ABSTRACT
Canada eliminated measles in 1998. We conducted a sero-epidemiology study to estimate population immunity to measles in the province of Ontario, Canada and to identify groups at higher risk of outbreaks. We used a previously developed modified enzyme immunoassay to test 1,199 residual sera from patients aged 1–39 years. We re-tested negative and equivocal sera using a plaque reduction neutralization assay. We interpreted our results in the context of Ontario’s immunization program and vaccine coverage data. Of 1,199 sera, 1035 (86.3%, 95% confidence interval (CI) 84.4, 88.2) were above the measles threshold for protection, 70 (5.8%, 95% CI 4.5, 7.2) were equivocal and 94 (7.8%, 95% CI 6.3, 9.4) were negative. The proportion of positive sera was highest for those 1–5 years, with 180/199 (90.5%, 95% CI 86.4, 94.5) positive sera, and lowest for those age 12–19 years, at 158/199 (79.4%, 95% CI 73.8, 85.0). Adjusted for age, females were more likely than males to have antibody titers above the threshold of protection (odds ratio = 1.60, 95% CI 1.14, 2.24). Most of the study cohort were eligible for two measles vaccine doses, and vaccine uptake in Ontario is >90% for school-aged cohorts. We observed a higher than expected proportion of sera with antibody levels below the threshold of protection, suggesting that immunity in some Ontario age-groups may be waning, despite high vaccine coverage. Alternatively, the traditional measles correlates of protection may not be an appropriate measure of population protection in measles-eliminated settings.
Introduction
Measles poses a major public health challenge as it is one of the most infectious diseases known,Citation1 requiring higher population immunity to achieve control than most other pathogens. In Canada, endemic transmission of measles was eliminated in 1998,Citation2 meaning that chains of transmission due to importation since then have terminated within <12 months.Citation3 To sustain measles elimination, a sufficient proportion of the population must be protected, either through previous infection or vaccine-induced immunity. This proportion is termed the “herd immunity threshold”.Citation4,Citation5 Herd immunity can protect those who are susceptible, including infants too young to be vaccinated, individuals with medical conditions that preclude measles vaccination, or those who choose not to be vaccinated.Citation1 However, if population immunity falls below this threshold, measles importations may lead to sustained endemic transmission.
Since measles is very transmissible, with a basic reproduction number (R0) of approximately 12–18,Citation6 and two-dose measles vaccine effectiveness is approximately 95%, immunization coverage targets are usually at least 95% in children.Citation7,Citation8 Measles vaccination is usually administered using the measles-mumps-rubella (MMR) vaccine, with a varicella antigen sometimes included (MMRV). Maintaining this high level of immunity is a challenge, making it important to identify susceptible cohorts and evaluate immunization programs.Citation5,Citation9 This can be difficult in Canada, where each of the 10 provinces and three territories determines its own schedule for administering vaccinations.Citation10,Citation11 There is no national vaccine registry, and each province and territory maintains its own immunization information system.Citation12 These vary considerably and often focus on specific age groups, such as school-aged children,Citation13 rather than the entire population. The Canadian population is very heterogeneous between and within provinces. Foreign-born individuals, who may have different measles immunity levels than those born in Canada,Citation14,Citation15 constitute over 20% of the population of Canada, rising above 50% in some metropolitan areas.Citation16 However, since many newcomers to Canada arrive as adults, a large proportion of the population is not included in coverage data. Furthermore, as there may be either primary or secondary vaccine failure,Citation17 vaccine coverage data may not reflect a population’s current immune status.
One way to address these challenges is through sero-epidemiology, which assesses population immunity by performing epidemiological analyses of cross-sectional antibody prevalence surveys.Citation5,Citation18 Sero-epidemiology is a particularly useful tool for vaccine-preventable diseases to enable evaluation of immunization programs, including optimal timing, the number of doses administered, vaccine coverage and vaccine failure. The objective of our study was to use sero-epidemiology to estimate population immunity to measles in Ontario, Canada (Canada’s most populous province), and determine which groups may be at higher risk of measles outbreaks.
