ABSTRACT
Estimates of medical care costs for cervical and other cancers associated with human papillomavirus (HPV) are higher in studies published in recent years than in studies published before 2012. The purpose of this report is (1) to review and summarize the recent cancer cost estimates and (2) to illustrate how the estimated cost-effectiveness of HPV vaccination might change when these recent cost estimates are applied. Our literature search yielded 6 studies that provided updated medical care cost estimates for 5 HPV-associated cancers. We found that applying the current cancer cost estimates had a notable impact on the estimated medical costs averted by HPV vaccination over an extended time frame (100 years), and a moderate impact on the estimated cost per quality-adjusted life year (QALY) gained by HPV vaccination. For example, for catch-up vaccination of teenagers and young adults, applying the more recent cancer costs reduced the estimated cost per QALY gained by about $12,400. The cost studies we identified in our literature review are up-to-date and based on reliable data sources from United States settings, and can inform future studies of HPV vaccination cost-effectiveness in the United States. However, careful consideration is warranted to determine the most appropriate cost values to apply.
Introduction
Estimates of the medical care costs for cervical and other cancers associated with human papillomavirus (HPV) are vital to inform studies of the cost-effectiveness of HPV vaccination strategies in the United States.Citation1-Citation11 Since 2012, several studies have suggested that cancer treatment costs may be higher than previously estimated, owing in part to newer, more resource-intensive treatments.Citation12-Citation15 These recent cost estimates are notably higher than the cost estimates that have been used in U.S.-based HPV vaccine cost-effectiveness studies.Citation1-Citation5, Citation7-Citation11
The purpose of this report is (1) to review and summarize the recent cancer cost estimates and (2) to illustrate how the estimated cost-effectiveness of HPV vaccination might change when these cost estimates are applied.
Results
Literature search
We found 260 studies common to all three searches that we performed, published since 2012 or later (). Of these, we excluded 235 studies based on title and abstract review: 163 studies that were not set in the United States; 34 studies that focused on the cost-effectiveness of specific treatment strategies (for example, we excluded a studyCitation16 that focused on the cost-effectiveness of adding paclitaxel or topotecan to cisplatin for the treatment of advanced cervical cancer, because it did not estimate the average medical care cost per case of cervical cancer); 23 studies that focused on the cost or cost-effectiveness of HPV vaccination or cancer screening (for example, we excluded a studyCitation17 that focused on the cost of providing cervical and breast cancer screening, because it did not estimate the average medical cost per case of cervical cancer); and 15 studies that did not present an original estimate of the average medical cost per case for an HPV-associated cancer.
Figure 1. Flow diagram illustrating the identification of studies providing updated medical care cost estimates for HPV-associated cancers in the United States.
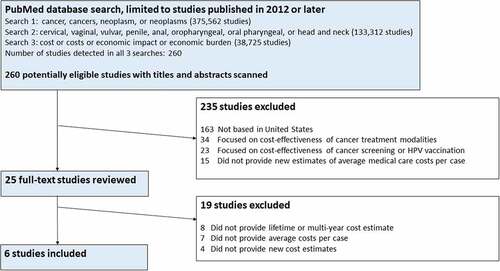
Of the 25 studies selected for full-text review, 8 were excluded because they did not provide lifetime or multi-year cost estimates, 7 were excluded because they did not provide average medical costs per case for an HPV-associated cancer, and 4 did not provide original cost estimates. The literature search yielded 6 studies that provided updated medical cost estimates for 5 HPV-associated cancers ().Citation12-Citation15,Citation18,Citation19 These 6 studies suggest a notably higher cost for cancer treatment than the previous studies, particularly for oropharyngeal and vaginal cancer. Our review did not find any studies that provided an average cost per case estimate for penile cancer.
Table 1. Medical care costs for HPV-associated cancers in the United States: Estimates from recent studies (2012 to 2018), compared to previous studies (before 2012) commonly cited in cost-effectiveness analyses of HPV vaccination.
