ABSTRACT
Frail older adults are at increased risk of poor clinical outcomes. Frailty assessment is therefore important in clinical trials to understand the benefits and harms of interventions. However, consensus is lacking on how frailty should be assessed.
We developed a prospectively specified index using a battery of formal tests and instruments and a retrospectively generated index using medical comorbidities and patient reported outcomes (PROs) within an adjuvanted recombinant zoster vaccine (RZV) trial (NCT02979639). For both frailty indices (FIs), a total deficit score was calculated as the accumulation of deficits and participants were categorized as non-frail, pre-frail and frail. We assessed (1) the feasibility and validity of both FIs; (2) the impact of RZV vaccine reactogenicity by frailty status on Short Form-36 [SF-36] physical functioning (PF) scores.
Of 401 participants, aged ≥50 years, 236 (58.9%) were categorized non-frail, 143 (35.7%), pre-frail, and 22 (5.5%) frail using the prospective FI. Corresponding numbers for the retrospective FI were 192 (47.9%), 169 (42.1%) and 40 (10.0%), respectively. Strong concordance was observed between the frailty status assessments (P < .001). The proportion defined as frail increased from 1.5%, to 10.4% in participants aged 50–59, and ≥70 years, respectively, for the prospective FI. Corresponding numbers for the retrospective FI were 3.7%, and 17.2%, respectively. RZV vaccination was associated with a transient, non-clinically meaningful, decrease on the SF-36 PF score in frail participants.
Both frailty indices provided similar results. The retrospectively generated FI offers the advantage of being easier to incorporate into vaccine clinical trials of older adults.
Introduction
Frailty is a term used in geriatric medicine to identify older adults who are at increased risk of poor clinical outcomes, such as disability, cognitive decline, falls, hospitalization, requiring long-term assisted care, or increased mortality.Citation1-Citation3 Frailty is defined as clinical vulnerability and is more predictive of health outcomes than chronological age. The aging process varies substantially between individuals because of unique features, such as genetic and environmental factors.Citation4 For example, frailty may be influenced by an age-related decline in innate and adaptive humoral and cell-mediated immunity (i.e. immunosenescence), which impairs the ability to resist infection and respond to interventions such as vaccination.Citation5-Citation8 Regulatory guidelines have identified the importance of demonstrating that interventions available for vulnerable groups, such as frail individuals, should be fully and appropriately studied for their effects in these specific groups.Citation9 This is of significance given the global trend of an aging population which will continue to increase global healthcare costs.Citation10
Health care professionals, policy makers and researchers have not achieved consensus on how to define frailty and how to quantitate the grading of frailty.Citation3 Mitnitski and Rockwood defined frailty status using a frailty index (FI) based on the accumulation of deficits (e.g. symptoms, signs, functional impairments, and laboratory abnormalities). This approach relies less on the nature of any deficit, but measures the cumulative effects of multiple deficits with age.Citation2 Considering that this approach is relatively simple, the results yielded by FIs measured in this manner have been consistent between studies and surveys. This is the case even though not every FI considered the same deficits, or even the same number of deficits, as long as these meet certain criteria. These criteria include that the FI increases with age, correlates with adverse outcomes, is not saturating (i.e. developed a too high prevalence at younger ages), and represents a range of systems or domains.Citation1 As such, the deficit accumulation approach offers the advantage of making frailty assessment accessible in the setting of clinical trials, if a robust frailty measure can be feasibly and reliably generated based on routinely collected data on health status and patient reported outcomes (PROs).
Although prior research on frailty focused on chronic conditions, general health status and physical frailty, more recently researchers have emphasized that frailty also involves psychological and social domains.Citation3 In addition, some studies have incorporated PROs, such as the Short Form-36 (SF-36), into the definition of frailty. For example, Ryb et al defined frailty as having an SF-36 physical functioning (PF) score <75.Citation11 Mansur defined frailty using weight loss and elements of the SF-36 scales as a measure of muscle weakness (SF-36 PF score), exhaustion (SF-36 vitality score), and physical inactivity (SF-36 questions on frequency of physical activity).Citation12 Other studies have explored the correlation between the SF-36 and other FIs.Citation13,Citation14 In general, the SF-36 scales have correlated very strongly with other frailty measures.