Results
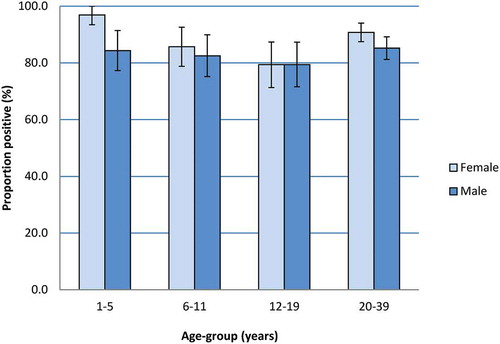
We included 1,199 sera in our study, half (600 or 50.0%) of which were from individuals 20–39 years (). The remainder were evenly distributed among the three younger age-groups. The proportion of sera from males and females was similar in each age-group (). Of the 1,199 sera tested using the BioPlex platform, 954 were positive. We tested the remaining 245 (20.4%) samples, which were equivocal (n = 210) or negative (n = 35) using BioPlex, by plaque reduction neutralization test (PRNT) to confirm their immunity status. Of these, 81, 70 and 94 sera were significantly above the threshold of protection, equivocal or below the threshold using PRNT, respectively (). Taking PRNT results over BioPlex results when available, 1035 sera overall (86.3%, 95% confidence interval (CI) 84.4, 88.2) had antibody titers above the threshold of protection, 70 sera (5.8%, 95% CI 4.5, 7.2) had equivocal antibody levels, and 94 sera (7.8%, 95% CI 6.3, 9.4) had a level of antibody significantly below the threshold of protection (). In persons under 20 years, the proportion of sera with antibody levels above the threshold of protection decreased linearly for each age-group (Cochran–Armitage test for trend two-sided p-value = .002) (). We found the highest proportion of positive sera in individuals aged 1–5 years, with 90.5% (95% CI 86.4, 94.5) samples above the threshold of protection. In comparison, the proportion of sera with antibody levels above the threshold of protection decreased in those age 6–11 years to 84.1% (95% CI 79.0, 89.1) (Chi2 p = .37), and in those age 12–19 years to 79.4% (95% CI 73.8, 85.0) (Chi2 p = .003).
Table 1. Characteristics of study sera
Table 2. BioPlex and PRNT test results. We first tested all sera using BioPlex, and then re-tested sera that with equivocal or negative BioPlex results using PRNT, taking PRNT results over BioPlex when available
Table 3. Number and proportion of sera that were above, equivocal or below the threshold of protection for measles virus antibody by age-group, Ontario 2013–2014
Figure 1. Measles antibody prevalence by age-group, Ontario 2013–2014. Figure 1 shows the proportion of sera that were above, equivocal or below the threshold of protection for measles virus antibody as well as BioPlex geometric mean titer (GMT) values for each age-group, along with GMT 95% confidence intervals (95% CI)
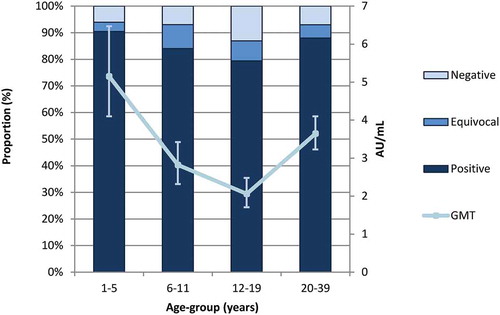
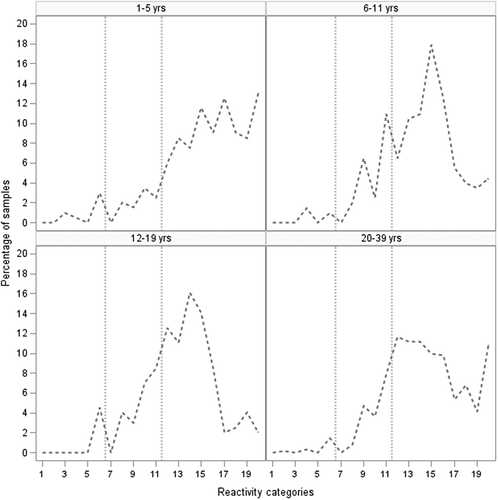
In adults aged 20–39 years, the proportion of positive sera was higher than in some of the younger age-groups, with 88.0% (95% CI 85.4, 90.6) of samples above the threshold of protection. When comparing to the youngest age-group, this difference did not reach statistical significance (Chi2 p = .17). Sera from the youngest and oldest age-groups had the lowest proportion of equivocal sera. Age-specific trends in geometric mean titer (GMT) values calculated using the BioPlex results were similar in pattern, with substantially different values for each age-group (). The GMT decreased from 5.15 antibody units (AU)/mL (95% CI 4.10, 6.47) in children 1–5 years to 2.82 AU/mL (95% CI 2.32, 3.42) in children 6–11 years (Tukey’s pairwise ANOVA p < .0001). There was a further decrease to 2.06 AU/mL (95% CI 1.71, 2.48) in those 12–19 years compared to those 1–5 years (Tukey’s pairwise ANOVA p < .0001). In adults age 20–39, the GMT values were higher, at 3.64 AU/mL (95% CI 3.23, 4.10); however, this difference was still statistically significant compared to the youngest age-group (Tukey’s pairwise ANOVA p = .02). When observing the distribution of titers grouped by reactivity band (), the highest proportion of antibody levels in all age-groups was within the protective range. However, the peak proportions in each age-group shifted slightly downwards as age increased. While for those aged 1–5 years, reactivity band 20 had the highest proportion of samples, this peak frequency decreased to reactivity categories 15 and 14 in those aged 6–11 years and 12–19 years, respectively, suggesting less robust humoral immunity. Sera from individuals aged 20–39 years exhibited a bimodal peak at reactivity categories 12–14 and 20.
