ABSTRACT
Human papillomavirus (HPV) infection is a major cause of cervical cancer. HPV vaccine has been shown to be highly effective in preventing HPV infection, and understanding the genotypes of HPV infection can guide the utilization of HPV vaccine. This epidemiological study aimed to investigate the genotype distribution of HPV in HPV-positive female patients in Changzhou. This study enrolled HPV-positive female patients admitted at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University were enrolled in this study. HPV genotypes were identified via standard HPV DNA testing. The cohort comprised 5738 patients, that the predominant genotypes were HPV52 (13.4%), HPV16 (9.6%), HPV58 (8.8%), HPV81 (8.6%), and HPV53 (7.7%). HPV infection prevalence was the highest among those aged 41–45 y (16.6%), followed by those aged 31–35 y (15.1%) and 36–40 y (15.1%). In total, 46.8% of infections were found in women aged 31–45 y. The main genotypes of HPV infection upon physical examination or diagnosis of cervicitis and cervical cancer were HPV52, 16, 58, 81, and 53. HPV52, 16, 58, 81, and 53 are the most predominant genotypes of HPV infection. Further, HPV infection is most prevalent among women aged 31–45 y. Our findings provide experimental evidence for guiding HPV vaccination policy.
KEYWORDS:
Introduction
Human papillomavirus (HPV) is the most frequent reproductive tract infection. It shows high human tropism and can immortalize normal epithelial cells while reside stably in the nucleus.Citation1 Most HPV infections are asymptomatic and resolve spontaneously,Citation2 but persistent HPV infections can cause paralysis or precancerous lesions.Citation3 The risk factors for persistent HPV infection include pregnancy, multiple sex partners, smoking, and aberrant immune function.Citation4 Moreover, persistent HPV infections increase the risk cervical, vulvar, vaginal, penile, and throat cancers.Citation5–Citation8
The primary modes of HPV transmission are sex and skin contact,Citation5 and HPV infection is the primary causative factor in >96% of cervical cancer cases.Citation9,Citation10 Over 200 HPV genotypes have been identified,Citation11 but HPV is mainly divided into high-risk and low-risk genotypes according to their association with cancers.Citation12 At least 17 HPV genotypes (i.e., 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) have been proven to cause >96% of cervical cancers and are thus considered as high-risk HPV genotypes.Citation13 Meanwhile, there are at least 6 HPV genotypes (i.e., 6, 11, 42, 43, 81, and 83) that cause benign or low-grade changes and are thus considered as low-risk HPV genotypes. The rate and genotypes of HPV infections vary in different countries.Citation6 HPV infection is prevalent in 11–12% of women without cervical abnormalities, while the prevalence rate in China, is 11.4–20.3%.Citation10 Approximately 561,000 new cancer cases worldwide annually (5.2% of all new cancers) are attributable to HPV, making HPV one of the most important infectious causes of cancer.Citation14
HPV vaccine is a major strategy for preventing HPV infection.Citation15 China has approved bivalent (2vHPV), quadrivalent (4vHPV), and 9-valent (9vHPV) vaccines, with 9vHPV being the successor to 4vHPV vaccine. The 2vHPV vaccine prevents infection from HPV16 and 18 genotypes; 4vHPV from HPV6, 11, 16, and 18 genotypes; and 9vHPV from HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 genotypes. Due to the lack of data on HPV genotypes, the rate of HPV vaccination is low and the incidence of HPV infection is increasing in China. Therefore, the detection of HPV genotypes in China will provide valuable information for guiding the development of HPV vaccination policies.
In this epidemiological study, we aimed to assess HPV DNA genotypes to determine the genotype distribution of HPV infection in HPV-positive female patients in China.
