ABSTRACT
Our laboratory has focused on Porin B (PorB), an outer membrane protein from Neisseria meningitidis and TLR2 ligand-based adjuvant, to characterize specific molecular and cellular pathways involved in improved immune responses induced by vaccine adjuvants. PorB’s ability to form micellar nanoparticular multi-molecular organized structures and its interaction with Toll-like receptor 2/1 complexes likely accounts for its potent adjuvant activity. Downstream from this stimulation, we have observed enhanced antigen uptake in antigen presenting cells (APC), greater antigen deposition in secondary lymphoid organs, and promotion of germinal center reactions. In mice, antigen-specific IgGs were increased after PorB adjuvanted vaccination using the model antigen ovalbumin (OVA). Likewise, this formulation resulted in more IL-4 and IFN-γ positive T cells. Mice that received PorB adjuvanted vaccinations benefitted from lower bacterial burdens when challenged with recombinant Listeria monocytogenes expressing OVA. Mouse models lacking MyD88 signaling in various APC types helped identify macrophages as an essential cell type for the adjuvant activity of PorB. We believe the work presented here provides examples of the mechanistic studies required to understand how vaccine adjuvants are contributing to the establishment of protective immunity.
Vaccines are arguably one of the most important public health breakthroughs in modern medicine. Since the observation that live cowpox infection in dairy maids conferred immunity to smallpox, the field of vaccine development has made great strides in understanding the mechanisms by which immunological protection and memory are induced. With this knowledge, an evolution and diversification of the components used in vaccine formulations occurred. Immunologists have used both live and attenuated or killed pathogens as the antigen component of vaccines. These formulations are not without their respective risks, which range from a reversion of live pathogen to its virulent form or a failure to establish protective immunity by killed-pathogen. Subunit vaccines avoid these potential pitfalls by utilizing component portions of pathogens (either native or recombinant) and adjuvants to provide immune protection to the individual. Adjuvants are substances included in vaccine preparations to help promote an immune response to poorly immunogenic antigens and possibly skew the type of adaptive immune response that develops, potentially improving protection.Citation1 Aluminum salts are one of the earliest adjuvants added to vaccines and remain widely used today despite an incomplete understanding of their mechanism. In the past two decades, interest in understanding the mechanism of action of aluminum salts brought into focus an important question for vaccine-induced protection: How does the innate immune system influence the adaptive immune system to confer immunological memory and how do adjuvants effect this interactionCitation2,Citation3? The discovery of pathogen-associated molecular patterns (PAMPs) and consequently, pattern recognition receptors such as Toll-like receptors (TLRs), provided the opportunity for well-defined cellular pathways to be exploited in order to gain further understanding about the cellular kinetics between innate and adaptive immunity.Citation4 Our laboratory has focused on the use of Porin B (PorB), an outer membrane protein from Neisseria meningitidis, as a TLR2 ligand-based adjuvant.Citation5,Citation6 This commentary will focus on different mechanisms of action for adjuvants and the advantages of PorB which represents a single adjuvant that targets multiple mechanisms of action.
Role and mechanism of vaccine adjuvants
From a biological systems standpoint, adjuvants have relevance in every aspect of the immune response to a vaccine.Citation7 During the initial injection of the vaccine, adjuvants can act as proinflammatory effector molecules which promote the local secretion of proinflammatory cytokines and recruitment of antigen-presenting cells (APCs).Citation8 One such example is MF-59, an oil-in-water emulsion of squalene oil used in the current flu vaccinations.Citation9 MF-59 has been shown to increase innate cell recruitment to the injection site.Citation10 However, this characteristic is not unique to MF-59 as investigators have shown an increase in innate cell recruitment after administrations of other oil-in-emulsion compoundsCitation11 or inorganic scaffolding.Citation12 The recruitment of innate cells to the injection site allows for antigen within the vaccine composition to be taken up via APCs and transported to secondary lymphoid organs such as lymph nodes, commencing the adaptive immune response. Accordingly, the extent of antigen trafficking to lymph nodes can be modulated using specific adjuvants. One such example is synthetic oligodeoxynucleotides containing unmethylated CpG which are used as TLR-9 ligand-based adjuvants.Citation13 When biochemically linked to a lipophilic tail, CpG has been shown to increase the accumulation of antigen within draining lymph nodes.Citation14 These studies emphasize the importance of the innate immune system during an immune response to a vaccine and highlight the need to determine how stimulation of these cells with adjuvants leads to better adaptive responses.
