ABSTRACT
Patients with anatomical or functional hypo-/a-splenia have a 10- to 50-fold higher risk of developing severe infectious diseases than does the general population. Thus, it is recommended to adhere to a specific vaccination schedule, including receiving influenza vaccine. During 2014, Bari Policlinico General Hospital approved a specific protocol to ensure that vaccines are actively offered to all splenectomized patients during their hospitalizations. The aim of this study is to evaluate the efficacy of this active recall protocol for performing influenza vaccination in the years following splenectomy among patients still involved in a specific vaccination program carried out by the hospital’s Hygiene department. From May 2014 to October 2016, 96 patients were involved in the vaccination program of the Hygiene department. In November 2017, 46/96 (48%) of patients received a specific invitation by phone to receive the annual influenza vaccine (intervention group), while 50/96 (52%) did not receive any such invitation (control group). At the end of the 2017 influenza season, 73/96 (76%; 95%CI = 66–84%) of patients reported having received the influenza vaccine; no differences were observed in the extent of vaccine coverage between the groups (intervention group = 80% vs. control group = 72%; p = 0.33). Older age, more recent splenectomy, hemo-lymphopathy and receiving the previous years’ doses of influenza vaccine are associated with receiving influenza vaccination during the 2017 season. These data indicate how effective communication at the time of the vaccine counseling results in good adherence to the vaccination program even after several years. Indeed, vaccination should be an opportunity not only limited to the administration of the vaccine but also for providing patient care.
Introduction
Splenectomized patients are at high risk of infectious diseases with symptoms including sepsis or meningitis, in particular due to encapsulated bacteria such as Streptococcus pneumoniae (responsible for more than 50% of infections), Haemophilus influenzae type b and Neisseria meningitidis .Citation1,Citation2
Patients with anatomic or functional hypo-/a-splenia have a 10- to 50-fold higher risk than does the general population of developing overwhelming post-splenectomy infection (OPSI).Citation1,Citation3 The estimated incidence of OPSI is 0.23–0.42% per year, with a lifetime risk of 5% .Citation4 Although the risk of OPSI has been reported as potentially lifelong,Citation5 it is commonly accepted that the highest frequency of life-threatening infectious episodes is observed during the first two years following splenectomy (˜30% of episodes occur within the first year and ˜50% within the first two years after splenectomy) .Citation1
Asplenic/hyposplenic subjects should be vaccinated as are healthy people according to international and national vaccination programs,Citation6,Citation7 and, indeed, they should receive additional vaccinations to prevent infections associated with splenic dysfunction. In particular, because of the increased risk of secondary bacterial infections, patients with anatomical or functional asplenia should receive the annual influenza vaccine.Citation8 Influenza vaccination is associated with a 54% reduced risk of death in vaccinated compared with unvaccinated asplenic individuals.Citation9 In Italy, official recommendations for asplenic/hyposplenic patients’ vaccination are reported in the National Immunization Plan and in the annual influenza prevention guidelines of Italian Ministry of Health .Citation7,Citation10
In 2014, Bari Policlinico General Hospital (Apulia, South Italy, ˜4,000,000 inhabitants) approved a specific protocol for actively offering vaccinations to splenectomized patients during their hospitalization. According to this protocol, for hospitalized patients who are splenectomized or candidates for splenectomy, the surgeon needs to consult a physician with expertise in vaccinology. The vaccinologist examines the patient and administers vaccines according to the following schedule: two doses of 13-valent pneumococcal conjugate vaccine (with a minimum interval of 8 weeks), two doses of meningococcal ACYW135 conjugate vaccine (with a minimum interval of 8 weeks), two doses of meningococcal B recombinant vaccine (with a minimum interval of 4 weeks), one dose of Haemophilus influenzae type b conjugate vaccine and (from October to December) one dose of annual influenza vaccine.
Vaccine administration begins during hospitalization (routinely 72 h after surgery). After discharge, patients are invited to come to the hygiene department to complete the vaccination schedule. This recommendation is specified in the hospital’s discharge letter, which includes the recommendation for annual influenza vaccination. At each appointment, the physicians of the hygiene department perform vaccination counseling activities in which they explain to patients and family members the importance of completing the vaccination schedule and the risks associated with the asplenia condition. Upon completion of the vaccination schedule, an updated vaccine certificate is issued, which indicates the vaccinations to be performed in the following years, such as annual influenza vaccination .Citation11
According to the Italian Vaccination framework, the administration of annual influenza vaccine is done by general practitioners (GPs). The protocol does not provide an active reminder, either for patients or for GPs.