Results
HPV infection genotypes
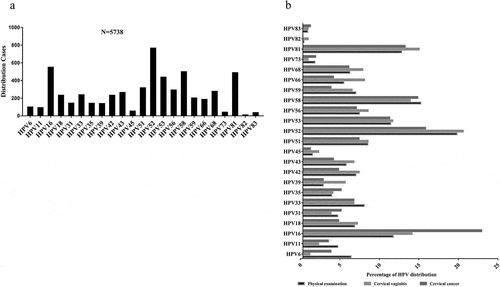
A total of 5738 HPV-positive female patients admitted at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University were enrolled in this study. In total, 23 different HPV genotypes were detected, including 17 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk HPV genotypes (6, 11, 42, 43, 81, and 83). The primary genotypes were HPV52 (13.4%), HPV16 (9.6%), HPV58 (8.8%), HPV81 (8.6%), and HPV53 (7.7%) (). To analyze the genotype distribution of HPV infection in different types of patients, we divided the HPV-positive patients into the voluntary physical examination group, cervicitis group, and cervical cancer group. The main genotypes of HPV infection in the physical examination group were HPV52 (19.5%), HPV58 (14.9%), HPV81 (12.5%), HPV16 (11.4%), and HPV53 (11.1%) patients. Similarly, HPV52 (20.3%), HPV81 (14.7%), HPV16 (13.8%), HPV58 (13.6%), and HPV53 (11.4%) were the main genotypes of HPV infections in cervicitis patients, although at a different distribution. Similarly, we found that HPV16 (22.7%), HPV52 (15.5%), HPV58 (14.6%), HPV81 (12.9%), and HPV53 (11.0%) were also the main genotypes of HPV infections in cervical cancer patients (). These results indicated that HPV52, 16, 58, 81, and 53 were the main genotypes of HPV infection in female patients in Changzhou, China.
Figure 1. HPV genotype distribution in the HPV-positive patients and the physical examination group, the cervicitis group, and the cervical cancer group. (a) HPV52, HPV16, HPV58, HPV81, and HPV53 were the most predominant genotypes in the HPV-positive patients. (b) HPV52, HPV16, HPV58, HPV81, and HPV53 were the main type of HPV infections in all the three groups (the physical examination group, the cervicitis group, and the cervical cancer).
Type of HPV infection
We analyzed the distribution of HPV infections in the physical examination group, the cervicitis group, and the cervical cancer group. As shown in , the proportion of high-risk HPV infection in the physical examination group, cervicitis group, and cervical cancer group was 76.7%, 79.9%, and 80.1%, respectively. Meanwhile, that the proportion of low-risk HPV infection in the physical examination group, cervicitis group, and cervical cancer group was 23.3%, 20.1%, and 19.9%, respectively. Regarding single and multiple genotype infection, the proportion of single HPV genotype infection in the physical examination, cervicitis group, and cervical cancer group was 65.2%, 61.6%, and 70.9%, respectively. Meanwhile, the proportion of multiple HPV genotype infection in the physical examination group, the cervicitis group, and the cervical cancer group was 34.8%, 38.4%, and 29.1%, respectively. These data suggested that high-risk HPV infection and single HPV infection were predominant in HPV-positive female patients (p < .05).
Table 1. Genotype distribution of HPV infection in the physical examination group, the cervicitis group, and the cervical cancer group.
Age distribution of HPV infection
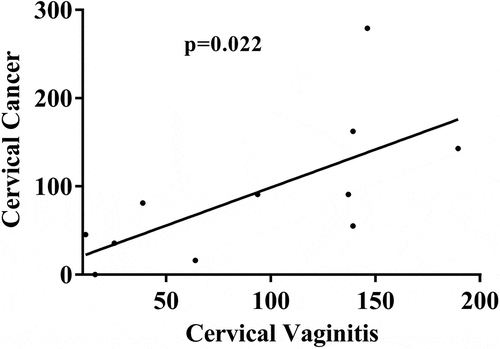
We also analyzed the age of prevalence for HPV infection in HPV-positive female patients and the age distribution of HPV-positive patients in the physical examination group, cervicitis group, and cervical cancer group. As shown in , the age of highest prevalence for HPV infection was 41–45 y (16.6%), and women aged 31–45 accounted for 46.9% of patients. The age of highest prevalence for female HPV infection in the physical examination group, the cervicitis group, and the cervical cancer group was 26–30 y (19.1%), 36–40 y (18.9%), and 41–45 y (27.9%), respectively. In addition, we found a linear positive association between age and HPV-positive cervicitis and between patient age and HPV-positive cervical cancer (P < .05; ). These results showed that the age of highest prevalence for female HPV infection was 31–45 y, and HPV-positive cervicitis increased the risk of cervical cancer.