One of the most important influences an adjuvant has on the adaptive pathway is its ability to enhance responses of B cells and T cells within the lymph node. This stimulation relies on specific signaling pathways which help activate APCs, B cells, and T cells. TLR ligands, particularly as adjuvants, have been very well studied for this purpose. Specifically, these molecules can increase the expression of co-stimulatory factors on the surface of each of these cell types. The TLR-ligand interaction on APCs initiates intracellular signaling which can result in increases in CD40, CD80, CD86, and MHCI/II expression.Citation15 CpG has been shown to increase the expression of CD40, CD80, and HLA-DR on naïve B cells when stimulated in vitro.Citation16 There is also evidence that CD4+ T cells can be directly activated by CpG and increase their expression of OX40 as well as the proliferative cytokine IL-2.Citation17 Furthermore, TLR ligands have been appreciated for their role in promoting the survival of activated CD4+ T cells.Citation18 Co-stimulatory factors alone, however, are insufficient to fully activate the adaptive immune response as cytokine expression and various modes of cytokine-dependent signaling are required. Cytokine expression, from both APCs and T/B cells, creates a unique microenvironment which allows a skewing of the adaptive immune response towards more humoral or cell-based effector functions.Citation19 Again, TLR-based adjuvants have been well studied in this area. Generally, extracellular TLR adjuvants (TLR1, TLR2, TLR5, and TLR6) produce inflammatory cytokines whereas intracellular TLR adjuvants (ligands for TLR3 and TLR7/8) primarily produce interferon cytokines, in vitro and in vivo.Citation15 With subunit vaccine research entering its third decade, there is now, more than ever, a need for studies that go beyond empirical observations and truly explore the mechanisms by which different vaccine compositions impart immunological memory. We are convinced that these studies are imperative for future vaccine development efforts and must include multiple adjuvants within their study designs to truly decipher mechanistic pathways.
Neisserial porin as a vaccine adjuvant
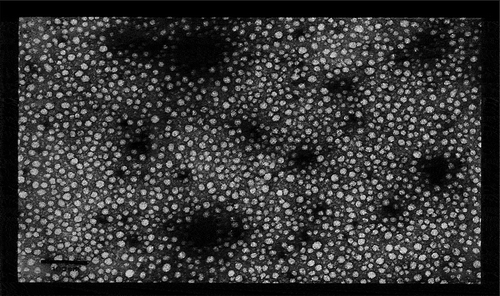
Our laboratory has focused on a specific TLR2-based ligand adjuvant, PorB, from Neisseria meningitidis. The past two decades of studies using PorB have elucidated how its structure influences its adjuvant activity in vitro and in vivo. In 2005, our lab enhanced a purification procedure to isolate PorB without contamination from lipooligosaccharide or other potential contaminants. This procedure, utilizing ion-exchange chromatography, gel filtration chromatography, and affinity chromatography, purified native PorB without any contaminants determined by silver stain and Western blot.Citation20 The final protein extraction was then dialyzed to remove detergent and to allow the formation of proteosomes (protein micelles devoid of detergent, approximately 10 nm in diameter (). From our studies, we have found that proteosome assembly is likely necessary for PorB’s activity as an adjuvant. These structures, described as “micellar multi-molecular organized structures”, nanoparticulate in nature, allow for PorB to have a similar structure as oil-in-water emulsion adjuvants and may be partially responsible for its potent adjuvant activity. In vitro studies indicated that PorB proteosomes signal through TLR1/TLR2 complexes and allow for IL-8 production from TLR2 expressing HEK293 cells.Citation6 Further studies examined the effects of PorB on APCs. We found that PorB can induce dendritic cell (DC) maturation, and enhances in vitro antigen presentation.Citation21 In addition, PorB stimulation of bone marrow-derived macrophages (BMDMs) in vitro was shown to increase the production of IL-6, IL-1β, and TNF-α. Interestingly, and similar to the DC studies, PorB treatment also increased surface expression of CD40, CD54, CD69, and CD86 costimulatory factors in BMDMs.Citation22
Figure 1. Electron micrograph of PorB proteosomes.
