ABSTRACT
Intravenous immunoglobulin (IVIG) is one of the widely used immunotherapeutic molecules in the therapy of autoimmune and inflammatory diseases. Previous reports demonstrate that one of the anti-inflammatory actions of IVIG implicates suppression of macrophage activation and release of their inflammatory mediators. However, macrophages are highly plastic and depending on the microenvironmental signals, macrophages can be polarized into pro-inflammatory classic (M1) or anti-inflammatory alternative (M2) type. This plasticity of macrophages raised additional questions on the role of IVIG towards macrophage polarization. In the present report, we show that IVIG affects the polarization of both classically and alternatively activated macrophages and this process is F(ab’)2-independent. Our data thus indicate the lack of reciprocal regulation of inflammatory and non-inflammatory macrophages by IVIG.
Introduction
Macrophages are heterogeneous and ubiquitous cell populations. They are the principal mediators of immune response to pathogens and inflammation. In addition, macrophages also play a pivotal role in tissue homeostasis and repair. In order to exert these diverse functions, macrophages acquire distinct features in response to local microenvironmental signals.Citation1 Thus, macrophages are broadly divided into two subtypes with opposing functions: classically activated macrophages or M1 and alternatively activated or M2 macrophages.Citation2,Citation3 M1 macrophages are induced by type I cytokines like interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα), or following recognition of pathogen-associated molecular patterns (PAMPs) like lipopolysaccharide (LPS). These macrophages contribute to inflammatory processes and macrophage-mediated tissue injury. In contrast, M2 macrophages are induced by Th2 cytokines like IL-4 and IL-13 and play a role in the resolution of inflammation.Citation1
Intravenous immunoglobulin (IVIG), a therapeutic preparation of human normal IgG purified from pooled plasma of thousands of donors is extensively used in the therapy of diverse autoimmune and inflammatory conditions including immune thrombocytopenic purpura, Guillain-Barré syndrome, Kawasaki disease, inflammatory myopathies, chronic inflammatory demyelinating polyneuropathy, and many others.Citation4,Citation5 The current evidence indicates that the therapeutic benefits of IVIG implicate several mutually nonexclusive mechanisms targeting the diverse arms of the inflammatory responses.Citation6-Citation8
Previous data show that IVIG suppresses the activation of monocytes/macrophages leading to inhibition of their inflammatory mediators. However, the plasticity of macrophages raises additional questions on the role of IVIG towards macrophage polarization. In the present report, we show that IVIG affects the polarization of both classically and alternatively activated macrophages thus indicating the lack of reciprocal regulation of inflammatory vs non-inflammatory macrophages by IVIG.
Results
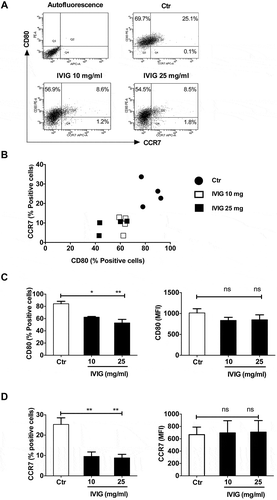
We first investigated whether IVIG could modulate M1 and M2 macrophage polarization. Human peripheral blood monocyte-derived macrophages were cultured with LPS and IFN-γ to obtain M1 macrophages. IVIG was added at two concentrations (10 and 25 mg) during this polarization process. We found that IVIG significantly downmodulated the proportion of M1 macrophages as demonstrated by the reduced percentage of cells positive for M1 markers CD80 (–) and CCR7 (, and ). However, the intensity of expression of both CD80 and CCR7 was not significantly modified by IVIG ( and ). Although polarized M1 macrophages were heterogeneous regarding the expression of CD80 and CCR7 (CD80+CCR7+ and CD80+CCR7− cells; and B), IVIG did not display preferential action on these populations. The proportions of both these populations were reduced by IVIG. As expected, M2 markers CD209 and CD206 were minimally expressed on M1 macrophages and were further reduced by IVIG.