Cost-effectiveness analyses
shows the estimated vaccination costs, number of quality-adjusted life years (QALYs) gained, medical costs averted, and cost-effectiveness estimates for two medical care cost scenarios for each HPV vaccination strategy: when applying the cancer cost estimates from 2012 to 2018 and when applying the cancer cost estimates from before 2012. Routine vaccination of adolescents (compared to no vaccination) was estimated to avert $5.1 billion in HPV-associated cancer costs and cost $4,500 per QALY gained when using the pre-2012 cancer cost estimates and to avert $13.1 billion in HPV-associated cancer costs and to be cost-saving (< $0 per QALY) when using the more recent cancer cost estimates. Adding catch-up vaccination through age 26 years to the adolescent vaccination program was estimated to avert an additional $3.8 billion in cancer costs and to cost $53,500 per QALY gained when using the pre-2012 cancer cost estimates, and to avert an additional $9.8 billion in cancer costs and to cost $41,100 per QALY gained when using the more recent cancer cost estimates.
Table 2. Estimated vaccination costs, number of quality-adjusted life years (QALYs) gained, medical costs averted, and cost-effectiveness estimates for two HPV vaccination strategies in the United States, over a 100-year time horizon.
shows the results of the sensitivity analysis, in which selected model parameters were varied. In all scenarios examined, routine vaccination of 12-year-olds was found to be cost-saving when applying the more recent (2012 to 2018) cancer cost estimates. The cost per QALY gained by catch-up vaccination ranged from $29,100 to $68,800 when applying the previous (pre-2012) cancer cost estimates and from $22,300 to $56,200 when applying the more recent (2012 to 2018) cancer cost estimates. The decrease in the estimated cost per QALY gained when applying the more recent (2012 to 2018) cancer cost estimates, compared to when applying the previous (pre-2012) costs, ranged from $6,800 to $15,800 in absolute terms and from 18% to 30% in relative terms.
Table 3. Sensitivity analysis: Estimated cost per quality-adjusted life year ($ per QALY) gained when applying cancer cost estimates from recent studies (2012 to 2018) and from older studies (before 2012), when other model parameters are varied.
Discussion
Current estimates of the medical care costs of HPV-associated cancer are notably higher than pre-2012 estimates commonly used in many U.S. studies of HPV vaccine cost-effectiveness. Researchers who generated the more recent cost estimates have noted potential reasons for these differences, including technologic or other changes in the standard of care over time.Citation13,Citation15 For example, the increase in the estimated cost per case of oropharyngeal cancer is likely due primarily to increased use of intensity-modulated radiotherapy (IMRT), an advanced type of radiation therapy.Citation13 The rise in estimated costs for oropharyngeal cancer, and the higher costs associated with IMRT, are supported by cost studies of head and neck cancers in general.Citation20,Citation21
We found that applying the current cancer cost estimates had a notable impact on the estimated medical costs averted by HPV vaccination over an extended time frame (100 years), and a moderate impact on the estimated cost per QALY gained by HPV vaccination. Adolescent vaccination was estimated to be cost-saving when applying current cancer medical cost estimates but not when applying the pre-2012 cancer cost estimates. The cost-effectiveness of adolescent HPV vaccination generally appears more favorable in more recent studies than in studies from a decade or more ago; this trend is not only because of higher cancer cost estimates but also because of the additional protection provided by 9-valent HPV vaccine and because of adoption of 2-dose vaccine schedules for adolescents initiating vaccination at ages 9 through 14 years.Citation22-Citation24 For catch-up vaccination of teenagers and young adults, applying the more recent cancer costs reduced the estimated cost per QALY gained by about $12,400. These findings are consistent with other modeling studies indicating the cost-effectiveness estimates for HPV vaccination are not particularly sensitive to reasonable variations in the assumptions regarding the medical costs per HPV-associated health outcome.Citation25 Still, it is important that cost-effectiveness analyses of HPV vaccination strategies in the United States incorporate the most accurate, up-to-date medical cost estimates available.Citation26
Our study has several important limitations. First, our review follows some but not all of the established recommendations for systematic literature reviews.Citation27 For example, we used only one literature database (PubMed), and we did not assess risk of bias in the individual studies in our review. However, limited or rapid reviews such as ours allow for a practical assessment of existing cost studies for use in cost-effectiveness analyses to inform timely decision-making in public health.Citation26,Citation28 Second, although we performed one-way sensitivity analyses to illustrate how changes in selected model assumptions affected our results, these sensitivity analyses were less comprehensive than is typically recommended for cost-effectiveness studies.Citation29,Citation30 The reason for this and other departures from standard cost-effectiveness