At the time of the writing of this study, the protocol activated in 2014 is being updated for adaptation to the latest scientific evidence and international recommendations.
The aim of our study is to evaluate the efficacy of the recommendation to perform influenza vaccination in the years following splenectomy and to evaluate whether a telephone reminder can increase influenza vaccination coverage.
Results
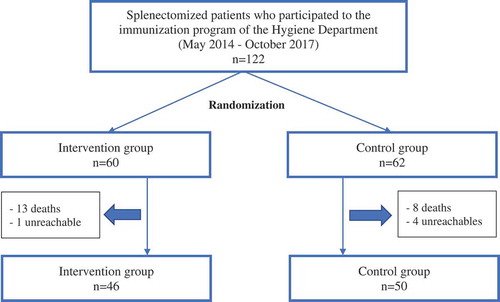
From May 2014 to October 2017, 122 splenectomized patients who participated in the immunization program of the Hygiene department were selected for this study, of which 60/122 (49.2%) belonged to the intervention group and 62/122 (50.8%) belonged to the control group.
26/122 (21.3%) subjects were excluded due to death or impossibility to reach them by phone, as described in ; of 96 patients reached, 46/96 (47.9%) belonged to the intervention group and 50/96 (52.1%) belonged to the control group.
Sixty of 96 subjects (62.5%) were male and the percentage of males did not differ between groups (intervention group = 28/46; 60.9% vs. control group = 32/50; 64.0%; X2 = 0.1; p = 0.752). The average age of patients was 50.9 ± 19.4 years (range: 4.0– 83.0), without statistically significant differences between groups (intervention group = 51.7 ± 20.0; range: 4.0– 83.0 vs. control group = 50.1 ± 29.0; range: 11.0– 77.0; z = 0.4; p = 0.689).
The average time from the entry in the immunization program to the start of the study was 40.4 ± 26.7 months (range: 1.0– 99.0), without differences between groups (intervention group = 44.7 ± 28.3; range: 1.0– 99.0 vs. control group = 36.4 ± 24.8; range: 1.0– 92.0; z = 1.4; p = 0.160).
The main proportion (n = 68/96; 70.8%) of subjects enrolled reported the splenectomy during elective surgery, while one third (n = 28/96; 29.2%) was splenectomized during emergency interventions; 9/68 (13.2%) of patients of elective surgery group received the first dose of the vaccines before the splenectomy.
The median time from the splenectomy to the start of the immunization program was 6.0 days (range IQR: 4.0– 13.0; range: 1.0– 107.0).
The causes of splenectomy were solid neoplasia (n = 43/96; 44.8%), trauma (n = 27/96; 28.1%), hemolymphopoietic diseases (n = 19/96; 19.8%) and other diseases (n = 7/96; 7.3%).
The average time from the first to the last vaccine required by the immunization program was 74.5 ± 23.8 days (range: 53.0– 176.0).
The distribution of enrolled subjects for type of surgery, the time from splenectomy to the start of the immunization program, the distribution for cause of splenectomy and the time from the first to the last vaccine required by the immunization program did not differ between groups (p > 0.05; ).
Table 1. Sample characteristics of surgery and timing of vaccine prophylaxis, per group (intervention/control).
All enrolled subjects completed the schedule provided by protocol (two doses of anti-pneumococcal vaccine, two doses of anti-meningococcal ACYW135 and B vaccines and one dose of anti-Hib vaccine) and 30/96 (31.3%) performed influenza vaccine during the hospitalization (in any influenza season previous the one investigated in our study), because the splenectomy was performed in the period from October to December; no statistically significant differences were observed in the proportion of subjects vaccinated against influenza during hospitalization between groups (intervention group = 14/46; 30.4% vs. control group = 16/50; 32.0%; X2 = 0.0; p = 0.869).
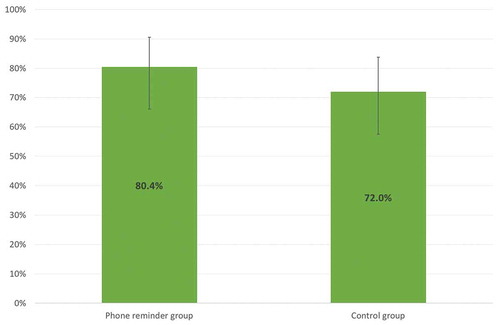
In the 2017 influenza season, 73/96 (76.0%; 95%CI = 66.3–84.2%) patients reported having performed the influenza vaccine; no statistically significant differences were observed in the comparison of vaccine coverage between groups (X2 = 0.9; p = 0.333; ).
