?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Ghana recorded the last case of poliomyelitis caused by wild poliovirus in 2008 and the country was declared polio-free in 2015. Polio-neutralizing-antibody levels in the population of three geographically representative regions of Ghana was determined, to identify possible immunity gaps.
Methods
Cross-sectional, hospital (1–70 years old) and school (primary, 1–15 years old)-based studies were undertaken in three regions in 2016. Individuals who visited the three teaching hospitals of the regions and were referred for haematology investigations were invited to participate in our study. Neutralizing-antibody titers to polio serotypes P1, P2, and P3 were assayed by WHO-standards. Antibody titers of ≥8 were considered protective. In the school lameness survey, clinical and epidemiological data were obtained from parents and their lamed children. Bivariate and multivariate analyses were conducted on subject characteristics, to assess potential factors for failure to seroconvert. P-values < 0.05 were considered statistically significant.
Results
Neutralizing-antibodies against poliovirus types 1, 2 and 3 were detected in 86% (264/307), 84% (258/307) and 75% (230/307) of the samples, respectively. Overall, 60.1% (185/307) were seropositive for the three polio serotypes and 2.9% (9/307) were seronegative. Polio neutralizing-antibodies (P1and P2) decreased with age (p < .001). Low seroprevalence of polio-neutralizing-antibodies was significantly associated with low school attendance of mothers (p < .001). Prevalence of residual paralysis was <1.0/1,000 among the school children.
Conclusion
Our study population is moderately protected against the three poliovirus serotypes. However, immunity appears to be lower with a higher age and low mother’s education. This may suggest the need for young-adult booster-dose to minimize the risk of wild poliovirus infection.
Introduction
Poliomyelitis is a highly infectious viral disease which can have crippling effects. The disease is caused by the poliovirus serotypes 1, 2 and 3. Polio generally affects children younger than 5 years, if exposed to the virus. According to Robert,Citation1 paralytic polio is observed in one out of 200 polio infections, while fatality is normally observed in 5–10% of paralytic polio cases in developing countries. Fecal-oral route is the primary mode of transmission and polio is vaccine preventable.
Since the introduction of the Global Polio Eradication Initiative (GPEI) in 1988, polio transmissions still exist in three countries namely: Pakistan, Afghanistan, and Nigeria. As of December 31st, 2018, 31 wild polioviruses (WPV) and 102 circulating vaccine-derived polioviruses (cVDPV) had been detected worldwide. Out of the cVDPV cases, 32.3% were recorded in Nigeria.Citation2 Ghana recorded the last cases of poliovirus in 2008.Citation3
Seroprevalence is the percentage of persons in a population who test positive for a specific disease, based on serology (blood serum) specimens. Important data on the performance of immunization programmes, the status of immunity against polio by the levels of polio neutralizing antibodies, groups susceptible to polio infection, and the populations facing at risk of future outbreaks can be obtained from seroprevalence studies.Citation4-Citation7
In a survey of poliovirus antibodies in 327 subjects in Kano-Northern Nigeria,Citation8 in terms of risk factors other than low vaccination histories, lower seroprevalence was associated with the female gender, lower maternal education, and fewer numbers of children in the household. In Pakistan, evaluated significant risk factors for failure to seroconvert were, the educational status of the respondent, stunting, and diarrhea in the past six months.Citation9 Low seroprevalence to poliovirus antibodies in a population, may contribute to an outbreak of polio in a community.Citation10-Citation12 Evidence of high polio seroprevalence in reducing the risk of poliomyelitis outbreak also abound.Citation13,Citation14
The sequelae of poliomyelitis are distinctive, and surveys of lame children can help to estimate the prevalence of the disease. Studies of lameness attributed to poliomyelitis in developing countries, have shown a higher prevalence of this problem as expected from earlier estimates. The prevalence rate of residual paralysis attributed to polio ranged from 1.05–19/1000 for school-aged children fromGhana,Citation15 Burma,Citation16 Egypt,Citation17 Philippines,Citation18 Indonesia,Citation19 ThailandCitation20 and Bangladesh.Citation21
Ghana continues to implement routine and mass immunization that includes polio vaccination, to prevent poliomyelitis. The country currently uses bOPV, which contains poliovirus serotypes 1 & 3. Four (4) doses of bOPV (at birth, 6, 10 and 14 weeks), are given in addition to inactivated polio vaccine (IPV) at 14 weeks. IPV does not replace the OPV vaccine but is used with OPV to strengthen a child’s immune system and to protect him from polio.