Herpes zoster (HZ) is the clinical manifestation of the reactivation of latent varicella-zoster virus (VZV), which presents, usually in adults with impaired immunity, as a vesicular dermatomal rash frequently accompanied by pain.Citation15 The incidence and severity of HZ increases with age, reflecting an age-related decline in VZV-specific T cell immunity.Citation16-Citation18 An adjuvanted recombinant HZ vaccine (RZV) containing glycoprotein E and the AS01B adjuvant system is highly efficacious.Citation19,Citation20 Still, RZV is associated with local and systemic reactions. The ZOSTER-063 study (NCT02979639) was consequently designed as an open-label phase III trial to determine the impact of reactogenicity after administration of RZV on SF-36 and EuroQol-5 Dimension (EQ-5D) PRO measures in older adults.Citation21 Frailty assessment following a previously described methodology was included in the study to evaluate possible inclusion in subsequent studies.Citation1,Citation22,Citation23 As there is no consensus in the field on how to assess frailty we also undertook a post-hoc analysis to evaluate an alternative and more easily implementable FI, which used the PRO instruments assessed within the clinical trial paired together with the baseline medical history of vaccine recipients.
We report on two objectives: (1) to assess the feasibility and validity of two FIs: a prospectively specified index developed using a battery of formal tests and instruments; and a second retrospectively generated index incorporating medical comorbidities and PROs, (2) as a practical example, i.e. to determine the impact of reactogenicity on SF-36 PF and quality of life (QoL) after administration of the first dose of RZV as a function of the frailty status classification at baseline.
Methods
Study design and participants
Frailty assessment was incorporated into a phase III, single-arm, open-label study, conducted in 13 centers in the United States. Adults aged ≥50 years at enrolment received two 0.5 ml doses of RZV at months 0 and 2, administered intramuscularly. A summary of the exclusion criteria for the study is provided in the supplementary material. In this manuscript, we describe data up to 7 days post-dose 1. The study protocol was approved by the investigational review boards at each study center and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Further details regarding the study are provided elsewhere.Citation21 Written informed consent was obtained from each participant prior to the performance of any study-specific procedures. The study is registered at http://www.clinicaltrials.gov (NCT02979639). The protocol is available at http://www.gsk-clinicalstudyregister.com (ID204928). Anonymized individual participant data and study documents are available for further research at www.clinicalstudydatarequest.com.
Frailty assessments
Prospectively defined frailty index
A frailty assessment was performed 7 days before dose 1. The following domains were assessed:
Disability: assessment of dependence on others to perform a list of specific daily activities (Supplementary Table S1, items 1 to 14);Citation1,Citation22
Cognition: the Montreal Cognitive Assessment is a 30-point questionnaire measuring cognitive impairment (Supplementary Table S1, item 15);Citation24
Self-rating of health and change in health (Table S1, items 16 and 17);Citation1
Physical status: the SF-36 PF score was used as a measure of the physical activity of the study participant (Supplementary Table S1, item 18);Citation11 and criteria for the definition of frailty developed by Fried et al.Citation25 were utilized to categorize the physical frailty phenotype (Supplementary Table S1, items 19 to 21);
Depression and exhaustion: the Center for Epidemiologic Studies Depression Scale – Revised was used to screen for depression and depressive disorders (Supplementary Table S1, item 22);Citation26
Multimorbidity: occurrence of 14 medical history conditions was based on the work of Song et al.Citation23 (Supplementary Table S1, items 23 to 36);
Further details on the deficits assessed and the scoring of the FI components are provided in Supplementary Table S1. A total deficit score was calculated as the accumulation of the individual deficits ranging from 0 to 36. The FI was then calculated by converting the deficit score into an index from 0 to 1 as follows:
FI = (deficit score/n)
where n is the number of non-missing components of the 36 items. Each study participant was then assigned to one of three categories based on the FI as follows: FI≤0.08 is classified as non-frail; 0.08< FI≤0.25 is classified as pre-frail; FI>0.25 is classified as frail.Citation23
Retrospectively generated frailty index
A retrospectively generated frailty assessment was based on a combination of the occurrence of specific age dependent diseases and data from two PRO questionnaires (SF-36 and EQ-5D) (see ).Citation23,Citation27,Citation28 The SF-36 is a multi-purpose health survey comprising 36 questions, including scales for PF, Role Physical, Bodily Pain, General Health, Vitality, Social Function, Role Emotional, and Mental Health.Citation27 EQ-5D is a generic measure of health status that defines health in terms of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. These five items are combined to generate health profiles, which are converted to a single index utility score, where a higher score represents a better QoL.Citation28 Both questionnaires were completed by study participants at Visit 1 (7 days pre-dose 1), Visit 2 (immediately before dose 1) and Visit 3 (7 days post-dose 1). Additionally, all participants completed the SF-36 PF component (questions 3a-3j) and the entire EQ-5D questionnaire at home daily for 6 days post-dose 1.