Table 2. Age of prevalence for HPV-positive patients and in the physical examination group, the cervicitis group, and the cervical cancer group.
Discussion
HPV is the most common sexually transmitted virus, and approximately 75% of sexually active women and men will acquire HPV infection in their lifetime.Citation16 Worldwide, approximately 20 million women have HPV infection.Citation17 HPV infection is an epitheliotropic infection.Citation18,Citation19 Most infections are subclinical and will cause no physical symptoms, but some cases of subclinical infections progress to clinical infections and may cause benign papillomas (such as warts or squamous cell papilloma) or cancers.Citation11,Citation20,Citation21 The HPV infection rate and genotypes vary between countries.Citation22 In our epidemiological study, we investigated the prevalence and genotype distribution of HPV infection in HPV-positive female patients admitted at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University. We found that HPV52, 16, 58, 81, and 53 were the most predominant genotypes in this population. Previous studies have shown that HPV16, 52, 58, 18, and 81 and HPV16, 58, and 52 are the main infectious genotypes in Guangdong and Xinjiang, China, respectively.Citation23,Citation24 We found that HPV53 was also the main HPV genotype in Changzhou, which could support the development of strategies to promote vaccination and help identify the specific vaccine valence needed in this area.
HPV vaccination is widely recommended as an effective strategy against HPV infection and has been recently implemented in most Western countries. The 2vHPV and 4vHPV vaccines are known to prevent most cervical cancers caused by HPV16/18 infection.Citation25 In China, three HPV vaccines are currently available; these are 2vHPV vaccine, 4vHPV vaccine, and 9vHPV vaccine. According to our epidemiological survey, HPV18 is not the main type of HPV infection, but HPV53 is the main type of HPV infection, in Changzhou, China. Therefore, our research can effectively guide local governments to develop HPV vaccination programs for targeted vaccination.
Persistent infection with high-risk HPV genotypes is the primary risk factor for cervical cancer.Citation26,Citation27 Previous studies have shown that the proportion of HPV16 and HPV18 infection is closely related with the incidence of cervical cancer.Citation9 In a Swedish cohort of cervical cancer patients, HPV16, 18, 31, 45, and 33 were the most frequently detected genotypes.Citation28 In this epidemiological study, we found that HPV16 (22.7%), HPV52 (15.5%), HPV58 (14.6%), HPV81 (12.9%), and HPV53 (11.0%) were the common genotypes of HPV infections in HPV-positive cervical cancer patients. Further, our epidemiological study showed that HPV53 accounted for a high proportion of infection in both HPV-positive female patients and HPV-positive cervical cancer patients. Given that HPV vaccines currently do not prevent HPV53 genotype infection, high-risk HPV vaccines should be developed.
A total of 70% of HPV infections are eliminated via autoimmunity within 2 y. However, the persisting infection can induce inflammation and further lead to malignancy. We found that the age of highest prevalence for HPV infection is 41–45 y (16.6%), and women aged 31–45 y accounted for 46.9% of patients. These results may be due to the diverse lifestyle of women at this age and because the rate of HPV screening was higher among women aged 31–45 y. In addition, there was a linear positive association between age and HPV-positive cervicitis and between age and HPV-positive cervical cancer. The findings of our epidemiological study are consistent with the fact that long-term persistent HPV infection can induce inflammation and malignant tumors.
This study also has some limitations. The collected samples were mainly from Changzhou, and the sample size was inadequate to be representative of the entire country. Further, we only detected the expression of 23 HPV genotypes, including 17 high-risk HPV genotypes and 6 low-risk HPV genotypes, which may lead to overestimation of the high-risk HPV genotype in the HPV-positive female patients. In addition, although we found that HPV53 accounted for a high proportion of infection in both HPV-positive female patients and HPV-positive cervical cancer patients, we only detected the expression of 23 HPV genotypes, including 17 high-risk HPV genotypes and 6 low-risk HPV genotypes, and this may have led to overestimation of the high-risk HPV genotype in the HPV-positive female patients. Further research focus on these aspects is needed to confirm these association and develop an effective vaccination policy for HPV infection.