methodology is that our analysis was not intended as a cost-effectiveness analysis of HPV vaccination, but rather as an illustration of the magnitude of the effect of incorporating the recent cancer cost estimates on the estimated cost per QALY gained by HPV vaccination. Third, we focused on studies providing estimates of the lifetime cost or the multi-year cost per case of cancer. Many of the recent cost estimates reflect medical care costs per case for only the first two yearsCitation13-Citation15,Citation18 after a cancer diagnosis, which nonetheless can be a useful approximation for lifetime costs. Although lifetime cost estimates are suitable for many models of HPV vaccine cost-effectiveness such as the one we applied, monthly cost estimates or annual cost estimates might be more applicable for some other HPV models. Finally, we reviewed estimates of the medical care cost of HPV-associated cancers, without regard to who pays these costs. Out-of-pocket costs and other expenses can impose substantial financial strain on affected individuals and their families, and evidence suggests that head and neck cancers can be especially burdensome in this regard.Citation31
Given that the cost studies we identified in our literature review are up-to-date and based on reliable data sources from U.S. settings, we encourage that these cost estimates be incorporated in future studies of HPV vaccination cost-effectiveness in the United States. However, limitations of these cost studies must also be considered. For example, Lairson’s estimates of the medical care costs for cervical cancer and oropharyngeal cancer were based on data from one state (Texas) and might not be applicable to the nation as a whole.Citation13,Citation14 As another example, Deshmukh’s estimate of the medical cost of anal cancer was calculated for patients aged 66 years or older, and might differ from the costs of younger patients.Citation12 Further, studies of medical claims data that compare cancer patients to controls might not be able to account for unobserved differences in these groups.Citation15 Another important consideration is that many of the recent cancer cost studies focus on data from commercially insured populations, whereas costs might differ among other populations, such as those with Medicaid.Citation32 Thus, although the studies we included in this review provide valuable new information about the current medical costs of HPV-associated cancers, careful consideration is warranted to determine the most appropriate values to apply in each future cost-effectiveness analysis of HPV vaccination in the United States.
Methods
First, we conducted a limited literature review to identify studies of the medical care costs for HPV-associated cancers published since January 1, 2012. Our review was limited in that it shared some but not all common features of a systematic literature review (e.g., we defined the search strategy and described eligibility and inclusion criteria, but did not assess risk of bias in individual studies or study quality).Citation27 We limited the analysis to studies published in 2012 or later so that our review (1) would include the most current cancer cost studies to date and (2) would supplement a 2012 assessment of the direct medical cost burden of HPV in the U.S.,Citation33 which summarized the cancer cost estimates commonly applied in HPV vaccine cost-effectiveness studies at the time. We performed the following three searches in PubMed on November 28, 2018. Search 1 included studies in which the title or abstract contained at least one of the following terms: cancer, cancers, neoplasm, or neoplasms. Search 2 included studies in which the title or abstract contained at least one of the following terms: cervical, vaginal, vulvar, penile, anal, oropharyngeal, oral pharyngeal, or head and neck. Search 3 included studies in which the title or abstract contained at least one of the following terms: cost, costs, economic impact, or economic burden. We initially selected articles that were (A) detected in all 3 of these searches, (B) published in 2012 or later, (C) listed in PubMed as of the November 28, 2018 search date. We then conducted title/abstract reviews and full-text reviews as needed to exclude articles that did not provide a medical care cost estimate for at least one HPV-associated cancer in a U.S. setting. In this process, we excluded studies of the cost-effectiveness of cancer screening or HPV vaccination, as model-based cost-effectiveness analyses typically do not involve primary cost data collection and instead apply cost estimates from existing data.Citation29 Similarly, we excluded cost-effectiveness studies of specific cancer treatment modalities when these studies focused on variations in cancer treatment strategies rather than the overall cancer medical care costs per patient. We included only studies that provided an original estimate of the average lifetime cost or multi-year cost estimate for cancer; we excluded other studies such as those that reported annual or monthly costs, because the HPV cost-effectiveness model we applied requires average lifetime cost per case estimates. We included only studies that provided new cost estimates for cervical, vaginal, vulvar, penile, anal, or oropharyngeal cancer. In doing so, we excluded studies that reported costs for head and neck cancers as a group without providing an estimate specifically for oropharyngeal cancer.