Figure 1. Vaccine coverage (%) of the influenza vaccine (season 2017), per group (intervention/control).
For 11/23 (47.8%) subjects, the reason why they did not perform vaccination is known, of which 4/11 (36.4%) by the choice of the GP, 2/11 (18.2%) by personal choice, 2/11 (18.2%) for forgetfulness, 2/11 (18.2%) because undergoing chemotherapy treatment and 1/11 (9.0%) for other reasons.
Multivariate analysis results are described in .
Table 2. Analysis of determinants of the influenza vaccination in the 2017 season in a multivariate regression model.
Discussion
Our study shows that there is a good adherence to the seasonal influenza vaccine (76%) in splenectomized patients who participated in a specific immunization program. The telephone reminder seems to be not associated to an increase of vaccination coverage and it seems to indicate that an effective communication at the time of the vaccinal counseling, based on the correct risk perception, involves a good adherence to the vaccination even after years; indeed, the vaccination coverage in the subjects belonging to the control group is equal to 72%, just less than 8 percentage points compared to the subjects of the intervention group, values that we consider satisfactory in this group of subjects at risk, although this difference is not statistically significative (p > 0.05) probably due to the small sample size. These assertions match with the experience described in a 2007 study on a population of subjects affected by cystic fibrosis who had already been recommended to receive the influenza vaccination in the context of ad hoc counseling; subsequently, the authors called by phone part of the patients to remember them to carry out the vaccination and, at the end of the influenza season, they obtained a coverage of ~96% in the called patients and ~80% in the non-called subjects.Citation12
The personal choice appears to be one of the main determinants of refusing vaccination; even this concept is found in several studies that underline how the skepticism toward the vaccine, the perception that the disease is not dangerous and the underestimation of the severity of one‘s own chronic state are deterrents for the influenza vaccination.Citation13–Citation15 Few patients reported the decision of the GP of not to execute vaccination, an evaluation that does not follow national and international guidelines and which puts the patient‘s health in concrete risk of danger; this event is consistent with general evidence from the literature that report that physicians are more likely to vaccinate chronic patients than healthy ones.Citation16 Another determinant of non-vaccination would appear to be the chemotherapy treatment, although there is no contraindication to influenza vaccination during chemotherapy if strategies that do not affect the effectiveness of immunization and treatment are applied (e.g. vaccination in the window times of therapy).Citation17,Citation18
The analysis of determinants confirms that the phone reminder is not essential for influenza vaccination in the years following the post-splenectomy prophylaxis, while a greater age, having been recently subjected to splenectomy immunization prophylaxis, hemolymphopoietic disease and having been previously vaccinated against influenza seem to be associated to the outcome. These findings are confirmed in many studies in literature; a 2013 review concludes that many factors influence the choice of vaccination, of which predisposing ones are, among others, higher age, having a chronic condition and positive prior experiences with the influenza vaccine, while a deterrent factor is forgetfulness .Citation19
Strength point of our study is the relevant cases of splenectomized patients; indeed, a 2017 study of our research team showed how, in 2015, 1 year after the implementation of the protocol activities, the vaccination coverage achieved among splenectomized patients of Bari Policlinico General Hospital, compared to that of 2013 splenectomized patients, increased by 10 times (from 5.7% to 66.7%; X2 = 42.4; p < 0.0001) and the time between the splenectomy and the beginning of vaccination protocol strongly decreased (from 84.7 to 7.5 days; t = 21.5; p < 0.0001).Citation11 Furthermore, the research topic, to our knowledge, is poorly studied in literature for this kind of chronic patient.
The main limitation is not being able to certify (if not in patients who have been vaccinated in the Hygiene department) if the vaccination was actually performed or if patients have reported untruthful news; in this context became fundamental the availability of an Immunization Database, also implemented for influenza vaccine.
It will be opportune for the future to repeat the study, on the one hand by increasing the number of patients enrolled and on the other by following the patients for several influenza seasons and performing an analysis by subgroups (for example, assessing if the vaccination adherence is different in patients operated for trauma, and therefore with a hypothetical higher life expectancy, compared to neoplastic subjects).