Conclusions
Our epidemiological study showed that HPV52, 16, 58, 81, and 53 were the main genotypes of HPV infection in Changzhou. Further, the age of highest prevalence for HPV infection was 41–45 y, and majority of patients were aged 31–45 y. Moreover, the age of highest prevalence for female HPV infection in patients who undergo physical examination, those with cervicitis, and those with cervical cancer was 26–30 y, 36–40 y, and 41–45 y. These findings will be valuable in developing strategies to promote HPV vaccination in different regions according to population characteristics.
Methods
Study subjects
This study enrolled HPV-positive female patients admitted at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University. The inclusion criteria were as follows: (1) aged from 17–80 y; (2) no previous HPV test, and (3) no history of vaginal drug use, no sexual intercourse 3 d before sample collection, or no previous HPV infection. The patients were divided into 3 groups as follows: the physical examination group comprised the healthy population who had normal physical examination findings; the HPV-positive group comprised patients diagnosed with cervicitis after gynecological examination, white blood cell examination, pathogen test, cervical scraping, and biopsy; and the cervical cancer group comprised HPV-positive patients diagnosed with cervical cancer via cervical smear cytology, cervical iodine test, colposcopy, cervical biopsy, cervical surgery, and pathological screening. This epidemiological study was approved by the Human Ethics Committees of the Affiliated Changzhou No. 2 People’s Hospital, Nanjing Medical University, and written informed consent was obtained from all the patients.
Specimen collection and storage
The cervical epithelial cells were collected using Cervix Epithelial cell Extraction Kit (Yaneng BioScience Co., Ltd. Shenzhen, China). Briefly, specimens were collected by inserting a speculum into the endocervical canal and rotating it unidirectionally four times. The exfoliated cervical epithelial cells then adhere to the flat sides of the bristles. The tip of the cervix brush is then placed into a vial containing transport medium and stored at 4°C.
DNA extraction
DNA from exfoliated cervical cells was collected using a DNA Extraction Kit (Yaneng BioScience Co., Ltd. Shenzhen, China) as per the manufacturer’s instructions. The cervical brush was fully eluted and transferred to a 1.5 ml centrifuge tube and then centrifuged at 13000 rpm for 10 min. After, the supernatant was discarded, and 50 µL of the lysate was added to suspend the pellet. This was then heated in a boiling water bath for 10 min and centrifuged again at 13000 for 10 min.
HPV DNA genotype testing
HPV DNA genotype testing was performed using HPV DNA testing Kit (Yaneng BioScience Co., Ltd. Shenzhen, China) following the manufacturer’s instructions.
The amplification system comprised 5 μl HPV-DNA and 20 μl reaction system (Yaneng BioScience Co., Ltd. Shenzhen, China). HPV was amplified using Roche LightCycler 480 instrument (Roche, USA) following the manufacturer’s guidelines as follows: 50°C for 15 min; 95°C, 10 min; 94°C, 30 sec; 42°C, 90 sec; 72°C, 30 sec (40 cycles); and 72°C, 5 min. HPV genotyping was performed via flow-through hybridization and using gene-chips that contained type-specific oligonucleotides (Yaneng BioScience Co., Ltd. Shenzhen, China). The gene-chip includes 23 type-specific oligonucleotides designed to detect 17 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk HPV genotypes (6, 11, 42, 43, 81, and 83). The final results were determined according to colorimetric change on the chip under direct visualization. Quality controls were performed throughout the experiments, including DNA extracting, amplification, and hybridization by applying positive and negative quality control products as control groups for PCR amplification.
Data analysis
Single and multiple HPV infections were defined as infection with one and with two or more subtypes of HPV infections, respectively. The proportion of women in different age groups with single and multiple infections was then analyzed. Data were compared using Pearson χ2 or Fisher exact tests. Descriptive and inferential statistical analysis were conducted using Statistical Package for the Social Sciences version 22 (SPSS Inc., Illinios, USA), and P < .05 was considered to be statistically significant.