Second, we compiled the recent HPV-associated cancer cost estimates from the studies found in the literature search, and updated these costs to 2018 U.S. dollars using the health care component of the personal consumption expenditures price index (https://bea.gov/). We compared these recent costs to selected, previously-reported costsCitation1,Citation33-Citation35 that have been cited in numerous HPV vaccine cost-effectiveness analyses in the United States.Citation1-Citation5, Citation7-Citation11
Third, we conducted a simple cost-effectiveness exercise to illustrate how the estimated cost-effectiveness of HPV vaccination in the United States might change when applying the more recent cancer costs. To do so, we examined the cost-effectiveness of HPV vaccination for two illustrative vaccine strategies: routine adolescent vaccination with and without catch-up vaccination for teenagers and young adults. We chose these two vaccination strategies because in the United States, HPV vaccination is routinely recommended for boys and girls at age 11–12 years, and the catch-up recommendations address populations through age 26 years.Citation22,Citation23,Citation36 Further, these two vaccination strategies offer a range in terms of the estimated cost per QALY gained by HPV vaccination, which in the existing literature is often estimated at <$25,000 for adolescent vaccination and >$25,000 for catch-up vaccination for young adults. For the adolescent vaccination strategy, we examined the cost-effectiveness of a gender-neutral HPV vaccination program of 12-year-olds vs. no vaccination. For the catch-up vaccination strategy, we examined the cost-effectiveness of a gender-neutral HPV vaccination program for 12-year-olds with catch-up vaccination through age 26 years for those not vaccinated previously vs. vaccination of 12-year-olds only.
For each of the above vaccination strategies, we estimated the cost-effectiveness of HPV vaccination using two medical costs scenarios: one using previous (pre-2012) cancer cost estimatesCitation33,Citation34,Citation35 that were frequently cited in previously-published cost-effectiveness studies, and one using the more recent (2012 to 2018) cost estimates from our literature review. Cost-effectiveness was examined using a published, dynamic HPV vaccination model.Citation10,Citation11 The model is described in detail in the Technical Appendix. Briefly, the model is a simplified compartmental model which tracks people in the population from ages 8 through 99 years. The high-risk HPV vaccine types (16/18/31/33/45/52/58) are modeled separately and the low-risk HPV types (6/11) are modeled as a single combined type. The model includes the following health outcomes that can be averted by HPV vaccination: cervical and other cancers (anal, oropharyngeal, penile, vaginal, and vulvar), genital warts, cervical intraepithelial neoplasia, and recurrent respiratory papillomatosis. We refer to the model as “simplified” because of three key simplifying features not typically found in other HPV models. First, cervical cancer screening is not explicitly modeled but is assumed to occur. Second, HPV transmission dynamics are approximated by applying age-specific annual probabilities of HPV acquisition in the “no vaccination” scenario, and modifying these HPV acquisition probabilities over time in scenarios with HPV vaccination in accordance with age-specific and sex-specific reductions in HPV acquisition due to HPV vaccination. Third, transition from HPV acquisition to HPV-associated disease is not explicitly modeled. Instead, the model approximates age-specific and sex-specific reductions in HPV-associated health outcomes due to HPV vaccination based on age-specific and sex-specific percentage reductions in cumulative HPV acquisition due to HPV vaccination.Citation4,Citation10,Citation11 Despite these and other simplifications, the model’s results have typically been fairly consistent with results from more complex models.Citation8,Citation37
We used the model to calculate the vaccination costs incurred, medical costs averted, QALYs gained, and cost-effectiveness ratios (cost per QALY gained) over a 100-year time horizon. Future costs and QALYs were discounted at an annual rate of 3%. We used a health system perspective in which we included all medical costs regardless of payer. We assumed vaccination uptake rates achieved in recent years would continue.Citation38,Citation39 We applied an HPV vaccination cost of $212 per dose, including administration costs.Citation11,Citation40 All cost assumptions and other model parameters are described in the Technical Appendix.