Table 1. Detail of SF-36 and EQ-5D components contribution to the retrospective frailty index
As in the prospectively defined frailty assessment described above, 14 comorbidities were assessed (see supplementary Table S1, items 23 to 36). In addition to the study participant’s medical comorbidity which contributes a maximum score of 14, the pre-vaccination SF-36 and EQ-5D questionnaires contribute a maximum score of 25 and 4, respectively (see ). Consequently, a total deficit score is calculated as the accumulation of the individual’s deficits, ranging from 0 to 43. As per the prospectively specified FI, the retrospectively generated FI was calculated by converting the deficit score into an index from 0 to 1 (i.e. FI = (deficit score/n) where n is the number of non-missing components of the 43 items) and frailty status was defined using the same cut-off points (i.e. 0.08 and 0.25).
Distributions and properties of both FIs were explored using descriptive methods. The association of both FIs with (1) age as both a categorical variable (i.e. age groups 50–59, 60–69 and ≥70 years) and as a continuous variable (assuming both a linear and an exponential distribution), and (2) the SF-36 PF score and the EQ-5D utility score were explored using descriptive statistics. The Kendall’s tau-b test was used to assess the concordance between the frailty status assessed using the 2 different methods. We investigated if any variable included in the FI saturated.
The secondary objective of this manuscript was to assess the impact of RZV vaccine reactogenicity on QoL, which was a pre-specified endpoint of the study protocol. We presented the mean SF-36 PF score and mean EQ-5D utility score by days post-dose 1 for the prospectively specified frailty status. A change of 3.3 in SF-36 PF score and a change of 0.074 in the EQ-5D utility score are considered clinically relevant.Citation29,Citation30
Results
Participants
401 participants received the first dose of RZV. Participants had a mean age of 64.6 years, and, as pre-defined, were equally distributed between three age groups: 50–59 years (33.4%), 60–69 years (33.2%), ≥70 years (33.4%). Most participants were Caucasian (82.8%) and there were more females than males (58.6 vs. 41.4%). Further details regarding the study results post-dose 1 are provided elsewhere.Citation21
Prospectively defined frailty index
Overall, 236 (58.9%) participants were categorized as non-frail, 143 (35.7%) as pre-frail and 22 (5.5%) as frail. presents the frailty status by age; 1.5% of participants 50–59 years of age (YOA) were considered frail, compared with 4.5% of those 60–69 YOA, and 10.4% of those ≥70 YOA.
Table 2. Distribution of age, SF-36 physical functioning score and EQ-5D scores by frailty status according to frailty index
The mean SF-36 PF scores at Day −7 were 90.7 (95% confidence interval [CI]: 89.0–92.4) in non-frail participants, 71.6 (95% CI: 67.7–75.5) in pre-frail participants and 40.7 (95% CI: 32.4–49.0) in frail participants. The mean EQ-5D utility scores at Day −7 were 0.92 (95% CI: 0.91–0.93) in non-frail participants, 0.84 (95% CI: 0.82–0.86) in pre-frail participants and 0.67 (95% CI: 0.60–0.74) in frail participants.