The compliance of influenza vaccination in splenectomized patients is a topic not studied in the literature. More evidence is found regarding the compliance of the chronic patients; the literature shows that chronic patients tend to be skeptical about the importance of vaccination and that they underestimate the consequences of the disease, with subsequently low vaccination coverage .Citation13–Citation15,Citation20 All studies agree that a careful and scrupulous information and education of the patient is necessary to increase adherence to vaccination;Citation13–Citation15,Citation20 the hospital setting, that is the setting of our immunization program, seems to be an ideal scenario for the vaccine promotion. The studies that have dealt with the issue of strategies to increase vaccine compliance assure that patients who receive preventive care are more likely to get their influenza shot than those who do not receive; furthermore, the health professionals involved in the care of patients affected by chronic conditions should encourage patients and family members to get immunized to prevent infectious diseases or minimize the risk.Citation12,Citation19,Citation21,Citation22 This consideration matches the conclusions of our study, as an effective vaccination counseling, with a careful education and training of the patient and the family members, involves a good adherence to the influenza immunization; furthermore, this compliance has been evidenced not only in the months after the counseling but also in the subsequent years.
The experience of our study teaches how proposing the vaccination in the hospital should be an opportunity not only limited to the administration of the vaccine, but also to patient education; it is therefore essential to dedicate time to the patients and the family members to train them on the importance of immunization (also and especially in relation to their basic clinical condition) and to the possible consequences of the splenectomy.
In the hospital, the patient is more prone to understand the importance of prophylaxis and the multidisciplinary approach is guaranteed. In fact, in our experience, the first recommendation to perform vaccination is formulated by a surgeon or oncologist, and after efforted by Public Health physician. This is the added value of our protocol. Indeed, the role of the physician in the patient‘s compliance to the vaccine, especially the chronic one, is one of the main determinants in the immunization process and more efforts should be made by Public Health authorities to boost influenza immunization of chronic patients .Citation19,Citation23
Methods
The study model is a prospective observational on a historical cohort.
We considered as eligible all splenectomized patients who participated in the vaccination program of the Hygiene department of Bari Policlinico General Hospital from May 2014 to October 2016 and completed the vaccination schedule provided by protocol (n = 122).
Eligible patients were randomized into two groups, homogeneous by gender, age at the time of vaccination prophylaxis and the time from the start of the vaccination program to the start of the study. Randomization was performed by Stata MP15 software.
A 60/122 (49.2%) patients were included in the intervention group and 62/122 (50.8%) in the control group.
Subjects of the intervention group were contacted by telephone in October 2017 by a physician of the Hygiene Department. The physician recommended to attend the GP clinic to perform influenza vaccination. Patients of the control group did not receive this recall.
In February 2018, the patients of the two groups (intervention and control) were contacted by phone and the influenza immunization in the 2017 season was investigated.
Using the hygiene department database, for each patient, a specific form has been set reporting the following variables:
surname and name
age
gender
time from the first vaccine performed to the start of the study
group (intervention/control)
condition for which splenectomy was necessary
type of surgery (election/emergency/to be programmed)
time from the splenectomy to the beginning of vaccine prophylaxis (days)
time from the first to the last vaccine dose (days)
influenza vaccine performed in at least one season previous our investigation during the hospitalization (YES/NO)
influenza vaccine performed in the 2017 season (YES/NO) and motivation for eventually not having performed the vaccine
Compiled forms were put in a database created by Excel spreadsheet and data analysis was performed by STATA MP15 software.
Continuous variables were described as mean±standard deviation and range or median, interquartile range and range, categorical variables as proportions, with the 95% confidence interval, where appropriate. The skewness and kurtosis test was used to evaluate the distribution of continuous variables, but for any variable was possible to set a normalization model. The Wilcoxon’s rank sum test (not parametric) was used to compare continuous variables between groups and the chi-square and exact Fisher tests were used to compare the proportions.
To assess the determinants of the influenza vaccination in the 2017 season, multivariate logistic regression was used, considering the performed vaccination as outcome and as determinant the group variable adjusted for the variables gender, age, condition for which splenectomy was requested, time from the start of prophylaxis protocol to the start of the study and the influenza vaccination performed in at least one previous seasons during hospitalization; the adjusted odds ratio values were calculated, with the 95%CI and were backed z score test. Chi-square Pearson was used to evaluate the goodness of fit of the multivariate logistic regression model.
For all the tests, a two-sided p-value<0.05 was considered statistically significant.
The research was carried out in accordance with the Helsinki declaration and approved by the ethical committee of Apulian Osservatorio Epidemiologico Regionale.
Flow chart 1. Sample size.
Abbreviations
| CDC | = | Center for Disease Control and Prevention |
| OPSI | = | Overwhelming Post-Splenectomy Infection |
| GIAVA | = | Regional Immunization Database |
| GP | = | General Practitioner |
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bonanni P, Grazzini M, Niccolai G, Paolini D, Varone O, Bartoloni A, Bartalesi F, Santini MG, Baretti S, Bonito C, et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunother. 2016;13(2):359–68. doi:10.1080/21645515.2017.1264797.