We conducted one-way sensitivity analyses to show how varying selected other key parameters (besides cancer cost estimates) influenced the impact of the updated cancer cost assumptions on the estimated cost-effectiveness of HPV vaccination strategies. That is, we estimated the cost per QALY gained when applying cancer cost estimates from the more recent studies (2012 to 2018) and from the previous studies (before 2012), when other model parameters are varied.
In the one-way sensitivity analyses, we varied each of the following parameters (or parameter sets) one at a time: vaccine price per series; the number of QALYs lost per case of each health outcome; the incidence rates of the health outcomes in the absence of vaccination; and the percentages of the health outcomes attributable to the HPV vaccine types.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download PDF (947.2 KB)Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–32. doi:10.1056/NEJMsa0707052.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–67. doi:10.1016/j.vaccine.2010.08.030.
- Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi:10.1136/bmj.b3884.
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29(46):8443–50. doi:10.1016/j.vaccine.2011.07.096.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–52. doi:10.1016/S1473-3099(10)70219-X.
- Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32(51):6941–47. doi:10.1016/j.vaccine.2014.10.052.
- Brisson M, Laprise JF, Chesson HW, Drolet M, Malagón T, Boily M-C, Markowitz LE. Health and economic impact of switching from a 4-valent to a 9-valent HPV vaccination program in the United States. J Natl Cancer Inst. 2015;108(1):djv282. doi:10.1093/jnci/djv282.
- Chesson HW, Laprise JF, Brisson M, Markowitz LE. Impact and cost-effectiveness of 3 doses of 9-valent human papillomavirus (HPV) vaccine among US females previously vaccinated with 4-valent HPV vaccine. J Infect Dis. 2016;213(11):1694–700. doi:10.1093/infdis/jiw046.
- Laprise JF, Markowitz LE, Chesson HW, Drolet M, Brisson M. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: A cost-effectiveness analysis. J Infect Dis. 2016;214(5):685–88. doi:10.1093/infdis/jiw227.
- Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: estimates from a simplified transmission model. Hum Vaccin Immunother. 2016;12(6):1363–72. doi:10.1080/21645515.2016.1140288.
- Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26 years in the United States. Vaccine. 2018;36(29):4362–68. doi:10.1016/j.vaccine.2018.04.071.
- Deshmukh AA, Zhao H, Franzini L, Lairson DR, Chiao EY, Das P, Swartz MD, Giordano SH, Cantor SB. Total lifetime and cancer-related costs for elderly patients diagnosed with anal cancer in the United States. Am J Clin Oncol. 2018;41(2):121–27. doi:10.1097/COC.0000000000000238.
- Lairson DR, Wu CF, Chan W, Dahlstrom KR, Tam S, Sturgis EM. Medical care cost of oropharyngeal cancer among Texas patients. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1443–49. doi:10.1158/1055-9965.EPI-17-0220.
- Lairson DR, Fu S, Chan W, Xu L, Shelal Z, Ramondetta L. Mean direct medical care costs associated with cervical cancer for commercially insured patients in Texas. Gynecol Oncol. 2017;145(1):108–13. doi:10.1016/j.ygyno.2017.02.011.
- Fu S, Lairson DR, Chan W, Wu CF, Ramondetta L. Mean medical costs associated with vaginal and vulvar cancers for commercially insured patients in the United States and Texas. Gynecol Oncol. 2018;148(2):342–48. doi:10.1016/j.ygyno.2017.12.019.
- Geisler JP, Swathirajan J, Wood KL, Manahan KJ. Treatment of advanced or recurrent cervical cancer with Cisplatin or Cisplatin containing regimens: a cost effective analysis. J Cancer. 2012;3:454–58. doi:10.7150/jca.4807.
- Ekwueme DU, Subramanian S, Trogdon JG, Miller JW, Royalty JE, Li C, Guy GP, Crouse W, Thompson H, Gardner JG. Cost of services provided by the National Breast and Cervical Cancer Early Detection Program. Cancer. 2014;120(Suppl 16):2604–11. doi:10.1002/cncr.28816.
- Wu CF, Xu L, Fu S, Peng HL, Messick CA, Lairson DR. Health care costs of anal cancer in a commercially insured population in the United States. J Manag Care Spec Pharm. 2018;24(11):1156–64. doi:10.18553/jmcp.2018.24.11.1156.