Figure 2. Distribution of measles antibody levels in each age-group as measured by BioPlex, Ontario 2013–14. The antibody titer distributions for each age-group are shown. To display the antibody titers we used reactivity categories, which are generated by dividing the log-transformed antibody titers into 20 bands of equal width. We then graphed the resulting range of titers. The vertical-dotted lines mark the equivocal range of ≥0.13 AU/mL and <1.10 AU/mL. Sera to the left of this zone are below the laboratory determined threshold of protection, while sera to the right are above it
When grouping positive and equivocal sera to investigate the proportion of individuals exposed to either measles vaccination or infection, the differences in proportion between age-groups were greatly reduced, with 187/199 (94.0%, 95% CI 90.7, 97.3) of sera from individuals aged 1–5 years, 187/201 (93.0%, 95% CI 89.5, 96.6) of sera from individuals aged 6–11 years, and 558/600 (93.0%, 95% CI 91.0, 95.0) of sera from individuals aged 20–39 years either equivocal or positive. The exception was sera from individuals aged 12–19 years, which had lower proportions classified as positive or equivocal, at 173/199 (86.9%, 95% CI 82.3, 91.6)
The proportion of sera with antibody titers above the threshold of protection varied by sex (). Overall, it was higher in females, with 530/595 (89.1%, 95% CI 86.6, 91.6) samples above the threshold of protection, compared to 505/604 (83.6%, 95% CI 80.7, 86.6) sera from males (Chi2 p = .006). This difference was most pronounced in the youngest age-group, in which 94/97 sera (96.9%, 95% CI 93.5, 100.0) from females were positive, compared to only 86/102 sera (84.3%, 95% CI 77.3, 91.4) in males. Children aged 12–19 years was the only age-group in which the proportion of positive sera from females was not higher than from males (both 79.4%). When controlling for age, we found that females were still more likely to have antibody levels above the measles threshold for protection compared to males (odds ratio = 1.60, 95% CI 1.14, 2.24).
Discussion
We have characterized population immunity to measles in residents of Ontario aged 1–39 years, a mostly-vaccinated population in an elimination setting. In Canada, the first measles vaccine was licensed in 1963Citation2 and, in 1983, an MMR immunization program was introduced for infants, with administration at 12 months of age.Citation19 A second dose of routine MMR was added to the program in 1996, and a large catch-up campaign provided a second measles vaccine dose to all school-aged children. The sera in our study were derived from individuals eligible for two doses of measles-containing vaccine, as per Ontario’s routine childhood immunization schedule or the 1996 measles catch-up campaign, apart from sera from individuals born in 1975 − 1976 and 2011–2013 (). Since the second dose of measles-containing vaccine is administered at age 4–6 years, it is possible that although eligible, some children born in 2008–2010 have yet to receive their second vaccine dose.