Retrospectively generated frailty index
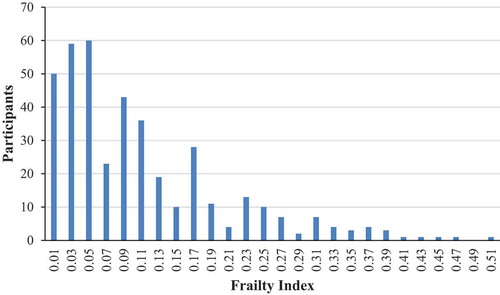
presents the distribution of the retrospectively generated FI score, which follows a gamma distribution tailing to the right with a maximum score of 0.50. As such, there is a consistent, submaximal upper limit to the percentage of deficits that any person can accumulate. Thirty-four individuals had a score of 0, while the median and mean were 0.08 and 0.11, respectively. Overall, 192 (47.9%) participants were categorized as non-frail, 169 (42.1%) as pre-frail and 40 (10.0%) as frail. presents the frailty status by age; 3.7% of participants 50–59 YOA were considered frail, compared with 9.0% of those 60–69 YOA, and 17.2% of those ≥70 YOA.
The mean SF-36 PF scores at Day −7 were 96.4 (95% CI: 95.8–97.0) in non-frail participants, 75.3 (95% CI: 72.8–77.8) in pre-frail participants and 42.8 (95% CI: 37.0–48.6) in frail participants. The mean EQ-5D utility scores were 0.96 (95% CI: 0.95–0.97) in non-frail participants, 0.87 (95% CI: 0.86–0.88) in pre-frail participants and 0.70 (95% CI: 0.66–0.74) in frail participants.
Comparison of frailty indices
A higher number of subjects were defined as frail using the retrospectively defined frailty index compared to the prospectively defined index. For both indices, the majority of subjects who were considered frail were aged ≥ 70 years, i.e. 63.6% and 57.5% for the prospectively and retrospectively defined frailty indices, respectively (see ). Although some differences can be seen between the two indices presented in , a similar trend was observed for both indices demonstrating that the SF-36 and EQ-5D scores decreased consistently with increasing frailty, and the number of deficits and the presence of a comorbidity deficit (see Supplementary Tables S2 and S3) increased consistently with increasing frailty. Both Supplementary Tables S2 and S3 demonstrate that high blood pressure was the most prevalent comorbidity with 202 (50.4%) participants reporting the condition. This suggests that no variable included in the FI was saturated.
compares the prospectively specified and retrospectively defined frailty categorizations. Note, both frailty status assessments were available for all 401 subjects who received the first dose of RZV. In total, 304 (75.8%) participants were assigned to the same retrospective and prospective frailty categories. Of the 236 study participants classified as non-frail with the prospective FI, only 1 was classified as frail using the retrospective FI. The Kendall’s tau-b statistic was significant (P < .001), indicating strong concordance between the frailty status assessed using the two different methods.
Table 3. Comparison of frailty status according to the retrospectively generated vs. prospectively specified frailty status
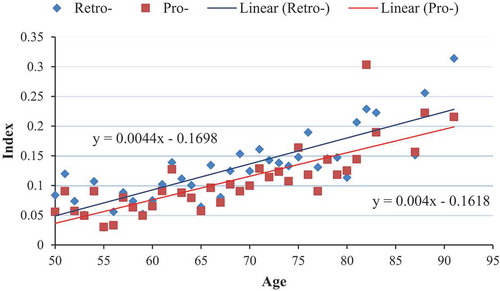
presents a scatterplot of the mean FI by age and type of FI. The intercept was −0.1618 and −0.1698 for the prospective and retrospective frailty indices, respectively. The indices increased on average by 0.0040 and 0.0044, respectively, each year. By the age of 90, the mean FI was approximately 0.2 for both indices, suggesting that most participants who are older are either frail or pre-frail. The linear equations presented in demonstrated a good fit for both the prospective and retrospective frailty indices (i.e. R-squared ≥0.60, P < .001). Figure S1 presents the same results assuming an exponential distribution resulting in a similar fit for both the prospective and retrospective frailty indices (i.e. R-squared ≥0.60, P < .001).