- Sher DJ, Fidler MJ, Tishler RB, Stenson K, al-Khudari S. Cost-effectiveness analysis of chemoradiation therapy versus transoral robotic surgery for human papillomavirus-associated, clinical N2 oropharyngeal cancer. Int J Radiat Oncol Biol Phy. 2016;94(3):512–22. doi:10.1016/j.ijrobp.2015.11.006.
- Razfar A, Mundi J, Grogan T, Lee S, Elashoff D, Abemayor E, St John M. IMRT for head and neck cancer: cost implications. Am J Otolaryngol. 2016;37(6):479–83. doi:10.1016/j.amjoto.2015.02.017.
- Sheets NC, Wheeler SB, Kohler RE, Fried DV, Brown PM, Chera BS. Costs of care in a matched pair comparison of intensity-modulated radiation therapy (IMRT) versus conventional radiation therapy (CRT) for the treatment of head and neck cancer. Am J Clin Oncol. 2014;37(6):539–44. doi:10.1097/COC.0b013e318282a850.
- Petrosky E, Bocchini JA Jr., Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–04.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–08. doi:10.15585/mmwr.mm6549a5.
- Chesson HW. Impact and economic analysis: summary of three models of 9-valent HPV vaccination among adults up to age 45 years in the United States. Meeting of the Advisory Committee on Immunization Practices; 2018 Oct 25; Atlanta,GA.
- Brisson M, Van de Velde N, De Wals P, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25(29):5399–408. doi:10.1016/j.vaccine.2007.04.086.
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. doi:10.1001/jama.2016.12195.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clinical Epidemiol. 2009;62(10):1006–12. doi:10.1016/j.jclinepi.2009.06.005.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91–108. doi:10.1111/j.1471-1842.2009.00848.x.
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine: second edition. New York (NY): Oxford University Press; 2016.
- Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. 2nd ed. New York (NY): Oxford University Press; 2002.
- Massa ST, Osazuwa-Peters N, Adjei, Boakye E, Walker RJ, Ward GM. Comparison of the financial burden of survivors of head and neck cancer with other cancer survivors. JAMA Otolaryngol Head Neck Surg. 2019. Epub ahead of print. doi:10.1001/jamaoto.2018.3982.
- Jacobson JJ, Epstein JB, Eichmiller FC, Gibson TB, Carls GS, Vogtmann E, Wang S, Murphy B. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, medicare, and medicaid. Head Neck Oncol. 2012;4:15. doi:10.1186/1758-3284-4-15.
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–19. doi:10.1016/j.vaccine.2012.07.056.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198(5):500.e1–500.e7. doi:10.1016/j.ajog.2008.03.064.
- Insinga RP, Ye X, Singhal PK, Carides GW. Healthcare resource use and costs associated with cervical, vaginal and vulvar cancers in a large U.S. health plan. Gynecol Oncol. 2008;111(2):188–96. doi:10.1016/j.ygyno.2008.07.032.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63:1–30.
- Brisson M, Benard E, Drolet M, Bogaards JA, Baussano I, Vänskä S, Jit M, Boily M-C, Smith MA, Berkhof J, et al. Population-level impact, herd immunity and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–17. doi:10.1016/S2468-2667(16)30001-9.
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, Markowitz LE, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–58. doi:10.15585/mmwr.mm6533a4.
- Williams WW, Lu PJ, O‘Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. doi:10.15585/mmwr.ss6501a1.
- Centers for Disease Control and Prevention. CDC vaccine price list. [accessed Dec 16 2018]. https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
- Maroun J, Ng E, Berthelot JM, Le Petit C, Dahrouge S, Flanagan WM, Walker H, Evans WK. Lifetime costs of colon and rectal cancer management in Canada. Chronic Dis Can. 2003;24:91–101.
- Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men. Am J Med. 2000;108:634–41.
- Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, Nefcy P. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–26.
- Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg. 2004;130(11):1269–75. doi:10.1001/archotol.130.11.1269.
- Fetters MD, Lieberman RW, Abrahamse PH, Sanghvi RV, Sonnad SS. Cost-effectiveness of pap smear screening for vaginal cancer after total hysterectomy for benign disease. J Low Genit Tract Dis. 2003;7:194–202.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004;103(4):619–31.