Table 4. Vaccine program eligibility and assessment of vaccine coverage for birth cohorts included in our study. percentage range indicates the minimum and maximum values for two-dose coverage estimate data from included birth-years
We observed a lower than expected proportion of sera above the measles threshold of protection, particularly in older children and teens. Sera from individuals 1–5 years of age had higher immunity levels and GMTs than older age groups, presumably due to recent vaccination with a first or second dose of vaccine. Older age-groups had a lower proportion of sera above the threshold of protection, and lower GMTs. Of most concern was the 12–19-year age-group. In this cohort, only 79.4% of sera were above the threshold of protection, and a further 7.5% were equivocal. Although we know that vaccination coverage in this group is very high, ranging from 90.4% to 96.7% for two doses of measles-containing vaccine,Citation13,Citation21,Citation22 many in this cohort were born into an elimination setting and are unlikely to have been exposed to measles antigens apart from during vaccination, which may result in waning immunity.Citation24,Citation25 Mixture modeling has been advocated as a useful tool to interpret the underlying “mixture” of immunity distributions within certain age groups, by categorizing serological test results into varying distributions (e.g., naturally infected individuals, vaccinated individuals, vaccinated individuals with antibody decay, susceptible individuals) that best reflect the different levels of reactivity observed within the population.Citation18 In the absence of such an analytic approach, a further indication of waning immunity in this study is the low GMTs in this group, with many individual sera titers only slightly above the threshold of protection. In the oldest age-group in our study (20–39 years), 88.0% of sera were positive, substantially higher than the levels in older children and teens. The proportion of samples with antibody titers well above the threshold of protection in this group, and two peaks in reactivity categories, one between reactivity categories 12–14, and one at the maximum reactivity category of 20 suggest a population with mixed vaccine- and infection-induced immunity.Citation25
We observed higher population immunity in females compared to males. Although this has been reported by other post-vaccination,Citation25,Citation26 the significance of this is unclear in relation to our study. In the 20–39 years age-group this difference is possibly related to pre- or post-natal MMR vaccine for rubella-susceptible women, or a higher proportion of female health-care workers submitting sera for immunity screening, who are more likely to be vaccinated. Interestingly, we did not see this phenomenon in those aged 12–19 years. It is possible that the sex difference in immunity would have been diminished had our sample size been larger.
Our results are concordant with recent findings from other jurisdictions that have eliminated measles. Gidding and colleagues used residual sera to perform a serosurvey in Australia in 2012–13, and found that the proportion of sera above the threshold of protection was lowest in older children and teens, who also had the largest proportions of equivocal sera.Citation27 Seagle et al. performed a longitudinal cohort study in the United States (US) and found that antibody titers of vaccinees declined by 6.3–9.7% per year after their second dose of MMR. However, despite this decline only a small proportion of seropositive participants became seronegative by the end of the 12-year follow-up period.Citation17 Interestingly, another US study, which used sera from individuals recruited into a study in 2009–10, found higher seroprevalence estimates than those reported in our study, with the lowest proportion of sera above the threshold of positivity in adults aged 30–39 years.Citation28 It is possible that this difference is due to varying testing algorithms and/or measles thresholds of protection between the studies, or a different population composition in the US, for example, relating to place of birth or socio-economic status, than that of our study.
The results of our study suggest that antibody titers to measles may be waning in Ontario residents. If this is the case, the lack of large measles outbreaks in Ontario is somewhat surprising. There are several possible explanations for our results. If some age-groups are experiencing waning immunity, this raises concerns for future measles control. However, it is possible that despite waning antibody titers, at least some of these individuals may be protected from measles by cellular responses or other immune mechanisms if exposed.Citation25,Citation29 The lack of large measles outbreaks in Ontario and high vaccine coverage estimates support this hypothesis. However, coverage estimates are not available for the oldest individuals in our cohort or foreign-born individuals who arrived in adulthood. Furthermore, although the risk of measles in Ontario is highest for those who are un-immunized, cases occasionally occur among even two-dose recipients.Citation30 Even if those with lower antibody titers are still protected from measles, inadequate humoral immunity in women of child-bearing age is still a concern. Since infants are protected from measles through transplacental antibody transfer, lower titers in these women will result in lower titers (and therefore increased susceptibility) in their infants, which is particularly important during their first year of life prior to MMR immunization.Citation31
A second possible explanation is that the sera used in our study are not representative of the general population. This may particularly be true for pediatric sera. This would explain the contrast between the lower than expected level of population immunity in the younger age-groups of our study and the high measles vaccine coverage in Ontario. Residual sera are routinely used for serosurveys in other jurisdictions, and have been shown by others to be comparable to results from sera collected from individuals recruited into a study for some infectious diseases, including measles.Citation32 A comparison between these methods in the Canadian context would be useful to clarify this issue.
Another possibility is that despite low antibody levels in our study sample, immunity is sufficiently high in Ontarians who are older or foreign-born, and are more likely to have robust measles immunity levels through previous infection. These individuals may contribute to a sufficient level of herd-immunity to avoid chains of measles transmission upon measles importation from abroad.Citation33 This could explain why, despite repeated importations, Ontario has not experienced sustained transmission or large outbreaks. Ontario is an international travel hub and travelers arriving from measles-endemic countries at international airports in Toronto and Ottawa (Ontario’s largest cities) frequently expose Ontario residents to measles through importation of virus.Citation33 However, the majority of international travelers remain in large cities, which have a high-proportion of foreign-born residents, to tour or visit family and friends rather than to travel to other Ontario regions. Nearly half of Toronto’s residents and one quarter of Ottawa’s residents are foreign-born, with large groups of immigrants from measles endemic countries such as India, China, and Philippines.Citation34 It is possible that these travel patterns contribute to the lack of large measles outbreaks.
