ABSTRACT
Pneumococci respond extremely rapidly to the vaccine pressure created by the conjugate vaccines (PCVs). Vaccine serotypes are disappearing, meanwhile new, often previously very rare types are emerging, and it is difficult to establish what makes certain serotypes more successful in replacement. The situation is very complex: every serotype has different antibiotic sensitivity or invasive capacity. However, despite this dynamic serotype rearrangement, the overall pneumococcal carriage rate remains quite stable, suggesting that these bacterial species can be considered as a commensal colonizer.
Introduction
Streptococcus pneumoniae (the pneumococcus) is a major human pathogenic bacterium, which can cause both invasive (sepsis, meningitis) and mucosal (pneumonia, otitis media, sinusitis) infections. Despite the successful vaccinations, it is still associated with a significant proportion of mortality worldwide, especially in children <5 years old.Citation1,Citation2 To prevent severe diseases, vaccines were developed against the pneumococcus. The 23-valent vaccine (PPV23) contains the purified capsular polysaccharide of 23 different serotypes, and it has been available since the early 1980s. The new generation conjugate vaccines include 10 (PCV10) or 13 (PCV13) different serotypes, covalently conjugated to strongly immunogenic bacterial carrier proteins. Unlike PPV23, these vaccines have completely changed the pneumococcal epidemiology all over the world, very soon after they were licensed and used wide-spread from the beginning of the 2000s onwards.
Reduction in pneumococcal disease incidence due to PCVs
The conjugate pneumococcal vaccines have been shown to have a remarkable effect, reducing the incidence of all types of pneumococcal diseases. As the PCVs were developed specifically against pediatric invasive pneumococcal diseases (IPDs), the most drastic reductions were observed in IPD among children <5 years of age. According to the US-wide CDC surveillance data, the overall IPD incidence (i.e., number of cases per 100,000 population) in this age category declined from 95 cases in 1998 to 9 cases in 2016; and IPD caused by PCV13 serotypes declined from 88 to 2 cases.Citation3
Although the PCV-vaccinated individuals are the children, transmission of pneumococci to unvaccinated populations can be prevented by this way, thank the so-called “herd effect”. A large-scale US study conducted by Moore et al. showed that the overall IPD incidence in adults also declined by 12–32%, depending on age.Citation4 In addition, reduction of mortality rates was also observed, particularly among the > 50-year-old patients. They estimated that due to the combined effect of PCV7 and PCV13, nearly 400,000 cases of IPD and about 30,000 deaths had been prevented between 2001 and 2012. So, the recommendation of PCVs was extended to adults as well the suggested basic regimen for them is a PCV first, later followed by PPV23 for higher serotype coverage.Citation5
Serotype replacement
However, this obvious decrease in disease incidence was largely due to decrease in vaccine serotypes, and parallel to this – although to a more modest ratio – emergence of non-vaccine serotypes occurred, most of which were previously hardly encountered. If we take the European IDP data – enhanced surveillance for IPD has been going on at ECDC since 2010 -, we can see that the prevalence of some serotypes is decreasing (e.g. serotype 7F), meanwhile others are on the rise (e.g. serotype 8 or 12F) ().
Figure 1. Changes in certain serotypes from IPD cases in Europe between 2010 and 2016, extracted from ECDC dataCitation6–11.
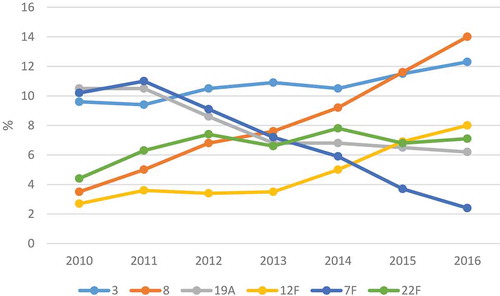
But this so-called serotype replacement is an extremely complex phenomenon. In case of pneumococci, each serotype possesses different properties, e.g. antibiotic resistance or capability to cause invasive infections.
Furthermore, the serotype rearrangement is occurring to different extent
in different age categories,
in different geographical locations,
in relation to vaccine coverage of the given country or region,
among invasive or mucosal infections, and finally
among disease-causing versus colonizing pneumococci.