Figure 1. IVIG suppresses the polarization of classically activated (M1) macrophages. Human monocyte-derived macrophages were treated with LPS and IFN-γ either alone (Ctr) or along with IVIG (10 and 25 mg/ml) for 72 h and analyzed for the expression of CD80 and CCR7 by flow cytometry. Representative dot-blots (A), scatter plots displaying the relative expression of markers (B), and the expression levels (% positive cells and mean fluorescence intensity, MFI) of (C) CD80 and (D) CCR7. Data are presented as mean ± SEM from four independent donors. Statistical significance (*, p< .05, **, p < .01) as analyzed by one-way ANOVA Tukey’s multiple comparison test. ns, not significant.
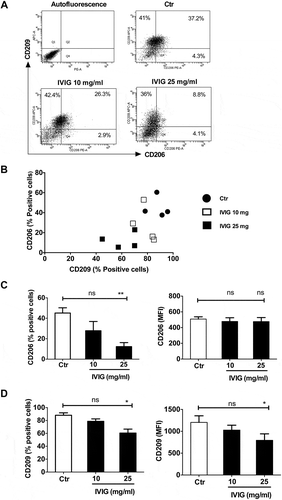
We then explored whether the inhibitory effects of IVIG on macrophage polarization are restricted only to M1 or if IVIG suppresses the polarization of M2 macrophage as well. Monocyte-derived macrophages were cultured with IL-13 and IL-4 for M2 macrophage polarization and IVIG was added at two concentrations during this polarization process. Although M2 macrophages are associated with anti-inflammatory process, we found that IVIG at high concentrations (25 mg) inhibited M2 macrophage polarization, shown by abrogated expression of CD206 (mannose receptor) (–) and CD209 (DC-SIGN) (, and ).
Figure 2. IVIG downregulates the surface markers of alternatively activated (M2) macrophages. Monocyte-derived macrophages were cultured for 72 h with IL-13 and IL-4 either alone or along with IVIG (10 and 25 mg/ml). The expression of CD206 and CD209 was analyzed by flow cytometry. Representative dot-blots (A), scatter plots displaying the relative expression of markers (B), and the expression levels (% positive cells and mean fluorescence intensity, MFI) of (C) CD206 and (D) CD209 was analyzed by flow cytometry. Data are presented as mean ± SEM from four independent donors. Statistical significance (*, p< .05, **, p < .01) as analyzed by one-way ANOVA Tukey’s multiple comparison test. ns, not significant.
Next, we then examined if suppressive effect of IVIG on macrophage polarization is also reflected on the expression of their cytokines. M1 macrophages express predominantly IL-12 while M2 macrophages display more of IL-10 (). A small proportion of cells (M1 or M2) expressed both the cytokines. However, the proportion of cells expressing IL-10 and IL-12 are low in M1 and M2 macrophages, respectively (). The low percentage of polarized macrophage populations positive for either IL-12 or IL-10 was possibly due to exhaustion of cells to produce cytokines (‘ex-producers’).
Figure 3. Inhibition of macrophage polarization by IVIG is associated with reduced intracellular expression of (A and B) IL-12 (M1 macrophages) and (C and D) IL-10 (M2 macrophages). Human monocyte-derived macrophages were treated (A and B) with LPS and IFN-γ either alone (Ctr) or along with IVIG (10 and 25 mg/ml) for 72 h (M1), or (C and D) with IL-13 and IL-4 either alone or along with IVIG (10 and 25 mg/ml) for 72 h (M2). The cells were then stimulated with phytohaemagglutinin-L for 18 h and with GolgiStop for additional 2 h. Representative dot-blots are presented in panels A and C. Data are presented as mean ± SEM from three to four independent donors. Statistical significance (**, p < .01) as analyzed by one-way ANOVA Tukey’s multiple comparison test. ns, not significant.
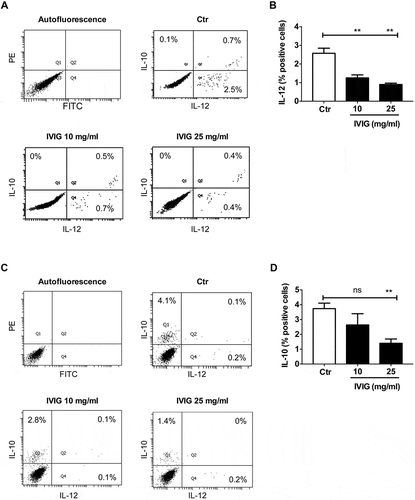
Our data revealed that IVIG dampens the expression of cytokines from both classically activated M1 macrophages as well as alternatively activated M2 macrophages as shown by reduced expression of IL-12 ( and ) and IL-10 ( and ) respectively. All together, these results suggest that irrespective of inflammatory or anti-inflammatory macrophage phenotype, IVIG hampers both M1 and M2 macrophage polarization.