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We would like to thank Pro. Sujian Wang at department of blood transfusion, the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University for his statistic assistance.
References
- Acevedo-Fontanez AI, Suarez E, Torres Cintron CR, Ortiz AP. Risk of anal cancer in women with a human papillomavirus-related gynecological neoplasm: puerto Rico 1987-2013. J Low Genit Tract Dis. 2018;22(3):225–30. doi:10.1097/LGT.0000000000000395.
- Adnan Ali SM, Awan MS, Atif S, Ali N, Mirza Y. Correlation of human papillomavirus infection and clinical parameters with five-year survival in oral squamous cell carcinoma. J Laryngol Otol. 2018;132(7):628–35. doi:10.1017/S0022215118000361.
- Mizukami A, Kaise T, Van Kriekinge G. Resource use and cost of treating human papillomavirus-related lesions in Japanese women. Value Health Reg Issues. 2018;15:56–62. doi:10.1016/j.vhri.2017.07.009.
- Bretagne CH, Jooste V, Guenat D, Riethmuller D, Bouvier AM, Bedgedjian I, Prétet JL, Valmary-Degano S, Mougin C. Prevalence and distribution of HPV genotypes and cervical-associated lesions in sexually active young French women following HPV vaccine. J Gynecol Obstet Hum Reprod. 2018;47:525–31. doi:10.1016/j.jogoh.2018.05.011.
- Ceccarelli G, Cavallari EN, Savinelli S, Bianchi L, Pierangeli A, Vullo F, Ciardi A, D‘Ettorre G. Clearance of human papillomavirus related anal condylomas after oral and endorectal multistrain probiotic supplementation in an HIV positive male: A case report. Medicine (Baltimore). 2018;97:e0329. doi:10.1097/MD.0000000000010329.
- Fuller C, Hudgins E, Finelt N. Human-papillomavirus-related disease in pediatrics. Curr Opin Pediatr. 2018;30:169–74. doi:10.1097/MOP.0000000000000561.
- Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–25. doi:10.1001/jamaoto.2018.0395.
- Zenga J, Pipkorn P, Graboyes EM, Martin EJ, Rich JT, Moore EJ, Haughey BH, Jackson RS. Oncologic outcomes of extended neck dissections in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck. 2018;40:955–62. doi:10.1002/hed.25044.
- Zeferino LC, Bastos JB, Vale D, Zanine RM, Melo Y, Primo WQSP, Corrêa FM, Val ICCD, Russomano F. Guidelines for HPV-DNA Testing for Cervical Cancer Screening in Brazil. Rev Bras Ginecol Obstet. 2018;40(6):360–68. doi:10.1055/s-0038-1657754.
- Long W, Yang Z, Li X, Chen M, Liu J, Zhang Y, Sun X. HPV-16, HPV-58, and HPV-33 are the most carcinogenic HPV genotypes in Southwestern China and their viral loads are associated with severity of premalignant lesions in the cervix. Virol J. 2018;15:94. doi:10.1186/s12985-018-1003-x.
- Serra I, Araujo ED, Barros GS, Santos F, Gurgel RQ, Batista MVA. Prevalence of human papillomavirus types associated with cervical lesions in Sergipe state, Northeastern Brazil: high frequency of a possibly carcinogenic type. Epidemiol Infect. 2018;146:1184–93. doi:10.1017/S095026881800122X.
- Orosco RK, Califano JA. HPV status, like politics, is local-evaluating p16 staining and a new staging system in a Dutch cohort of oropharynx cancer. Ann Oncol. 2018;29:1089–90. doi:10.1093/annonc/mdx807.
- Flanagan MB. Primary High-Risk Human Papillomavirus Testing for Cervical Cancer Screening in the United States: is It Time?. Arch Pathol Lab Med. 2018;142:688–92. doi:10.5858/arpa.2018-0001-RA.
- Mena M, Taberna M, Tous S, Marquez S, Clavero O, Quiros B, Lloveras B, Alejo M, Leon X, Quer M, et al. Double positivity for HPV-DNA/p16(ink4a) is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral Oncol. 2018;78:137–44. doi:10.1016/j.oraloncology.2018.01.010.