Every serotype that has a high prevalence among IPD cases, obviously has high invasive potential. In addition, however, ECDC provided serotype-specific case fatality rates in the 2012 annual report.Citation7 According to this, serotypes 9N, 11A, 19F, and 23A had the highest ranking with 23%, 21%, 20% and 18%, respectively. At the same time, those two serotypes, which have been showing the sharpest emergence in the last few years (), were associated with lower case fatality rates: 9.5% for serotype 8 and only 3% for serotype 12F.
The two serotypes with the worst reputation: 19A and 3
One of the major concerns was the unexpectedly rapid emergence of serotype 19A after the introduction of PCV7. It soon took very elegant positions on the prevalence list, but luckily, it started declining again after the introduction of PCV13, which included this serotype. This serotype is associated not only with strong invasive capacity, but also with multiple antibiotic resistance. For instance throughout our carriage studies, serotype 19A not just increased its prevalence from 1.4% in the pre-PCV era to 11.5% within three years, but also accounted for a significant proportion of both penicillin-nonsusceptibility and macrolide resistance in the latter period (36.3% and 52.1%, respectively).Citation12 But, its prevalence decreased back again to 0.5% by 2015–2016.Citation13
The other serotype that has raised concerns is serotype 3. Meanwhile, it is almost entirely sensitive to antibiotics, it has high invasive potential and case-fatality rate (>15%). Despite it is part of PCV13, it is not just firmly keeping its second position among IPDs in Europe, but it is slightly increasing even (). On the other hand, it is seen less in carriage.
IDP versus carriage
A very elegant study from Sweden compared the colonizing isolates with those causing IPD in the same time period (2011–2015). Their results showed that certain serotypes were almost entirely found in IPDs and rarely in colonization (i.e., having high invasive potential). The most significant differences were found with serotypes 7F, 8 and 3.Citation14 We in Hungary have obtained very similar results: there were only two isolates each of serotype 7F and 8 over the last 10 years of investigation (nearly 1000 carriage isolates in total) and even more promisingly serotype 3 was not isolated in the recent few years anymore from colonization.Citation12,Citation13 On the other hand, some serotypes, such as 11A, 15B/C, 35B/F or 21 are more associated with colonization.
A further twist in the story could be that serotype changes are detected earlier in colonization compared to clinical samples.Citation13 A rational explanation for this could be that young children are the typical carriers of pneumococci. Carriage rate peaks at 2–3 years of age, i.e. in those who are directly targeted by vaccination. But I am not sure we could state that prevalence in carriage predicts directly for what happens sooner or later among clinical isolates.
Antibiotic susceptibility
The replacing serotypes are usually more sensitive to antibiotics, especially compared to the pre-PCV era. The formulation of PCV7 was designed to eliminate the seven most frequent and quite resistant serotypes that were ruling the pneumococcal palette for decades.Citation5 According to Moore et al., although the emergence of serotype 19A has lead to a temporary increase in resistance of IDP isolates, significant reductions in resistance rates were observed in the subsequent years.Citation4 But if we look at the IPD data in Europe, a little increase could be detected in the prevalence of non-susceptibility to penicillin and erythromycin between 2014 and 2016.Citation11 But the two serotypes currently leading the invasive top list (): serotypes 8 and 3, are almost fully sensitive to all kinds of antibiotics.
Why are certain serotypes more successful in replacement than others?
One possible explanation could be that certain serotypes have a wider variety of virulence factors or more potent ones. However, Gámez et al. have investigated the fully annotated genomes of 25 pneumococcal strains belonging to 11 different serotypes and identified a considerable number of virulence factors, but the majority of them were shown quite conserved across the pneumococcal population and none of them could be made responsible for increased success.Citation15
Clonal spread could well play an important role in the rapid emergence of certain serotypes. For instance, serotype 3 is highly clonal all over the world, and some wide-spread clones were identified among serotype 19A isolates as well.
Trzciński et al. have infected mice intranasally with several pneumococci of different serotypes at the same time and monitored changes in the serotype ratios using qPCR. They found that always the same hierarchy was built up between serotypes. They identified the role of the capsule (metabolic cost of capsule synthesis) and surface charge as predictors of serotype-specific fitness in carriage.Citation16
Is it surely a good idea to try to fully eradicate pneumococci by vaccination?