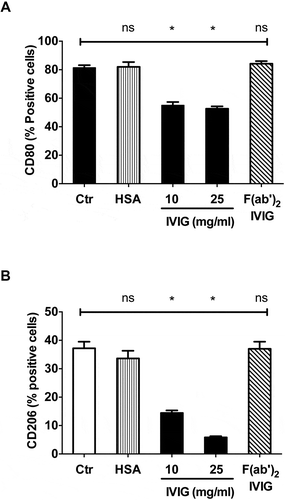
We explored the mechanism by which IVIG inhibits macrophage polarization. IgG contains Fab region that recognizes specific antigen while Fc exerts effector functions by binding Fcγ receptors. Certain mechanisms of IVIG were reported to be F(ab’)2-dependent while others were Fc-dependent.Citation6 Therefore, we investigated if F(ab’)2 fragments of IVIG were able to inhibit macrophage polarization similar to that of intact IVIG. Flow cytometric analyses of the expression of M1 (CD80; ) and M2 macrophage (CD206; ) surface markers revealed that F(ab’)2 fragments were dispensable for the IVIG-mediated inhibition of macrophage polarization thus indicating that this effect is mediated either by Fc-domain or by whole IVIG (). Human serum albumin (HSA), used as a protein control for IVIG did not affect polarization of macrophages, thus confirming the specificity of IVIG action on macrophages ().
Figure 4. Inhibition of M1 and M2 macrophage polarization by IVIG is F(ab’)2-independent. Monocyte-derived macrophages were cultured under either (A) M1 or (B) M2-polarizing conditions for 72 h along with equimolar concentrations of IVIG, F(ab’)2 or HSA. The expression (% positive cells, mean ± SEM from three independent donors) of (A) CD80 and (B) CD206 was analyzed by flow cytometry. Statistical significance (*, p< .05) as analyzed by one-way ANOVA Tukey’s multiple comparison test. ns, not significant.
Discussion
Although monoclonal antibodies (MAbs) and recombinant proteins have revolutionized the management of autoimmune diseases, and several other novel immunotherapies are in pipeline,Citation9-Citation18 immunotherapy with IVIG is still attractive and has several advantages because of broad-spectrum action and safety profiles. These mechanisms of IVIG represent functions of circulating normal IgG in the regulation of immune tolerance and immune homeostasis. The mechanisms of action of IVIG vary depending on the pathologies. Several reports have demonstrated that IVIG imparts tolerogenic properties to innate cells and effector T cells. In addition, IVIG regulates the functions of B cells and neutralizes pathogenic autoantibodies, inflammatory cytokines and complements.Citation6-Citation8,Citation19-Citation28
Previous data from several laboratories including ours have demonstrated that IVIG targets monocytes/macrophages to suppress the inflammation both in humans and mice models. IVIG suppresses the production of several inflammatory mediators from the monocytes and macrophages, and enhances the anti-inflammatory cytokines like IL-1RA.Citation29-Citation32 The suppressive effects of IVIG on the activation of monocytes/macrophages are associated modulation of intracellular signaling events and in particular reduced ERK1/2, P38 MAPK and NF-κB pathways.Citation33 The microarray data from the monocytes of IVIG-treated patients also indicate suppression of inflammatory genes.Citation34,Citation35
The reciprocal regulation of cells implicated in inflammation and anti-inflammation has been reported with IVIG. Thus, IVIG reciprocally regulates immunoprotective regulatory T cells and pathogenic Th1-Th17 cells.Citation19,Citation21,Citation24-Citation28 Our current data with macrophages however show that this reciprocal regulation is not applicable to all immune cells. Thus, under specific in vitro polarization conditions, IVIG suppresses the polarization of both inflammatory and anti-inflammatory macrophages. Data from previous reports both in vitro and in vivo have demonstrated that the phenotype and cytokine profiles of polarized M1 and M2 macrophages are reversible.Citation36,Citation37 By suppressing both M1 and M2 polarization, IVIG thus ensures global suppression of macrophage-mediated inflammatory responses. Although M2 macrophages are implicated in eliciting Th2 responses and thus suppress effector Th1 or Th17 cytokines in autoimmune and inflammatory conditions, our data suggest that enhanced Th2 responses observed following IVIG therapy in autoimmune pathologies do not implicate regulation of cytokine profiles of macrophages.Citation38