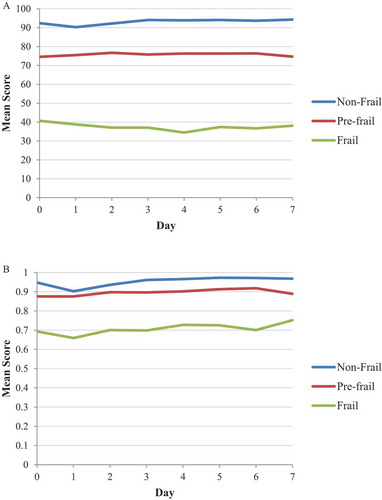
As per the secondary objective of this manuscript, presents the mean SF-36 PF score and mean EQ-5D utility score by day post-dose 1 by frailty status for the prospectively specified FI. There was a small (i.e. non-clinically meaningful) transient decrease in the SF-36 PF score in non-frail participants, i.e. the score decreased from 92.4 on Day 0 to 90.3 on Day 1, but had returned to baseline by Day 2 (i.e. 92.2). No apparent decrease was observed in SF-36 PF score in pre-frail participants, whereas, there was a gradual decrease until Day 4 with a subsequent increase until Day 7 for frail participants. The mean EQ-5D utility score decreased by 0.045 in non-frail participants and 0.035 in frail participants on Day 1, with values returning to baseline by Day 2. These decreases were considered not clinically relevant.Citation30 No apparent decrease was observed in EQ-5D utility score in pre-frail participants.
Discussion
We present the results from both a prospectively specified and a retrospectively generated FI. Both frailty assessments were completed for all 401 participants who received the first dose of RZV in the study. The frailty status generated from both FI differentiated participants by age, SF-36 PF score and EQ-5D utility scores. The frailty status based on the retrospective analysis of the comorbidity status and SF-36 and EQ-5D scores demonstrated strong concordance with the prospective frailty status that was based on a previously validated methodology. We demonstrated that none of the comorbidity status items saturated. For the SF-36 and EQ-5D items, although ceiling effects may be present, it is unlikely that these measures saturate for the general population at younger ages.
As a secondary objective of this manuscript we demonstrated using a practical application, how subjects can be classified into frail categories to investigate clinical outcomes. The study results suggested that reactogenicity associated with RZV had a transient, non-clinically meaningful, impact on SF-36 PF and EQ-5D utility scores in frail participants. Frailty assessment will become more important in the future to characterize populations for which new therapies or vaccines are being developed, since many such products will target older adults and frail populations. It is therefore important to note that even without specific attempts to recruit frail subjects, we identified pre-frail and frail participants, which demonstrates that if relatively nonrestrictive in/exclusion criteria are applied (see supplementary material for details on the exclusion criteria), one can expect a relatively broad range of participants.
A complete evaluation (i.e. a ‘gold standard’) of frailty requires a multidimensional, interdisciplinary assessment, including domains such as physical and cognitive function, nutritional status, multimorbidity, concomitant medications, and socio-economic factors.Citation9 The recent European Medicines Agency (EMA) guidelines focus primarily on physical frailty, recognizing that due to the complexity of frailty assessment, a complete evaluation was beyond the scope of the guidelines. In practice, including a complete evaluation of frailty assessment in a clinical study is complex and burdensome for study participants and investigators, and may impact the feasibility of the study. This is particularly true for phase III vaccine clinical trials where thousands of participants are recruited and followed to observe a predefined number of cases who develop disease. In this study we explored the feasibility of including a prospective FI, to establish a workable (from an operational perspective) methodology.
We proposed additionally a FI based on medical comorbidities that are generally collected as part of the medical history in clinical studies, in association with standard PRO instruments (i.e., SF-36 and EQ-5D). Our work shows that it can be used retrospectively in studies already including the SF-36 and EQ-5D questionnaires. Although the proposed retrospective index may have limitations, it can also be implemented prospectively in clinical trials conducted in older adults.
The EMA guidelines emphasize that treatments for vulnerable groups, such as frail individuals, should be appropriately studied for unique treatment effects. Therefore, it is important to be able to characterize the frailty level of the study population, either descriptively, or to allow a targeted or stratified recruitment of study participants with different levels of frailty. Our work confirms this is feasible, via 2 different methods of FI. Clinical events such as a fall or the onset of diseases such as influenza may result in a decline of physical function in frail individuals.Citation31 As such, it is also important to study the impact of preventive interventions (e.g. diet, exercise, fall prevention and vaccination) on the reduction of risk of individuals becoming frailer, thereby promoting active and healthy aging.Citation32,Citation33 The growth of this vulnerable population increases the need for closer attention to long-term care policies and public health decision-making.