The last possibility is that population immunity data contradict coverage data and disease incidence data because the currently accepted correlate of protection, determined several decades ago in a measles-endemic setting,Citation35 is not optimal and may need to be re-evaluated in measles-eliminated settings, particularly when conducting serosurveys.Citation27 In the context of waning vaccine-mediated humoral immunity, other immune parameters (i.e., – either cell-mediated immunityCitation25 or antibody avidityCitation36,Citation37) may prove to be useful measures to estimate individual or population-level protection, either in combination or instead of an absolute humoral correlate of protection. Furthermore, as has been suggested by others, perhaps lower population immunity may be acceptable in elimination settings provided that immunity is high in some age-groups, specifically school-aged children, who have the largest role in transmission.Citation38
The use of PRNT as a reference method for all sera that tested negative or equivocal using the BioPlex is a strength of our study. This approach differs from that of many other sero-epidemiology networks, including most European,Citation39–Citation41 Australian,Citation41 and USCitation28 serosurveys, which use solely an EIA to test sera. Although this approach is sufficient in some settings, we believe it is not optimal in settings where many individuals exhibit lower titers, clustered around the threshold of protection.Citation42,Citation43 We therefore chose to incorporate PRNT into our testing to ensure that we obtained sensitive and accurate test results from our serum collection, that likely represented mostly vaccinated individuals in a measles elimination setting, and may therefore exhibit lower antibody titers.Citation25 Since PRNT is labor intensive and not feasible to perform in every laboratory setting, we believe that our testing algorithm, which mixes EIA and PRNT testing, maximizes the sensitivity of our results but still remains efficient and cost-effective. Interestingly, while 32/35 (91.4%) sera that were negative by BioPlex remained negative when tested using PRNT, 81/210 (38.6%) of the sera that were equivocal by BioPlex were positive using PRNT, raising the proportion of our sera with antibodies above the threshold of protection by approximately 7% compared to BioPlex results alone, thus demonstrating the utility of PRNT reference testing in our setting.
There are several limitations to our study. We did not characterize measles population immunity in those age 40 years and older. Although we assumed that people born before 1970 (corresponding to those 43 years and older in our study) are likely immune through previous infection, estimates of immunity that included those >40 years would have been optimal. Since residual sera lack associated data, it was not possible to determine individual vaccination status or to ascertain whether individuals were foreign-born and may have come from jurisdictions with different measles epidemiology and/or vaccine schedules. The age-groups for the sera used in our study were originally chosen to enable comparisons to another Ontario serosurvey with a different sample source.Citation44 These broad age-groups did not allow us to differentiate between cohorts with varying vaccination eligibility. For example, the 1–5 years age-group includes children eligible for either one or two doses or measles vaccine (). As described above, there may be selection bias in this kind of sampling. Residual sera may be derived from populations being screened for immunity, such as health-care workers and prenatal women, who may be healthier than the general population. Conversely, there may be a selection bias towards populations with co-morbidities, who may exhibit suppressed immune responses, or different reasons for laboratory testing. This is particularly true for pediatric sera, since healthy children seldom have blood drawn. Lastly, the relatively small sample size, which limits our ability to generate estimates standardized to the population of Ontario, and the fact that serum samples originally submitted for measles immunity testing were not removed from eligibility for our study, are also limitations.
Our findings underscore the importance of maintaining high vaccine coverage, disease surveillance and ongoing serosurveillance to avoid outbreaks and sustain elimination.
Methods
Population and sample source
We used anonymized residual sera from Ontario for this study. Ontario is Canada’s largest province, and its population of nearly 14.2 million residents comprised 39% of Canada’s population in 2017.Citation45 Sera were collected between November 2013 and May 2014 from a large private diagnostic laboratory, which conducts much of the physician-requested outpatient testing in the province, as well as testing of hospitalized patients. Any serum sample with a minimum volume of 2 mL, collected in a serum separator tube, and processed within 48 h was eligible for archiving. Information provided with the serum included age group, sex, and region in Ontario where the sample was collected. We used sera from individuals aged 1–39 years, as we assumed most of those born before 1970 (aged over 43 years of age at the time these sera were collected) would have immunity through previous infection, consistent with advice outlined in Canada’s national measles control guidelines.Citation10 We used sera from approximately equal proportions of males and females, in four age-groups (1–5 years, 6–11 years, 12–19 years and 20–39 years), collected in these specific age-groups to enable comparison to another serum sources. We aimed to include samples from throughout Ontario.