Of interest, we have observed variations among the individual donors regarding the expression of macrophage markers. For example, the expression of M2 marker CD206 was varying from 33% to 60.4%. It is well recognized that genetic, epigenetic and environmental factors influence the ability of immune cells to respond to stimuli. This individual variation is also reflected among the patients treated with IVIG wherein all the patients treated with this immunotherapy do not respond in a similar way.Citation39
A recent report shows that IVIG prevents infiltration of M1 macrophages in a rat model of chemotherapy-induced peripheral neurotoxicity.Citation40 Another report also found significant reduction in inflammatory cytokines in M1 macrophages upon IVIG exposure thus validating our observations.Citation41 Similar to this report, we did not observe enhancement of anti-inflammatory cytokine IL-10 in M1 macrophages upon IVIG treatment. Moreover, we have extended our investigation by analyses of surface markers and confirm suppressive effect of IVIG on M1 macrophages based on the expression of CD80 and CCR7. However, in contrast to the report of Dominguez-Soto et al.Citation41 we did not notice enhancement of M1 macrophage features in IVIG-treated M2 macrophages. These discrepancies are mainly due to experimental conditions. We have used specific polarizing cytokines to explore the effect of IVIG on macrophage polarization while Dominguez-Soto et al. have used LPS stimulation in their experiments.
In our experimental conditions, the uniform expression of CD80 or CD209 indicates differentiation of cells into M1 or M2 macrophages. However, the expression of CCR7 and CD206 in corresponding macrophage populations was not uniform. Thus, we observed CD80+CCR7+ and CD80+CCR7− M1 macrophages, and CD209+CD206+ and CD209+CD206− M2 macrophages. These data suggest heterogeneity in polarized M1 or M2 macrophages. Whether cells double positive for the indicated markers are functionally potent compared to single positive cells remains to be explored in the future.
We found that suppressive effects of IVIG on macrophage polarization does not implicate F(ab’)2 fragments indicating that either Fc-fragment or entire IgG molecules are required for these effects. These data also indicate that the suppressive effects of IVIG on macrophage polarization are not due to passive neutralization of polarizing cytokines used in the experiments. Though controversy,Citation42 Fc-mediated effects of IVIG are reported to be mediated mainly by α(2,6)-sialic acid-linkagesCitation43 that are recognized by lectin receptors such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), dendritic cell immunoreceptor (DCIR) and CD22.Citation20,Citation44-Citation46 M2 macrophages are positive for DC-SIGN (CD209) and further work is necessary to identify the nature of receptor(s) on M1 macrophages that mediate anti-inflammatory action of IVIG.
Methods
Buffy coats of healthy donors were purchased from Centre Necker-Cabanel (Etablissement Français du Sang, Paris, France). Ethical approval (Institut National de la Santé et de la Recherche-EFS Ethical Committee Convention N° 15/EFS/012/and 18/EFS/033) was obtained for the use of such materials. Monocytes were isolated from peripheral blood mononuclear cells (PBMC) of buffy coats by using CD14 microbeads (Miltenyi Biotec, Paris, France), and subsequently cultured for 6 d in complete RPMI 1640 supplemented with recombinant M-CSF (1000 IU/106 cells, Miltenyi Biotec). The obtained nonpolarized macrophages were stimulated with LPS (200 ng/106 cells, Escherichia coli, Sigma-Aldrich, St. Quentin Fallavier, France) and IFN-γ (40 ng/106 cells) (Immunotools, Friesoythe, Germany) for 72 h to polarize M1 macrophages. Alternatively, nonpolarized macrophages were cultured with IL-4 (500 IU/106 cells, Miltenyi Biotec) and IL-13 (400 ng/106 cells, Immunotools) to obtain M2 macrophages. IVIG (10 or 25 mg/ml/0.5x106 cells; Tegeline®, LFB Biomedicaments, Les Ulis, France) or equimolar concentrations of F(ab’)2 fragments of IVIG (16 mg) or HSA (10 mg; LFB Biomedicaments, France) were added during this 72 h of polarization of nonpolarized macrophages into M1 or M2.