Our study is not without limitations. We assessed feasibility based on our ability to define frailty status for all subjects. We did not ask the investigators or subjects about how burdensome the work was, etc. Our frailty measures are based on self-reported variables rather than being based on objective measurements. However, frailty indices based on self-reporting have been shown to be valid across many settings, and the approach represents a means of generating a holistic measure of health and vulnerability that explicitly considers the participant’s experience (i.e. of symptoms, functional impacts) in clinical trials.Citation1,Citation2,Citation7,Citation22,Citation23
Traditionally, research on frailty focused on chronic conditions, general health status and physical frailty. Commonly used methods of assessing physical frailty are the Short Physical Performance Battery (SPPB),Citation34 which measures three separate tests (i.e. standing balance, gait speed, and ability to rise from a chair) and the Fried Frailty phenotype which assesses five criteria exploring the presence/absence of physical signs or symptoms (i.e. involuntary weight loss, exhaustion, slow gait speed, poor handgrip strength, and sedentary behavior).Citation25 However, some researchers have suggested that the required contact between the individual and the assessor for formally assessing physical frailty may suggest that other assessments (e.g. self-reported questionnaires) may be preferred in the first estimation of the individual’s frailty profile.Citation35 More recently, researchers have emphasized that frailty also involves psychological and social domains.Citation3 In addition to the medical comorbidities and SF-36 PF, the proposed index included items from both the SF-36 and EQ-5D questionnaires assessing general health (e.g. ‘my health is excellent’, ‘I get sick easier than other people’, ‘I expect my health to get worse’), vitality (e.g. feeling full of life, calm, peaceful) and mental health (e.g. feeling depressed, anxious and worn out).
In summary, we used information gathered in our study to assess the feasibility and validity of two FIs. Both indices provided similar results, although from a feasibility perspective, the FI generated retrospectively using medical comorbidities and PROs, can be more easily incorporated as an assessment in future clinical trials conducted in older adults. In addition, we demonstrated that reactogenicity associated with dose 1 of RZV had a transient, non-clinically meaningful, impact on SF-36 PF and EQ-5D utility scores in frail participants.
Abbreviations
| EQ-5D | = | EuroQol-5 Dimension |
| FI | = | frailty index |
| HZ | = | herpes zoster |
| PF | = | physical functioning |
| PROs | = | patient reported outcomes |
| QoL | = | quality of life |
| RZV | = | adjuvanted recombinant herpes zoster vaccine |
| SF-36 | = | Short Form-36 |
| VZV | = | varicella-zoster virus |
Authors’ contribution
The list of contributors who conceived and designed the study, as well as those who collected, performed and analyzed the active phase study data can be found in the contributor sections here 21.
Generated the frailty analyses data: MKA, DC, KG, MJL, LO, KES, and ET.
Interpreted the frailty analyses data: MKA, DC, KG, NPK, SM, LO, and KES.
Disclosure of potential conflict of interest
MKA reports grant from the GSK group of companies (GSK) for research on frailty in relation to shingles during the conduct of the study, as well as grants from GSK, Pfizer and Sanofi for reasearch on frailty in relation to influenza or pneumococcal illness outside the submitted work. KES reports grants from GSK during the conduct of the study. MJL reports grants and fees as an Advisory Board member from GSK during the study, as well as grants and fees as an Advisory Board member from Merck outside the submitted work. DC, KG and ET are employees and LO former employee of GSK. DC and LO own GSK stock options as part of their current or former employee remuneration. LO is employee of CureVac AG and is inventor on a patent owned by GSK and relevant to RZV. SM is a freelance consultant for GSK. NPK reports grants from GSK during the study, as well as grants from Merck, Pfizer, Sanofi Pasteur, MedImmune, Protein Science and Dynavax outside the submitted work. CF and MN report no potential conflict of interest.
Supplemental Material
Download MS Word (33 KB)Acknowledgments
The authors would like to thank the study participants, investigators and study teams involved in this trial, as well as Brecht Geeraerts (GSK; supervised the protocol development) and Pierre Simonet (GSK; participated in the initial drafting of the prospective frailty index).
Editorial assistance and publication coordination were provided by Quentin Deraedt (XPE Pharma and Science c/o GSK).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24.
- Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood, K. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–89. doi:10.1111/j.1532-5415.2005.00506.x.
- Looman WM, Fabbricotti IN, Blom JW, Jansen APD, Lutomski JE, Metzelthin SF, Huijsman, R and on behalf of the TOPICS-MDS Research Consortium. The frail older person does not exist: development of frailty profiles with latent class analysis. BMC Geriatr. 2018;18:84. doi:10.1186/s12877-018-0776-5.