- Huang SH, O‘Sullivan B, Waldron J. The current state of biological and clinical implications of human papillomavirus-related oropharyngeal cancer. Semin Radiat Oncol. 2018;28:17–26. doi:10.1016/j.semradonc.2017.08.007.
- Williamson AL, Garland S, Palefsky J, Rybicki E, Stanley M, de Sanjose S. The Cape Town declaration on human papillomavirus related disease. Papillomavirus Res. 2018;5:59–60. doi:10.1016/j.pvr.2017.12.003.
- Vazquez-Otero C, Vamos CA, Thompson EL, Merrell LK, Griner SB, Kline NS, Catalanotto FA, Giuliano AR, Daley EM. Assessing dentists’ human papillomavirus-related health literacy for oropharyngeal cancer prevention. J Am Dent Assoc. 2018;149:9–17. doi:10.1016/j.adaj.2017.08.021.
- Stiebing BN, Rosado FG, Vos JA. Human papillomavirus-related malignancies in the setting of posttransplantation immunosuppression. Arch Pathol Lab Med. 2018;142:711–14. doi:10.5858/arpa.2017-0586-RA.
- Siddiq S, Cartlidge D, Stephen S, Sathasivam HP, Fox H, O’Hara J, Meikle D, Iqbal MS, Kelly CG, Robinson M, et al. Robotic lateral oropharyngectomy following diagnostic tonsillectomy is oncologically safe in patients with high risk human papillomavirus related squamous cell cancer. Eur Arch Otorhinolaryngol. 2018;275:1853–60. doi:10.1007/s00405-018-4968-6.
- Shah AA, Lamarre ED, Bishop JA. Human papillomavirus-related multiphenotypic sinonasal carcinoma: A case report documenting the potential for very late tumor recurrence. Head Neck Pathol. 2018;12(4):623–28. doi:10.1007/s12105-018-0895-5.
- Osazuwa-Peters N, Arnold LD, Loux TM, Varvares MA, Schootman M. Factors associated with increased risk of suicide among survivors of head and neck cancer: A population-based analysis. Oral Oncol. 2018;81:29–34. doi:10.1016/j.oraloncology.2018.03.017.
- Ozkaya O, Karsidag S, Canli M, Soydan T, Sakiz D. A rare anatomical location of human papillomavirus-related penile warty carcinoma: simultaneous axillary involvement. Indian J Dermatol Venereol Leprol. 2018;84:336–38.
- Zhao P, Liu S, Zhong Z, Hou J, Lin L, Weng R, Su L, Lei N, Hou T, Yang H. Prevalence and genotype distribution of human papillomavirus infection among women in northeastern Guangdong Province of China. BMC Infect Dis. 2018;18:204. doi:10.1186/s12879-018-3109-6.
- Wang J, Tang D, Wang J, Zhang Z, Chen Y, Wang K, Zhang X, Ma C. Genotype distribution and prevalence of human papillomavirus among women with cervical cytological abnormalities in Xinjiang, China. Hum Vaccin Immunother. 2019. doi:10.1080/21645515.2019.1578598.
- Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199:919–22. doi:10.1086/598985.
- Owosho AA, Wiley R, Stansbury T, Gbadamosi SO, Js R. Trends in human papillomavirus-related oropharyngeal squamous cell carcinoma incidence, Vermont 1999-2013. J Community Health. 2018;43(4):731–37. doi:10.1007/s10900-018-0477-1.
- Robadi IA, Pharaon M, Ducatman BS. The importance of high-risk human papillomavirus types other than 16 and 18 in cervical neoplasia. Arch Pathol Lab Med. 2018;142:693–95. doi:10.5858/arpa.2017-0563-RA.
- Kaliff M, Sorbe B, Mordhorst LB, Helenius G, Karlsson MG, Lillsunde-Larsson G. Findings of multiple HPV genotypes in cervical carcinoma are associated with poor cancer-specific survival in a Swedish cohort of cervical cancer primarily treated with radiotherapy. Oncotarget. 2018;9:18786–96. doi:10.18632/oncotarget.v9i27.