- Chong J, Jones P, Spelman D, Leder K, Cheng AC. Overwhelming post-splenectomy sepsis in patients with asplenia and hyposplenia: a retrospective cohort study. Epidemiol Infect. 2017;145(2):397–400. doi:10.1017/S0950268816002405.
- Martino C, Gallone MS, Quarto M, Germinario C, Tafuri S. Immunization coverage among splenectomized patients: results of an ad hoc survey in Puglia Region (South of Italy). Hum Vaccin Immunother. 2016;12(5):1277–79. doi:10.1080/21645515.2015.1138025.
- Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Pathol. 2001;54:214–18.
- Newland A, Provan D, Myint S. Preventing severe infection after splenectonomy. BMJ. 2005;331:20–27.
- CDC. Asplenia and adult vaccination [accessed 2019 Jan 10]. https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/asplenia.html.
- Italian Ministry of Health. National plan of vaccinal prevention (PNPV) 2017-2019 [accessed 2019 Jan 12]. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Davies JM, Barnes R, Milligan D; British Committee for Standards in Haematology. Working Party of the Haematology/Oncology Task Force. Update of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Clin Med (Lond). 2002;2(5):440–43.
- Langley JM, Dodds L, Fell D, Langley GR. Pneumococcal and influenza immunization in asplenic persons: a retrospective population-based cohort study 1990-2002. BMC Infect Dis. 2010;10:219. doi:10.1186/1471-2334-10-219.
- Italian Ministry of Health. Prevention and control of influenza: recommendations for the 2018-2019 season [accessed 2019 Jan 14]. http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=64381&parte=1%20&serie=null.
- Gallone MS, Martino C, Quarto M, Tafuri S; Bari Policlinico General Hospital. Active offer of vaccinations during hospitalization improves coverage among splenectomized patients: an Italian experience. Am J Infect Control. 2017;45(8):e87–e89. doi:10.1016/j.ajic.2017.02.039.
- Tran C, Pitts J. Improving influenza vaccine compliance through patient education for patients with cystic fibrosis. J Pediatr Health Care. 2007;21(1):57–61. doi:10.1016/j.pedhc.2006.08.014.
- Bödeker B, Remschmidt C, Schmich P, Wichmann O. Why are older adults and individuals with underlying chronic diseases in Germany not vaccinated against flu? A population-based study. BMC Public Health. 2015;15:618. doi:10.1186/s12889-015-1970-4.
- Sampson R, Wong L, Macvicar R. Parental reasons for non-uptake of influenza vaccination in young at-risk groups: a qualitative study. Br J Gen Pract. 2011;61(588):e386–91. doi:10.3399/bjgp11X583155.
- Sagor KH, AlAteeq MA. Beliefs, attitudes, and barriers associated with the uptake of the seasonal influenza vaccine among patients visiting primary healthcare clinics. Saudi Med J. 2018;39(7):690–96. doi:10.15537/smj.2018.7.22293.
- Villacorta R, Sood N. Determinants of healthcare provider recommendations for influenza vaccinations. Prev Med Rep. 2015;2:355–70. doi:10.1016/j.pmedr.2015.04.017.
- Ariza-Heredia EJ, Chemaly RF. Practical review of immunizations in adult patients with cancer. Hum Vaccin Immunother. 2015;11(11):2606–14. doi:10.1080/21645515.2015.1062189.
- American Cancer Society. Should people with cancer get a flu shot? [accessed 2019 Jan 16]. https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/infections/should-i-get-a-flu-shot.html.
- Nagata JM, Hernández-Ramos I, Kurup AS, Albrecht D, Vivas-Torrealba C, Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13:388. doi:10.1186/1471-2458-13-388.
- Yu MC, Chou YL, Lee PL, Yang YC, Chen KT. Influenza vaccination coverage and factors affecting adherence to influenza vaccination among patients with diabetes in Taiwan. Hum Vaccin Immunother. 2014;10(4):1028–35. doi:10.4161/hv.27816.
- Nessler K, Krztoń-Królewiecka A, Chmielowiec T, Jarczewska D, Windak A. Determinants of influenza vaccination coverage rates among primary care patients in Krakow, Poland and the surrounding region. Vaccine. 2014;32(52):7122–27. doi:10.1016/j.vaccine.2014.10.026.
- Schoefer Y, Schaberg T, Raspe H, Schaefer T. Determinants of influenza and pneumococcal vaccination in patients with chronic lung diseases. J Infect. 2007;55(4):347–52. doi:10.1016/j.jinf.2007.06.002.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14(6):1311–22. doi:10.1080/21645515.2018.1445446.