ABSTRACT
Since 1983 the world has been introduced to four vaccines combating disease caused by Streptococcus pneumoniae bacteria. However, despite vaccination programs disease caused by S. pneumoniae continues to lead to high morbidity and mortality worldwide. Surprisingly, instances of invasive pneumococcal disease (IPD) are still highly attributed to serotypes found in the current vaccine, such as serotypes 3 and 19A. Conversely, non-conjugate vaccine serotypes, such as 35B, are increasing and of rising interest. The persistence of vaccine type serotypes and the increase in non-conjugate vaccine type serotypes show the need for further research into conjugate vaccine design and the need for novel strategies to combat IPD.
Abbreviation: IPD: invasive pneumococcal disease
We would like to thank Ozkaya-Parlakay et al. for their response to our article and for further emphasizing a continuing cause for concern in invasive pneumococcal disease (IPD) caused by vaccine type Streptococcus pneumoniae strains. Despite the use of conjugate vaccines, we are seeing an increase in both non-conjugate vaccine type and vaccine type serotypes (),Citation1 with the persistence or increase of vaccine types being particularly alarming. Of the vaccine type serotypes, types 3 and 19A are of great concern. The drastic increase seen in 19A (), is of interest as it is included in the current marketed vaccine, PCV13, indicating a reduced efficacy of the vaccine to protect against this serotype. Additionally, this particular serotype has noted antibiotic resistance, more so than other serotypes.Citation2-Citation4 The rise in antibiotic resistant strains is becoming greater cause for concern and action as it is recently estimated that 30% of IPD cases are caused by S. pneumoniae resistant to one or more antibiotics.Citation5
Figure 1. Serotype distribution before and after vaccine introduction, all ages.
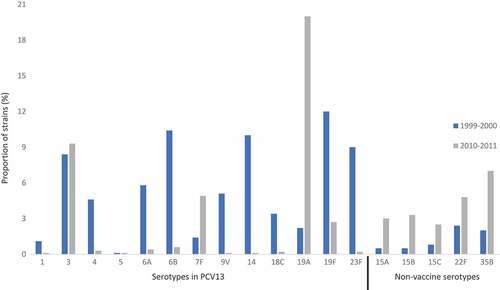
The prevalence of disease caused by serotype 3, on the other hand, has not increased but rather stayed the same despite current vaccination programs (). This indicates reduced effectiveness of the vaccine against this serotype as well. Owing to its increased virulence and mortality rate, serotype 3 has raised global attention.Citation6-Citation9 In fact, serotype 3 is the second most common isolate of adult IPD and currently accounts for about 10% of all disease with that number ever increasing.Citation6 In the recent study by Silva-Costa et al. surveying pediatric pneumonia cases in Portugal between 2010 and 2015, they found that serotypes 3,1, and 19A accounted for 62% of all cases.Citation10 With more and more data suggesting reduced vaccine effectiveness against type 3 it is imperative to seek new strategies to combat this serotype. Recently, work has been done to implement a capsule degrading enzyme against type 3 as a therapeutic agent.Citation11,Citation12
It is our opinion that both vaccine type and non-conjugate vaccine type serotypes are emerging concerns in human health. This is due to increased antibiotic resistance seen in a growing number of serotypes,Citation5 capsular switching and replacement, and the persistence of vaccine serotypes.Citation4 This brings to light the need for novel strategies to fight IPD and the need for truly protective, new-generation conjugate vaccines that utilize effective immune mechanisms.Citation13-Citation15 However, it is imperative that these issues should not deter use of the current conjugate vaccines against S. pneumoniae. Numerous studies have shown the effectiveness of these vaccines,Citation4 including recent work studying the administration of PCV13 in adults over the age of 65 and demonstrating its effectiveness against IPD.Citation16 Remarkably, the prevalence of most vaccine-type serotypes has decreased since the introduction of conjugate vaccines (), which manifests the power of conjugate vaccines in combating infectious diseases.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
References
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerg Infect Dis. 2013;19:1074–83. doi:10.3201/eid1907.121830.
- Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–34. doi:10.1126/science.1198545.
- Dagan R. Serotype replacement in perspective. Vaccine. 2009;27 Suppl 3:C22–4. doi:10.1016/j.vaccine.2009.06.004.
- Wantuch PL, Avci FY. Current status and future directions of invasive pneumococcal diseases and prophylactic approaches to control them. Hum Vaccin Immunother. 2018;14:2303–09. doi:10.1080/21645515.2018.1470726.
- Kim L, McGee L, Tomczyk S, Beall B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev. 2016;29:525–52. doi:10.1128/CMR.00058-15.
- Sugimoto N, Yamagishi Y, Hirai J, Sakanashi D, Suematsu H, Nishiyama N, Koizumi Y, Mikamo H. Invasive pneumococcal disease caused by mucoid serotype 3 Streptococcus pneumoniae: a case report and literature review. BMC Res Notes. 2017;10:21. doi:10.1186/s13104-016-2353-3.
- Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect Dis. 2004;4:21. doi:10.1186/1471-2334-4-21.
- Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Rückinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51:692–99. doi:10.1086/655828.
- Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–16.
- Silva-Costa C, Brito MJ, Pinho MD, Friães A, Aguiar SI, Ramirez M, Melo-Cristino J. Pediatric complicated pneumonia caused by streptococcus pneumoniae serotype 3 in 13-valent pneumococcal conjugate vaccinees, Portugal, 2010-2015. Emerg Infect Dis. 2018;24:1307–14. doi:10.3201/eid2407.180029.
- Middleton DR, Zhang X, Wantuch PL, Ozdilek A, Liu X, LoPilato R, Gangasani N, Bridger R, Wells L, Linhardt RJ, et al. Identification and characterization of the Streptococcus pneumoniae type 3 capsule-specific glycoside hydrolase of Paenibacillus species 32352. Glycobiology. 2018;28:90–99. doi:10.1093/glycob/cwx097.
- Middleton DR, Paschall AV, Duke JA, Avci FY. Enzymatic hydrolysis of pneumococcal capsular polysaccharide renders the bacterium vulnerable to host defense. Infect Immun. 2018;86. doi:10.1128/IAI.00316-18.
- Avci FY, Li XM, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature Medicine. 2011;17:1602–10. doi:10.1038/nm.2535.
- Sun L, Middleton DR, Wantuch PL, Ozdilek A, Avci FY. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology. 2016;26:1029–40. doi:10.1093/glycob/cww062.
- Middleton DR, Sun L, Paschall AV, Avci FY. T cell-mediated humoral immune responses to type 3 capsular polysaccharide of. J Immunol. 2017;199:598–603. doi:10.4049/jimmunol.1700026.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi:10.1056/NEJMoa1408544.
