
- Gil-Prieto R, Viguera-Ester P, Álvaro-Meca A, San-Martín-Rodriguez M, Gil de Miguel Á. The burden of hospitalizations for head and neck neoplasm in Spain (1997–2008): an epidemiologic study. Hum Vaccin Immunother. 2012;8:788–798. doi:10.4161/hv.19819.
- Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23:1997–2008. doi:10.1158/1055-9965.EPI-14-0410.
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13:28–41. doi:10.3201/eid1301.060438.
- Ultsch B, Damm O, Beutels P, Bilcke J, Brüggenjürgen B, Gerber-Grote A, Greiner W, Hanquet G, Hutubessy R, Jit M, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: A consensus framework from a european vaccine economics community. Pharmacoeconomics. 2016;34:227–244. doi:10.1007/s40273-015-0335-2.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–6867. doi:10.1016/j.vaccine.2010.08.030.
- Chesson HW. Overview of cost-effectiveness of 9-valent HPV vaccination. Atlanta (GA, United States): Meet Advis Comm Immun Pract (ACIP). 2015 Feb 26.
- Instituto Nacional de Estadistica (INE). Demographic phenomena 2014. 2016 Jun [accessed 2017 Mar 12]. http://www.ine.es/en.
- Instituto Nacional de Estadística. Health and Sexual Habits Survey 2003. General Report. Madrid (Spain); 2005 [ accessed 2017Mar 12]. http://www.ine.es/en.
- Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, Macdowall W, Lewis R, Field N, Datta J, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the national surveys of sexual attitudes and lifestyles (Natsal). Lancet. 2013;382:1781–1794. doi:10.1016/S0140-6736(13)62035-8.
- Instituto Nacional de Estadística. European survey of Health in Spain 2014. Madrid (Spain); 2015 [ accessed 2017 Mar 12]. http://www.ine.es/en.
- Bergeron C, Breugelmans J-G, Bouée S, Lorans C, Bénard S, Rémy V. [Cervical cancer screening and associated treatment costs in France]. Gynecol Obstet Fertil. 2006;34:1036–1042. doi:10.1016/j.gyobfe.2006.09.005.
- Bergeron C, Largeron N, McAllister R, Mathevet P, Remy V. Cost-effectiveness analysis of the introduction of a quadrivalent human papillomavirus vaccine in France. Int J Technol Assess Health Care. 2008;24:10–19. doi:10.1017/S0266462307080026.
- Marqués Espí J, Peiró I Gregori S, Mendrano Heredia J. València: Subsecretaria per a l’Agència Valenciana de la Salut [Variations in standardized rates of surgery in Alicante], Valencia, Spain. 2003.
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5–a population-based study. Lancet Oncol. 2014;15:23–34. doi:10.1016/S1470-2045(13)70546-1.
- Cancer Research UK. Cervical cancer survival statistics. 2014 [ accessed 2017 Mar 1]. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/survival.
- Ault KA. Future II study group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi:10.1016/S0140-6736(07)60852-6.
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GWK, Ferris DG, Steben M, Bryan J, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi:10.1056/NEJMoa061760.
- Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–1702. doi:10.1016/S0140-6736(07)60777-6.
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi:10.1056/NEJMoa1010971.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–411. doi:10.1056/NEJMoa0909537.
- Ministerio de Sanidad. [Vaccine coverages. Statistical data]. 2015 [ accessed 2017 Mar 10]. http://www.msssi.gob.es/en/profesionales/saludPublica/prevPromocion/vacunaciones/coberturas.htm.
- Instituto Nacional de Estadistica (INE). Harmonised index of consumer prices 2016. [accessed 2017 Mar 10, 15]. http://www.ine.es/en.
- Consejo General de Colegios Oficiales de Farmacéuticos. [accessed 2017 Oct 31]. https://botplusweb.portalfarma.com/botplus.aspx.