IVIG and HSA were dialyzed before their use. F(ab’)2 fragments of IVIG were obtained by pepsin digestion (2% wt/wt; Sigma Aldrich). The digested antibodies were passed through protein G Sepharose column to remove intact IgG and SDS-PAGE analysis was done to confirm the purity of F(ab’)2 fragments.
Following 72 h of culturing the cells in the presence or absence of IVIG, the phenotype of macrophages was analyzed by flow cytometry (LSR II, BD Biosciences, Le Pont de Claix, France) using fluorochrome-conjugated monoclonal antibodies (MAbs) and data were analyzed by BD FACS DIVA software (BD Biosciences). For intracellular staining of cytokines, macrophages following 72 h of culturing under polarizing conditions were stimulated with phytohaemagglutinin-L (10 µg/ml, Sigma-Aldrich) at 37°C for 18 h and with GolgiStop (BD Biosciences) for additional 2 h. Cells were fixed and permeabilized using Foxp3 Fixation/Permeabilization kit (eBioscience, Paris, France) and incubated at 4°C with fluorochrome-conjugated MAbs.
The antibodies used for flow cytometry were PE-conjugated MAbs to CD80, CD206, IL-10, FITC-conjugated MAbs to IL-12, APC-conjugated MAb to CD209 (all from BD Biosciences), and APC-conjugated MAbs to CCR7 and IL-12 (all from eBioscience).
Data were analyzed by one-way ANOVA with Tukey’s multiple comparison test (*P < .05, ** p< .01) using Prism 6 software (GraphPad Software, Inc, La Jolla, USA).
Author contributions
CS, PK, SVK, JB were involved in the study design. All authors participated in the acquisition and interpretation of the data. All authors had full access to the data and gave final approval before submission.
Disclosure of potential conflicts of interest
The work is supported in part by research grant from Laboratoire Français du Fractionnement et des Biotechnologies (LFB) Biomedicaments, France.
Acknowledgments
We thank the staff of Centre d‘Histologie, d‘Imagerie et de Cytométrie, Centre de Recherche des Cordeliers for the help.
Additional information
Funding
References
- Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66. doi:10.1146/annurev-physiol-022516-034339
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi:10.1038/nri1733
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi:10.1172/JCI59643
- Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1–S46. doi:10.1016/j.jaci.2016.09.023
- Joao C, Negi VS, Kazatchkine MD, Bayry J, Kaveri SV. Passive serum therapy to immunomodulation by IVIG: a fascinating journey of antibodies. J Immunol. 2018;200:1957–63. doi:10.4049/jimmunol.1701271
- Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–98. doi:10.1093/intimm/dxx039
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–89. doi:10.1038/nri3401
- Seite JF, Shoenfeld Y, Youinou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev. 2008;7:435–39. doi:10.1016/j.autrev.2008.04.012
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–16. doi:10.1038/nri2761
- Casan JML, Wong J, Northcott MJ, Opat S. Anti-CD20 monoclonal antibodies: reviewing a revolution. Hum Vaccin Immunother. 2018;14:2820–41. doi:10.1080/21645515.2018.1508624
- Bayry J. Repressing immunity in autoimmune disease. N Engl J Med. 2016;374:2090–92. doi:10.1056/NEJMcibr1602864
- Abbas AK, Trotta E, Simeonov DR, Marson A, Bluestone JA. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 2018;3:eaat1482. doi:10.1126/sciimmunol.aat1482
- Wraith DC. The future of immunotherapy: A 20-year perspective. Front Immunol. 2018;8:1668. doi:10.3389/fimmu.2017.01668