Micellar multi-molecular organized clusters of PorB termed “proteosomes”. The scale bar is in the lower left corner and measures 100nm. Each proteosome is approximately 10nm in diameter. This electron micrograph was generated by Dr Lee Wetzler
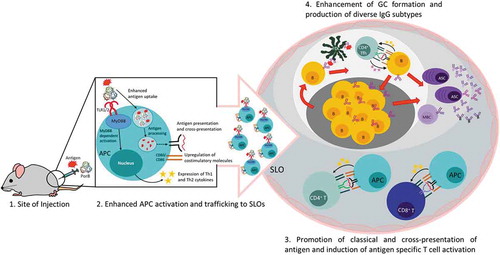
PorB has also been shown to increase total antigen-specific IgGs including IgG1, IgG2b, and IgG3 subtypes when used as an adjuvant.Citation23 These data suggest that PorB promotes both a Th1and Th2 antibody response, which is an ideal situation for producing a diverse array of protective antibodies. To decipher which innate cell type(s) were required for the PorB-induced increase in antibody response, our lab used transgenic mice to conditionally remove TLR-signaling in multiple professional antigen-presenting cell types. Specifically, we used Cre-Lox recombination to prevent MyD88 expression, the essential adaptor molecule for all extracellular TLRs, in APCs: macrophages (Lysmcre), dendritic cells (CD11ccre) or B cells (CD19cre). PorB, CpG, and aluminum salts (alum) were all used to delineate cellular kinetics and mechanisms. Loss of MyD88 signaling resulted in a significant decrease of antigen-specific IgGs from all cre-floxed mice treated with TLR-based adjuvants with the greatest decrease observed in animals without macrophage MyD88 signaling.Citation23 Intriguingly, the germinal centers of these mice were also significantly decreased in both size and number when compared to wild-type control animals with intact MyD88 signaling. These studies emphasized the role of MyD88 signaling within macrophages for antibody production and germinal center formation, regardless of the TLR-based adjuvant used. A recent publication from our lab focused on the ability of PorB to increase antigen trafficking to the lymph node and cross-presentation of antigen by dendritic cells.Citation24 This observation led to our exploration of PorB’s effect on CD4+ and CD8+ T cell populations. Most recently, our group has shown that PorB adjuvanted vaccination of mice using the model antigen ovalbumin (OVA) results in an increase of IL-4 and IFNγ positive CD4+ and CD8+ T cells. Most strikingly, when challenged with a recombinant intracellular pathogen expressing OVA, Listeria monocytogenes (rLmOVA), mice that received PorB adjuvanted OVA vaccination benefitted from lower bacterial burdens and an increase in survival when compared to OVA vaccination alone.Citation25 Together, these data highlight the unique characteristics of PorB which interacts with TLR1/2 complexes and signals in a MyD88-dependent fashion. As a vaccine adjuvant, PorB increases the expression of co-stimulatory factors and cytokines by APCs, increases antibody production, improves antigen trafficking to the lymph node, and promotes a broad T cell response including effective cross-presentation allowing for both CD4 and CD8 T cell responses.Citation23–Citation25 A visual representation of PorB’s effects in relation to vaccine adjuvanticity is displayed in .
Figure 2. Effects of PorB as an adjuvant during vaccination.