Sample size calculations
Sample size calculations indicate that for 200 sera, the precision of immunity estimates would be ±3.4% if immunity was 95%, ±5.4% if immunity was 85%, and ±6.5 if immunity was 75%, with a type I error rate of α = 0.05. For 600 sera the precision of estimates would be ±1.9%, ±3.0%, or ±3.6% for immunity of 95%, 85% or 75%, respectively. Sample size calculations were performed using PASS 13 sample size software (Kaysville, Utah, US).
Laboratory methods
We tested all sera using the BioPlex 2200 MMRV IgG assay (Bio-Rad Laboratories), which is a microsphere-based enzyme immunoassay (EIA). Sera with negative (<0.13 AU/mL) or equivocal (≥0.13 and <1.10 AU/mL) results using the BioPlex assay were tested using PRNT, which is the gold-standard reference test for measles antibody, according to methods previously described by others.Citation42 Sera with levels of 112–192 mIU/mL using PRNT were considered equivocal. Titers above this range were considered above the threshold for protection,Citation35 and all those below were considered to be susceptible, as per a previously published in-house validation using the World Health Organization (WHO) 3rd Anti-Measles International Standard.Citation43
Epidemiological analysis
We performed descriptive analyses to investigate the proportion of sera that had a concentration of anti-measles antibodies above the threshold of protection (i.e.,- seropositive in the BioPlex assay or with PRNT titer>192 mIU/mL).Citation43 We calculated the proportion of sera classified as positive, negative and equivocal by age-group, and by age-group and sex. In addition, we also calculated proportions with positive and equivocal sera combined, because although equivocal sera have antibody titers below the threshold of protection, these sera contain detectable antibody titers that suggest measles exposure or more likely, vaccination. To generate 95% Wald CIs for proportions we assumed a binomial approximation, unless the cell count was less than five, in which case we used exact methods. We used chi-squared testing to investigate differences in proportions between groups and Cochran-Armitage tests to determine whether there was a trend in immunity by age-group. We performed all statistical analysis using SAS version 9.3 (The SAS Institute, Cary, NC, US) software.
We used logistic regression analysis to investigate differences in measles immunity between males and females, adjusting for age group. Immunity status was defined as immune (above the threshold of protection) or non-immune (either negative or equivocal).
To generate GMTs we used BioPlex results only expressed as AU/mL. We took this approach because using the 3rd Anti-Measles international standard for our laboratory validation yields titers that are approximately two-fold higher for EIAs compared to PRNT,Citation46 preventing us from reporting BioPlex titers in mIU/mL with confidence. We therefore focused on BioPlex results alone, as this was the only method used to test every sample. The BioPlex platform rounds all test results to one decimal point, resulting in test values of 0 AU/mL for any titer <0.05 AU/mL. We therefore replaced any antibody results of 0 AU/mL with random values that were <0.05 using a beta distribution, with parameters of ∝ = 5 and β = 1. We then divided the log10-transformed antibody titers into 20 bands of equal width (referred to as reactivity categories), and graphed the resulting range of titers.Citation18
To interpret our findings, we triangulated our epidemiological analyses with data from other Ontario sources on vaccine program eligibility and immunization coverage.Citation13,Citation19–Citation23 The routine surveillance of immunization coverage in Ontario is focused on school pupils, using immunization information collected at the local level, with centralized analysis at the provincial level. Some changes in methodology, including age groups of focus, have occurred over time. More recently, the analysis and reporting of immunization coverage have focussed on the key milestone ages of 7 and 17 years-of-age, in accordance with Canadian recommendations.
Ethics
Our study received ethics approval from the Public Health Ontario Ethics Review Board, as well as the University of Manitoba Health Research Ethics Board.
Disclosure of potential conflicts of interest
There are no conflicts of interest to declare.
Additional information
Funding
References
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford, New York: Oxford University Press; 2010.
- King A, Varughese P, De Serres G, Tipples GA, Waters J. Working group on measles elimination. Measles elimination in Canada. J Infect Dis. 2004;189:S236–42.
- Dabbagh A, Patel MK, Dumolard L, Gacic-Dobo M, Mulders MN, Okwo-Bele JM, Kretsinger K, Papania MJ, Rota PA, Goodson JL. Progress toward regional measles elimination - worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep. 2017;66:1148–53. doi:10.15585/mmwr.mm6642a6.
- Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi:10.1093/oxfordjournals.epirev.a036121.
- Durrheim DN. Measles elimination, immunity, serosurveys and other immunity gap diagnostic tools. J Infect Dis. 2018;218:341–43. doi:10.1093/infdis/jiy138.
- Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. The basic reproduction number (R0) of measles: A systematic review. Lancet Infect Dis. 2017;17:e420–e428. doi:10.1016/S1473-3099(17)30307-9.