To cover more and more of the emerging serotypes, new generation conjugate vaccines are in the pipeline. The new 15-valent and 20-valent PCVs will include the following new serotypes: + 22F, 33F (PCV15) and + 8, 10A, 11A, 12F, 15B (PCV20).Citation17 However, these are still representing only a relatively small proportion of the nearly 100 different capsular serotypes, and – as pneumococci respond extremely rapidly to the vaccine pressure – increasing vaccine valency continuously, based on the newly emerging serotypes, would lead to an endless battle between us and the pneumococci. Therefore, alternative approaches in vaccine development have also been considered. These include (i) protein-based vaccines which target antigens that are conserved across all serotypes,Citation18 and (ii) whole-cell vaccines containing either killedCitation19 or even live attenuated pneumococci.Citation20 The results of these studies indicate that these vaccines may be able to completely eliminate the pneumococcus even from the nasopharynx.Citation21
But is it really what we want to achieve?
Pneumococci are apparently well adapted to the human nasopharynx and their presence can be considered a normal phenomenon.Citation21,Citation22 Meanwhile a drastic serotype-rearrangement among both clinical and carried isolates has been seen all over the world due to the selective pressure of conjugate vaccines, the overall pneumococcal prevalence in carriage seems to remain more or less stable. There are many studies found in the literature supporting the commensal manner of pneumococci.
Oikawa et al. have investigated the short-term effect of the PCV7 vaccination among healthy young children.Citation23 They found that the proportion of pneumococcal carriers was 55% before vaccine implementation, and although this figure decreased to 43% by six month post-PCV7, but it was 50% again when tested 18 months post-PCV7. A long-term follow-up was conducted in the Netherlands, where Spijkerman and colleagues have sampled the vaccinated children until 6.5 years post-PCV7.Citation24,Citation25 In both countries, despite certain fluctuations due to different reasons (e.g. PCV10 was introduced in the Netherlands meanwhile), the prevalence of pneumococci remained in the same interval. In the above-mentioned Swedish study, Lindstrand et al. found an extremely stable colonization rate between 2011 and 2015 (i.e., 4–8 years after PCV introduction), among 3024 children screened: carriage was between 29.6% and 31.1%.Citation14
We have found very similar results also in Hungary.Citation12 When testing 2262 healthy children aged 3–6 years, attending 39 different day-care centers (DCCs) between 2009 and 2012, in the period of rapidly increasing vaccination uptake in the country, the overall pneumococcus carriage remained almost unchanged, independent of the vaccination rate in the DCC group ().
Figure 2. Despite increasing vaccination rate per DCC group, the carriage rate of the children remained essentially the sameCitation12.
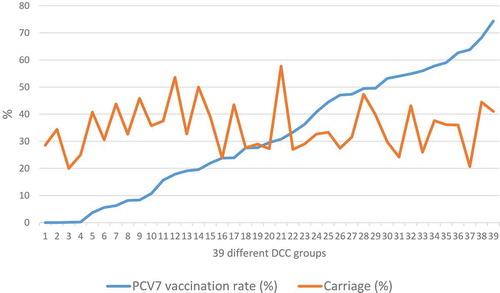
In summary, we shall be careful by targeting the complete elimination of pneumococci from the nasopharynx, because we can not foresee what could be the unwelcomed results of this intervention. Among other possibilities, other pathogenic species might be able to occupy the vacated niche. Instead, a better approach would be to develop vaccines that allow the pneumococcus to remain in the nasopharynx but prevent its dissemination to normally sterile body sites (blood, meninges, lungs, middle ear), where it could cause infection.Citation21,Citation22 One thing is for sure: as long as the (otherwise extremely successful) conjugate vaccines are used, we must keep on monitoring the pneumococcal serotype replacement closely, as the situation is dynamically changing from year to year.
Disclosure of potential conflicts of interest
There were no potential conflicts of interest.
References
- WHO Publications. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Weekly Epidemiological Report, 2019;94:85–104. http://www.who.int/wer
- WHO Publication. Progress in introduction of pneumococcal conjugate vaccine - worldwide, 2000–2012. MMWR Morb Mortal Wkly Rep. 2013;62:308–11.
- available online [accessed 2019 Mar 15]: http://www.cdc.gov/pneumococcal/surveillance.html
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015 Mar;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc. 2016;13(6):933–44. doi:10.1513/AnnalsATS.201511-778FR.