- Bayry J, Kaveri SV. Kill ‘Em All: efgartigimod immunotherapy for autoimmune diseases. Trends Pharmacol Sci. 2018;39:919–22. doi:10.1016/j.tips.2018.08.004
- Pozsgay J, Szekanecz Z, Sarmay G. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol. 2017;13:525–37. doi:10.1038/nrrheum.2017.107
- Stephen-Victor E, Bayry J. Multimerized IgG1 Fc molecule as an anti-inflammatory agent. Nat Rev Rheumatol. 2018;14:390–92. doi:10.1038/s41584-018-0013-9
- Cousens L, Najafian N, Martin WD, De Groot AS. Tregitope: immunomodulation powerhouse. Hum Immunol. 2014;75:1139–46. doi:10.1016/j.humimm.2014.10.012
- Lai Y, Suo S, Wang R, Kong X, Hu Y, Tang D, Shi H, Chen S, Hu H. Trends involving monoclonal antibody (mAb) research and commercialization: A scientometric analysis of IMS lifecycle R&D focus database (1980–2016). Hum Vaccin Immunother. 2018;14:847–55. doi:10.1080/21645515.2017.1420445
- Othy S, Hegde P, Topçu S, Sharma M, Maddur MS, Lacroix-Desmazes S, Bayry J, Kaveri SV. Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J Immunol. 2013;190:4535–41. doi:10.4049/jimmunol.1201965
- Fiebiger BM, Maamary J, Pincetic A, Ravetch JV. Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc Natl Acad Sci U S A. 2015;112:E2385–2394. doi:10.1073/pnas.1505292112
- Maddur MS, Stephen-Victor E, Das M, Prakhar P, Sharma VK, Singh V, Rabin M, Trinath J, Balaji KN, Bolgert F, et al. Regulatory T cell frequency, but not plasma IL-33 levels, represents potential immunological biomarker to predict clinical response to intravenous immunoglobulin therapy. J Neuroinflammation. 2017;14:58. doi:10.1186/s12974-017-0818-5
- Sharma M, Das M, Stephen-Victor E, Galeotti C, Karnam A, Maddur MS, Bruneval P, Kaveri SV, Bayry J. Regulatory T cells induce activation rather than suppression of human basophils. Sci Immunol. 2018;3:eaan0829. doi:10.1126/sciimmunol.aan0829
- Galeotti C, Stephen-Victor E, Karnam A, Das M, Gilardin L, Maddur MS, Wymann S, Vonarburg C, Chevailler A, Dimitrov JD, et al. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J Allergy Clin Immunol. 2019. epub ahead of print. doi:10.1016/j.jaci.2018.10.064
- Maddur MS, Kaveri SV, Bayry J. Circulating normal IgG as stimulator of regulatory T cells: lessons from intravenous immunoglobulin. Trends Immunol. 2017;38:789–92. doi:10.1016/j.it.2017.08.008
- Su Y, Rossi R, De Groot AS, Scott DW. Regulatory T cell epitopes (Tregitopes) in IgG induce tolerance in vivo and lack immunogenicity per se. J Leukoc Biol. 2013;94:377–83. doi:10.1189/jlb.0912441
- Saha C, Das M, Patil V, Stephen-Victor E, Sharma M, Wymann S, Jordi M, Vonarburg C, Kaveri SV, Bayry J. Monomeric immunoglobulin A from plasma inhibits human Th17 responses in vitro independent of FcαRI and DC-SIGN. Front Immunol. 2017;8:275. doi:10.3389/fimmu.2017.00275
- Massoud AH, Kaufman GN, Xue D, Béland M, Dembele M, Piccirillo CA, Mourad W, Mazer BD. Peripherally generated Foxp3+ regulatory T cells mediate the immunomodulatory effects of IVIg in allergic airways disease. J Immunol. 2017;198:2760–71. doi:10.4049/jimmunol.1502361
- Bozza S, Kasermann F, Kaveri SV, Romani L, Bayry J. Intravenous immunoglobulin protects from experimental allergic bronchopulmonary aspergillosis via a sialylation-dependent mechanism. Eur J Immunol. 2019;49:195–98. doi:10.1002/eji.201847774
- Ruiz de Souza V, Carreno MP, Kaveri SV, Ledur A, Sadeghi H, Cavaillon JM, Kazatchkine MD, Haeffner-Cavaillon N. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin). Eur J Immunol. 1995;25:1267–73. doi:10.1002/eji.1830250521
- Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. FcγRIII-dependent inhibition of IFNγ responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi:10.1016/j.immuni.2006.11.010
- Galeotti C, Hegde P, Das M, Stephen-Victor E, Canale F, Muñoz M, Sharma VK, Dimitrov JD, Kaveri SV, Bayry J. Heme oxygenase-1 is dispensable for the anti-inflammatory activity of intravenous immunoglobulin. Sci Rep. 2016;6:19592. doi:10.1038/srep19592
- Kozicky LK, Menzies SC, Zhao ZY, Vira T, Harnden K, Safari K, Del Bel KL, Turvey SE, Sly LM. IVIg and LPS co-stimulation induces IL-10 production by human monocytes, which is compromised by an FcγRIIA disease-associated gene variant. Front Immunol. 2018;9:2676. doi:10.3389/fimmu.2018.02676
- Kozicky LK, Zhao ZY, Menzies SC, Fidanza M, Reid GS, Wilhelmsen K, Hellman J, Hotte N, Madsen KL, Sly LM. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol. 2015;98:983–94. doi:10.1189/jlb.3VMA0315-078R
- Abe J, Jibiki T, Noma S, Nakajima T, Saito H, Terai M. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J Immunol. 2005;174:5837–45. doi:10.4049/jimmunol.174.9.5837
- Damås JK, Gullestad L, Aass H, Simonsen S, Fjeld JG, Wikeby L, Ueland T, Eiken HG, Frøland SS, Aukrust P. Enhanced gene expression of chemokines and their corresponding receptors in mononuclear blood cells in chronic heart failure–modulatory effect of intravenous immunoglobulin. J Am Coll Cardiol. 2001;38:187–93. doi:10.1016/S0735-1097(01)01335-3
- Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–86. doi:10.1016/j.imbio.2006.05.017
- Marino S, Cilfone NA, Mattila JT, Linderman JJ, Flynn JL, Kirschner DE. Macrophage polarization drives granuloma outcome during mycobacterium tuberculosis infection. Infect Immun. 2015;83:324–38. doi:10.1128/IAI.02494-14
- Tjon AS, van Gent R, Geijtenbeek TB, Kwekkeboom J. Differences in anti-onflammatory actions of intravenous immunoglobulin between mice and men: more than meets the eye. Front Immunol. 2015;6:197. doi:10.3389/fimmu.2015.00197
- Galeotti C, Kaveri SV, Bayry J. Molecular and immunological biomarkers to predict IVIg response. Trends Mol Med. 2015;21:145–47. doi:10.1016/j.molmed.2015.01.005
- Meregalli C, Marjanovic I, Scali C, Monza L, Spinoni N, Galliani C, Brivio R, Chiorazzi A, Ballarini E, Rodriguez-Menendez V, et al. High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats. J Neuroinflammation. 2018;15:232. doi:10.1186/s12974-018-1270-x
- Domínguez-Soto A, de Las Casas-Engel M, Bragado R, Medina-Echeverz J, Aragoneses-Fenoll L, Martín-Gayo E, van Rooijen N, Berraondo P, Toribio ML, Moro MA, et al. Intravenous immunoglobulin promotes antitumor responses by modulating macrophage polarization. J Immunol. 2014;193:5181–89. doi:10.4049/jimmunol.1303375
- von Gunten S, Shoenfeld Y, Blank M, Branch DR, Vassilev T, Käsermann F, Bayry J, Kaveri S, Simon HU. IVIG pluripotency and the concept of Fc-sialylation: challenges to the scientist. Nat Rev Immunol. 2014;14:349. doi:10.1038/nri3401-c1
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–73. doi:10.1126/science.1129594
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–78. doi:10.1073/pnas.0810163105
- Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, Mourad W, Piccirillo CA, Mazer BD. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. 2014;133:853–63. doi:10.1016/j.jaci.2013.09.029
- Séïté JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA, Hillion S. IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood. 2010;116:1698–704. doi:10.1182/blood-2009-12-261461