(1) PorB recognition by TLR1/2 on antigen presenting cells (APCs) at the site of injection leads to TLR2 and MyD88-dependent signaling for increased costimulatory molecule expression and secretion of Th1 and Th2 cytokines as shown by Platt et alCitation22 and Mosaheb et al.Citation23 (2) Enhanced APC activation promotes trafficking of APC-antigen to secondary lymphoid organs (SLOs) and (3) antigen uptake and processing for classical and cross-presentation inducing activation of antigen-specific CD4+ (as shown in green) and CD8+ T cells (as shown in blue).Citation24 (4) PorB enhances germinal center (GC) formation and production of both Th1 and Th2 IgGs.Citation23,Citation24Follicular dendritic cell (FDC); CD4+ T follicular helper cells (Tfh); Memory B cell (MBC); Antibody secreting cell (ASC)
Conclusion
Optimization of vaccine compositions for the protection of humans against disease is an ongoing effort with the long-term goal of global disease eradication. Adjuvants play a critical role in this optimization, particularly for weakly immunogenic antigens like those used in subunit vaccines. Accordingly, there is a need to reveal the potentially diverse mechanisms by which novel adjuvants influence both the innate and adaptive arms of the immune response. Understanding how adjuvants stimulate a specific immune response will serve to both inform vaccine design efforts and answer some of the most fundamental questions in immunology. Here, we have described PorB, a molecule with unique characteristics as an adjuvant that improves both humoral and cell-based immunity. Beyond this, our studies of PorB have yielded insights regarding intracellular signaling, cellular dynamics, and cross-presentation of antigen during an adjuvant associated immune response. By continuing these types of studies, and communicating findings in a consistent manner, the vaccine field will continue to advance, allowing for improved vaccines. The prospect of reducing disease burden is exciting and of utmost importance especially when considering diseases for which there is no efficient vaccine such as HIV, TB, and malaria.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Vaccine Adjuvants | NIH: National Institute of Allergy and Infectious Diseases. 2018 [accessed 12 Mar 2019] https://www.niaid.nih.gov/research/vaccine-adjuvants.
- Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines. 2015;3(2):320–43. doi:10.3390/vaccines3020320.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–93. doi:10.1038/nri2510.
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197–216. doi:10.1146/annurev.immunol.20.083001.084359.
- Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: immune stimulation by Neisserial porins is toll-like receptor 2 and MyD88. J Immunol. 2002;168(4):1533–37. doi:10.4049/jimmunol.168.4.1533.
- Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol. 2006;176(4):2373–80. doi:10.4049/jimmunol.176.4.2373.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–608. doi:10.1038/nm.3409.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi:10.3389/fimmu.2013.00114.
- Kostova D, Reed C, Finelli L, Cheng PY, Gargiullo PM, Shay DK, Singleton JA, Meltzer MI, Lu PJ, Bresee JS. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One. Goldstein E, ed. 2013;8(6):e66312. doi:10.1371/journal.pone.0066312.
- O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 – an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–48. doi:10.1016/J.VACCINE.2011.09.061.
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29(13):2461–73. doi:10.1016/j.vaccine.2011.01.011.
- Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33(1):64–72. doi:10.1038/nbt.3071.
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. doi:10.1586/erv.10.174.
- Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–22. doi:10.1038/nature12978.
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta - Mol Cell Res. 2002;1589(1):1–13. doi:10.1016/S0167-4889(01)00182-3.
- Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naïve B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37(8):2205–13. doi:10.1002/eji.200636984.
- Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45(1):25–36. doi:10.1007/s12026-009-8113-x.
- Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172(10):6065–73. [accessed 7 Feb 2019]. http://www.ncbi.nlm.nih.gov/pubmed/15128790.
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29(2):272–82. doi:10.1016/J.IMMUNI.2008.05.016.
- Massari P, King CA, MacLeod H, Wetzler LM. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr Purif. 2005;44(2):136–46. doi:10.1016/J.PEP.2005.04.021.
- Singleton TE, Massasri P, Wetzler LM. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol. 2005;176(9):3545–50. doi:10.4049/jimmunol.174.6.3545.
- Platt A, MacLeod H, Massari P, Liu X, Wetzler L. In vivo and in vitro characterization of the immune stimulating activity of the neisserial porin PorB. Das G, ed. PLoS One. 2013;8(12):e82171. doi:10.1371/journal.pone.0082171.
- Mosaheb MM, Reiser ML, Wetzler LM. Toll-like receptor ligand-based vaccine adjuvants require intact MyD88 signaling in antigen-presenting cells for germinal center formation and antibody production. Front Immunol. 2017;8:225. doi:10.3389/fimmu.2017.00225.
- Reiser ML, Mosaheb MM, Lisk C, Platt A, Wetzler LM. The TLR2 binding neisserial porin PorB enhances antigen presenting cell trafficking and cross-presentation. Sci Rep. 2017. doi:10.1038/s41598-017-00555-4.
- Mosaheb M, Wetzler LM. Meningococcal PorB induces a robust and diverse antigen specific T cell response as a vaccine adjuvant. Vaccine. 2018;36(50):7689–99. doi:10.1016/J.VACCINE.2018.10.074.