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe, 2011. Stockholm, Sweden: ECDC; 2013.
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe, 2012. Stockholm, Sweden: ECDC; 2015.
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2016 – Invasive pneumococcal disease. [Internet]. Stockholm, Sweden: ECDC; 2016 [accessed 2019 Mar 19]. http://ecdc.europa.eu/en/healthtopics/invasivepneumococcaldisease/Pages/Annualepidemiologicalreport2016.aspx.
- European Centre for Disease Prevention and Control. Annual epidemiological report 2014 – Vaccine-preventable diseases – invasive bacterial diseases. Stockholm, Sweden: ECDC; 2015.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease. In: ECDC. Annual epidemiological report for 2015. Stockholm, Sweden: ECDC; 2017.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease. In: ECDC. Annual epidemiological report for 2016. Stockholm, Sweden: ECDC; 2018.
- Tóthpál A, Laub K, Kardos S, Tirczka T, Kocsis A, van der Linden M, Dobay O. Epidemiological analysis of pneumococcal serotype 19A among healthy children following PCV7 vaccination. Epidemiol Infect. 2016;144:1563–73.
- Kovács E, Sahin-Tóth J, Tóthpál A, Kristóf K, van der Linden M, Tirczka T, Dobay O. Vaccine-driven serotype-rearrangement is seen with latency in clinical isolates: comparison of carried and clinical pneumococcal isolates from the same time period in Hungary. Vaccine. 2019;37:99–108. doi:10.1016/j.vaccine.2018.11.026.
- Lindstrand A, Galanis I, Darenberg J, Morfeldt E, Naucler P, Blennow M, Alfvén T, Henriques-Normark B, Örtqvist Å. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8years after vaccine introduction in Stockholm, Sweden. Vaccine. 2016;34:4565–71. doi:10.1016/j.vaccine.2016.10.045.
- Gámez G, Castro A, Gómez-Mejia A, Gallego M, Bedoya A, Camargo M, Hammerschmidt S. The variome of pneumococcal virulence factors and regulators. BMC Genomics. 2018;19:10. doi:10.1186/s12864-017-4376-0.
- Trzciński K, Li Y, Weinberger DM, Thompson CM, Cordy D, Bessolo A, Malley R, Lipsitch M. Effect of serotype on pneumococcal competition in a mouse colonization model. MBio. 2015;6(5):e00902–15. doi:10.1128/mBio.00902-15.
- US patent: United States Patent Application. Immunogenic compositions comprising conjugeted capsular saccharide antigens and uses thereof; 2015. http://patents.com/us-20150202309.html
- Darrieux M, Goulart C, Briles D, Leite LC. Current status and perspectives on protein-based pneumococcal vaccines. Crit Rev Microbiol. 2015;41(2):190–200. doi:10.3109/1040841X.2013.813902.
- Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010;17:1005–12. doi:10.1128/CVI.00036-10.
- Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Swords WE, et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014;6:141–54. doi:10.1002/emmm.v6.1.
- McDaniel LS, Swiatlo E. Should pneumococcal vaccines eliminate nasopharyngeal colonization? mBio. 2016;7(3):e00545–16. doi:10.1128/mBio.00545-16.
- Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Rev Nat Rev Microbiol. 2018;16(6):355–67. doi:10.1038/s41579-018-0001-8.
- Oikawa J, Ishiwada N, Takahashi Y, Hishiki H, Nagasawa K, Takahashi S, Watanabe M, Chang B, Kohno Y. Changes in nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among healthy children attending a day-care centre before and after official financial support for the 7-valent pneumococcal conjugate vaccine and H. influenzae type b vaccine in Japan. J Infect Chemother. 2014;20:146–49. doi:10.1016/j.jiac.2013.10.007.
- Spijkerman J, Prevaes SMPJ, van Gils EJM, Veenhoven RH, Bruin JP, Bogaert D, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One. 2012;7(6):e39730. doi:10.1371/journal.pone.0039730.
- Bosch ATM, van Houten MA, Bruinc JP, Wijmenga-Monsuur AJ, Trzciński K, Bogaert D, Rots NY, Sanders EAM. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the pneumococcal conjugate vaccine in the Netherlands. Vaccine. 2016;34:531–39. doi:10.1016/j.vaccine.2016.10.045.