- Public Health Agency of Canada. Vaccination coverage goals and vaccine preventable disease reduction targets by 2025; 2017. [accessed 2018 Oct 5] https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html#1.1.3;.
- Pan American Health Organization (PAHO), World Health Organization (WHO) Regional Office for the Americas. 29th Pan American Sanitary Conference. 69th session of the Regional Committee of WHO for the Americas. Washington (DC): Pan American Health Organization. 2017 Sep 25–29. Final Report.
- Dimech W, Mulders MN. A 16-year review of seroprevalence studies on measles and rubella. Vaccine. 2016;34:4110–18. doi:10.1016/j.vaccine.2016.06.002.
- Public Health Agency of Canada (PHAC). Canadian immunization guide, Part 4, active vaccines, measles vaccines. Ottawa (ON): Government of Canada; 2015.
- Government of Canada. Canada’s provincial and territorial routine (and catch-up) vaccination programs for infants and children. 2018. [accessed 2019 May 27] https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html
- Wilson SE, Quach S, MacDonald SE, Naus M, Deeks SL, Crowcroft NS, Mahmud SM, Tran D, Kwong JC, Tu K. Immunization information systems in Canada: attributes, functionality, strengths and challenges. A Canadian immunization research network study. Can J Public Health. 2017;107:575–82. doi:10.17269/CJPH.107.5679.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Immunization coverage report for school pupils in Ontario: 2013–14, 2014–15 and 2015–16 School Years. Toronto: Queen’s Printer for Ontario; 2017.
- Ng E, Sanmartin C, Elien-Massenat D, Manuel DG. Vaccine-preventable disease-related hospitalization among immigrants and refugees to Canada: study of linked population-based databases. Vaccine. 2016;34:4437–42. doi:10.1016/j.vaccine.2016.06.079.
- Greenaway C, Dongier P, Boivin JF, Tapiero B, Miller M, Schwartzman K. Susceptibility to measles, mumps, and rubella in newly arrived adult immigrants and refugees. Ann Intern Med. 2007;146:20–24.
- Ontario Ministry of Finance. Office of economic policy labour and demographic analysis branch. 2011 National household survey highlights. Vol. 2018. 2013. p. 2. [accessed 2018 Oct 18] https://www.fin.gov.on.ca/en/economy/demographics/census/nhshi11-1.html
- Seagle EE, Bednarczyk RA, Hill T, Fiebelkorn AP, Hickman CJ, Icenogle JP, Belongia EA, McLean HQ. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine. 2018;36:818–26. doi:10.1016/j.vaccine.2017.12.075.
- Vyse AJ, Gay NJ, Hesketh LM, Pebody R, Morgan-Capner P, Miller E. Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol Infect. 2006;134:1303–12. doi:10.1017/S0950268806006340.
- Canadian Public Health Association. Immunization timeline; 2018. [accessed 2018 Apr 26] https://www.cpha.ca/immunization-timeline.
- Public Health Agency of Canada. Vaccine coverage in canadian children: results from the 2013 childhood national immunization coverage survey (CNICS). Ottawa, ON: Public Health Agency of Canada; 2016.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Immunization coverage report for school pupils in Ontario: 2016–17 School year. Toronto: Queen’s Printer for Ontario; 2018.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Immunization coverage report for school pupils: 2012–13 School year. Toronto: Queen’s Printer for Ontario; 2014.
- Ontario Ministry of Health and Long-Term Care, Public Health Division. Immunization coverage report for school pupils - school years 2004/05 to 2007/08. Queen’s printer for Ontario; 2009.
- Whittle H, Aaby P, Samb B, Cisse B, Kanteh F, Soumare M, Jensen H, Bennett J, Simondon F. Poor serologic responses five to seven years after immunization with high and standard titer measles vaccines. Pediatr Infect Dis J. 1999;18:53–57.
- World Health Organization (WHO). The immunological basis for immunization series module 7: measles. Geneva, CH: WHO Press; 2009.
- Green MS, Shohat T, Lerman Y, Cohen D, Slepon R, Duvdevani P, Varsano N, Dagan R, Mendelson E. Sex differences in the humoral antibody response to live measles vaccine in young adults. Int J Epidemiol. 1994;23:1078–81. doi:10.1093/ije/23.5.1078.
- Gidding HF, Quinn HE, Hueston L, Dwyer DE, McIntyre PB. Declining measles antibodies in the era of elimination: Australia’s experience. Vaccine. 2018;36:507–13. doi:10.1016/j.vaccine.2017.12.002.