- Diaz M, de Sanjose S, Ortendahl J, O’Shea M, Goldie SJ, Bosch FX, Kim JJ. Cost-effectiveness of human papillomavirus vaccination and screening in Spain. Eur J Cancer. 2010;46:2973–2985. doi:10.1016/j.ejca.2010.06.016.
- Castellsagué X, Rémy V, Puig-Tintoré LM, de la Cuesta RS, Gonzalez-Rojas N, Cohet C. Epidemiology and costs of screening and management of precancerous lesions of the cervix in Spain. J Low Genit Tract Dis. 2009;13:38–45. doi:10.1097/LGT.0b013e318182cd89.
- Morano R, Torné A, Castellsagué X. [Healthcare and economic impact of vaccination against cervical cancer and precursor lesions in Spain]. Progr Obstet Ginecol. 2012;55:299–303. doi:10.1016/j.pog.2012.02.004.
- Georgalis L, de Sanjosé S, Esnaola M, Bosch FX, Diaz M. Present and future of cervical cancer prevention in Spain: a cost-effectiveness analysis. Eur J Cancer Prev. 2016;25:430–439. doi:10.1097/CEJ.0000000000000202.
- Cortés J, Hurtado P, Castellsagué X. M356. Burden of disease due to vulvar and vaginal cancers in Spain. Int J Gynecol Obstet. 2012;119:S644–5. doi:10.1016/S0020-7292(12)61547-1.
- Castellsagué X, Cohet C, Puig-Tintoré LM, Acebes LO, Salinas J, San Martin M, Breitscheidel L, Rémy V. Epidemiology and cost of treatment of genital warts in Spain. Eur J Public Health. 2009;19:106–110. doi:10.1093/eurpub/ckn127.
- Hughes O, Tsikoudas A, Auerbach R, Barr G, Bateman N, Blaney S, Bosman D, Bridger M, Clarke P, Clement A, et al. The burden of recurrent respiratory papillomatosis in the United Kingdom: results from the BAPO and ENT-UK National Survey. In: ORS Spring Meeting, London 18th March 2011. Clin Otolaryngol. 2011;36:409.
- Trapero-Bertran M, Acera Pérez A, de Sanjosé S, Manresa Domínguez JM, Rodríguez Capriles D, Rodriguez Martinez A, Bonet Simó JM, Sanchez Sanchez N, Hidalgo Valls P, Díaz Sanchis M. Cost-effectiveness of strategies to increase screening coverage for cervical cancer in Spain: the CRIVERVA study. BMC Public Health. 2017 Feb 14;17(1):194. doi:10.1186/s12889-017-4115-0.
- Esalud. 2017 rate for colposcopy in Andalusia (Spain). [accessed 2018 Jun 4]. http://esalud.oblikue.com/.
- Esalud. 2017 rate for biopsy in Basque Country (Spain). [accessed 2018 Jun 4]. http://esalud.oblikue.com/.
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi:10.1016/j.ijgo.2009.02.012.
- Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht (The Netherlands): Springer Netherlands; 2014.
- Elbasha EH, Dasbach EJ. An integrated economic evaluation and HPV disease transmission models - Technical report accompanying the manuscript “Impact on vaccinating boys and men against HPV in the United States”. Vaccine. 2010;28(42):6858–6867.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Mak. 2011;31:800–804. doi:10.1177/0272989X11401031.
- Hu D, Goldie S. The economic burden of noncervical human papilomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500–507. doi:10.1016/j.ajog.2008.03.064.
- International Agency for Research on Cancer (IARC). GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [accessed 2017 Mar 15]. http://globocan.iarc.fr/Pages/summary_table_pop_sel.aspx.
- Instituto Nacional de Estadística. [Mortality by cause of death]. [ Accessed 2017 Mar 15]. http://msssi.gob.es/gl/estadEstudios/estadisticas/estadisticas/estMinisterio/mortalidad/home.htm.
- Hartwig S, Syrjänen S, Dominiak-Felden G, Brotons M, Castellsagué X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi:10.1186/1471-2407-12-30.