- Rodríguez-Rodero S, Fernández-Morera JL, Menéndez-Torre E, Calvanese V, Fernández AF, Fraga MF. Aging genetics and aging. Aging Dis. 2011;2:186–95.
- McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis. 2008;198:632–34. doi:10.1086/590435.
- Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WW, Zhang JH, Chan ISF, Yeh -S-S, Levin MJ, Harbecke RM, et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc. 2010;58:1634–41. doi:10.1111/j.1532-5415.2010.03021.x.
- Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, McElhaney JE, Ambrose A, Boivin G, Bowie W, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216:405–14. doi:10.1093/infdis/jix282.
- Curran D, Oostvogels L, Heineman T, Matthews S, McElhaney J, McNeil S, Diez-Domingo J, Lal H, Andrews C, Athan E, et al. Quality of life impact of a recombinant zoster vaccine in adults >/=50 years of age. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly150.
- European Medicines Agency: points to consider on frailty: evaluation instruments for baseline characterisation of clinical trial populations. [accessed 2019 Jan 29]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/12/WC500199243.pdf.
- Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17:30. doi:10.1186/s12877-017-0420-9.
- Ryb GE, Dischinger PC, Burch CA, Kerns TJ, Kufera J, Andersen D. Frailty and injury causation. Ann Adv Automot Med. 2012;56:175–81.
- Mansur HN, Colugnati FA, Grincenkov FR, Bastos MG. Frailty and quality of life: a cross-sectional study of Brazilian patients with pre-dialysis chronic kidney disease. Health Qual Life Outcomes. 2014;12:27. doi:10.1186/1477-7525-12-27.
- Masel MC, Graham JE, Reistetter TA, Markides KS, Ottenbacher KJ. Frailty and health related quality of life in older Mexican Americans. Health Qual Life Outcomes. 2009;7:70. doi:10.1186/1477-7525-7-70.
- Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253:1223–29. doi:10.1097/SLA.0b013e318214bce7.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255–63. doi:10.1056/NEJMcp1302674.
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi:10.1086/528696.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57. doi:10.1007/82_2010_31.
- Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201:1024–30. doi:10.1086/651199.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–32. doi:10.1056/NEJMoa1603800.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. doi:10.1056/NEJMoa1501184.
- Schmader KE, Levin MJ, Grupping K, Matthews S, Butuk D, Chen M, Idrissi ME, Fissette LA, Fogarty C, Hartley P, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine upon the physical functioning and quality of life of older adults: an open phase III trial. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly218.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36. doi:10.1100/tsw.2001.58.
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–87. doi:10.1111/j.1532-5415.2010.02764.x.
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings, J L., Chertkow, H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–99. doi:10.1111/j.1532-5415.2005.53221.x.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi:10.1093/gerona/56.3.m146.
- Bjorgvinsson T, Kertz SJ, Bigda-Peyton JS, McCoy KL, Aderka IM. Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment. 2013;20:429–36. doi:10.1177/1073191113481998.
- Ware JE Jr, Kosinski M. SF-36 Physical and mental health summary scales: a manual for users of Version 1. 2nd. Lincoln (RI): Quality Metric Incorporated; 2001.
- Kind P. The EuroQL instrument: an index of health-related quality of life. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia, New York: Lippincott-Raven Publishers; 1996:191.
- Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi:10.1002/1529-0131(200108)45:4<384::aid-art352>3.0.co;2-0.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–32. doi:10.1007/s11136-004-7713-0.
- Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med. 1998;158:645–50. doi:10.1001/archinte.158.6.645.
- Zorzoli E, Pica F, Masetti G, Franco E, Volpi A, Gabutti G. Herpes zoster in frail elderly patients: prevalence, impact, management, and preventive strategies. Aging Clin Exp Res. 2018;30:693–702. doi:10.1007/s40520-018-0956-3.
- Lionis C, Midlov P. Prevention in the elderly: A necessary priority for general practitioners. Eur J Gen Pract. 2017;23:202–07. doi:10.1080/13814788.2017.1350646.
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr, P A., Wallace, R B. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
- Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi:10.1093/ageing/aft160.