- Lebo EJ, Kruszon-Moran DM, Marin M, Bellini WJ, Schmid S, Bialek SR, Wallace GS, McLean HQ. Seroprevalence of measles, mumps, rubella and varicella antibodies in the United States population, 2009–2010. Open Forum Infect Dis. 2015;(2):ofv006. doi:10.1093/ofid/ofv006.
- Bautista-Lopez N, Ward BJ, Mills E, McCormick D, Martel N, Ratnam S. Development and durability of measles antigen-specific lymphoproliferative response after MMR vaccination. Vaccine. 2000;18:1393–401.
- Thomas S, Hiebert J, Gubbay JB, Gournis E, Sharron J, Severini A, Jiaravuthisan M, Shane A, Jaeger V, Crowcroft NS, et al. Measles outbreak with unique virus genotyping, Ontario, Canada, 2015. Emerg Infect Dis. 2017;23:1063–69. doi:10.3201/eid2307.161145.
- Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi:10.1136/bmj.c293.
- Kelly H, Riddell MA, Gidding HF, Nolan T, Gilbert GL. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine. 2002;20:3130–36.
- Wilson SE, Khan K, Gilca V, Miniota J, Deeks SL, Lim G, Eckhardt R, Bolotin S, Crowcroft NS. Global travel patterns and risk of measles in Ontario and Quebec, Canada: 2007–2011. BMC Infect Dis. 2015;15:341–015–1039–0. doi:10.1186/s12879-015-1039-0.
- Statistics Canada. Census profile, 2016 census. 2017. Statistics Canada Catalogue no. 98-316-X2016001.
- Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–42. doi:10.1093/infdis/162.5.1036.
- Sowers SB, Rota JS, Hickman CJ, Mercader S, Redd S, McNall RJ, Williams N, McGrew M, Walls ML, Rota PA, et al. High concentrations of measles neutralizing antibodies and high-avidity measles IgG accurately identify measles reinfection cases. Clin Vaccine Immunol. 2016;23:707–16. doi:10.1128/CVI.00268-16.
- Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J Infect Dis. 2012;206:1542–48. doi:10.1093/infdis/jis568.
- Funk S, Knapp J, Lebo E, Reef S, Dabbagh A, Kretsinger K, Jit M, Edmunds WJ, Strebel PM. Target immunity levels for achieving and maintaining measles elimination. bioRxiv. 2017;201574. http://dx.doi.org/10.1101/201574
- de Melker H, Pebody RG, Edmunds WJ, Levy-Bruhl D, Valle M, Rota MC, Salmaso S, van Den Hof S, Berbers G, Saliou P, et al. The seroepidemiology of measles in Western Europe. Epidemiol Infect. 2001;126:249–59.
- Del Fava E, Shkedy Z, Bechini A, Bonanni P, Manfredi P. Towards measles elimination in italy: monitoring herd immunity by Bayesian mixture modelling of serological data. Epidemics. 2012;4:124–31. doi:10.1016/j.epidem.2012.05.001.
- Tischer A, Andrews N, Kafatos G, Nardone A, Berbers G, Davidkin I, Aboudy Y, Backhouse J, Barbara C, Bartha K, et al. Standardization of measles, mumps and rubella assays to enable comparisons of seroprevalence data across 21 European countries and Australia. Epidemiol Infect. 2007;135:787–97. doi:10.1017/S0950268807008266.
- Cohen BJ, Doblas D, Andrews N. Comparison of plaque reduction neutralisation test (PRNT) and measles virus-specific IgG ELISA for assessing immunogenicity of measles vaccination. Vaccine. 2008;26:6392–97. doi:10.1016/j.vaccine.2008.08.074.
- Hatchette TF, Scholz H, Bolotin S, Crowcroft NS, Jackson C, McLachlan E, Severini A. Calibration and evaluation of quantitative antibody titers for measles virus by using the BioPlex 2200. Clin Vaccine Immunol. 2017;24:e00269–16. doi:10.1128/CVI.00269-16.
- Canadian Immunization Research Network. Population immunity to measles in Canada; 2016. [accessed 2019 May 27] http://cirnetwork.ca/research-study/population-immunity-to-measles-in-canada/.
- Statistics Canada C. Statistics canada. table 17- 10-0005-01.population estimates on July 1st, by age and sex. 2018. [accessed 2019 May 28] https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501
- Bentley M, Christian P, Heath A, National institute for biological standards and control. Report on a collaborative study to investigate the relationship between the 1st IRP and the 2nd and 3rd international standards for anti-measles Serum/ Plasma,in both ELISA and PRNT. Expert Committee on Biological Standardization 2007; WHO/BS.07/2076:1-17.