The mass polio vaccination has stalled since 2015 and the Regional Polio Certification Committee declared Ghana a polio-free country in 2015.Citation16 Nevertheless, there are a few areas of inherent transmission in Africa (Nigeria) and Asia which threaten the global attempt to eradicate polio. The probability of importation of wild poliovirus to countries that are almost polio-free still exists.Citation17,Citation18 There were two major polio outbreaks in 2003 and 2008 and the country still records polio compatibles, 26 cases in 2017.Citation3 Weaknesses in health system infrastructure, inadequate service delivery of oral poliovirus vaccine (OPV), suboptimal OPV efficacy, social-cultural beliefs and low seroprevalence to polio neutralising antibodies are some of the possible explanations for these observations.Citation19-Citation21 Low neutralizing poliovirus antibodies may lead to polio outbreak that could result in permanent lameness and possible death. This study determined the seroprevalence of poliovirus neutralizing antibodies serotypes 1, 2 and 3 in sampled populations selected from three regions of Ghana prior to the global switch from tOPV to bOPV. It also explored the factors that predicted low poliovirus neutralising antibodies among the respondents. Serological surveys are a useful tool for assessing population immunity and for identifying areas with low immunity.
Results
Seroprevalence of polio neutralizing antibodies
Of the respondents (307), 153 (49.8%) were females. The median and intra-quartile age range of respondents was 4 (1–14) years old. There were 85, 123 and 99 samples from the Northern, Ashanti and Greater Accra regions respectively. Neutralising polio antibodies against poliovirus serotypes 1, 2 and 3 poliovirus were detected in 86.0% (264/307) [95% confidence intervals CI: 82–90%] for poliovirus type1, 84% (258/307) [95% CI 79.4–87.9%] for type 2 and 75% (230/307) [95% CI 70–80%] for poliovirus type 3 of samples. Approximately, 60.1% (185/307) of the sera of respondents were seropositive for the three polio serotypes and nine (2.9%) sera had no antibodies to the three poliovirus serotypes.
Distribution of poliovirus serotypes neutralizing antibodies by sex, age, and place
The proportion of males compared to females was higher among the seropositive in all the age groups in the three polio antibody serotypes 1(137/264 = 51.9%,) 2(133/258 = 51.6%), 3 (121/230 = 52.6%) in all three regions. Neutralizing poliovirus antibodies (PV1) was found in females 92.86% (79.77–97.72) in the Northern region. PV2 and PV3 were recorded highest among males 91.83% (79.95–96.95) in Greater Accra and males 83.87% (72.39–91.16) in the Ashanti region respectively ().
Table 1. Distribution of polio antibodies that neutralized the three polioviruses with respect to sex and place.
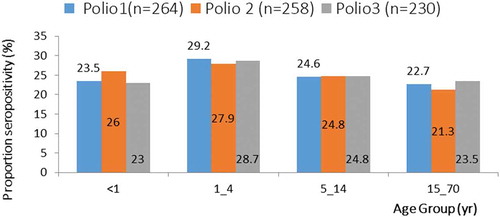
Children within age group 1–4 years reflecting the seropositivity rates for the three different polioviruses recorded the highest (PV1 = 29.2%; PV2 = 27.9%; PV3 = 28.7%) ().
Seroprevalence of poliovirus type 1, 2, 3 neutralizing antibodies by geographical location
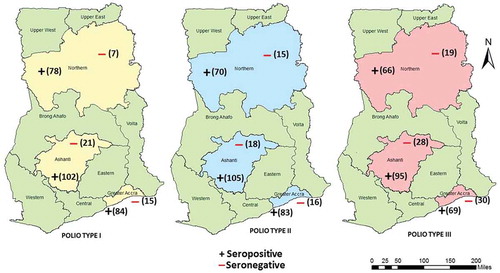
The proportion of samples testing positive for polio neutralizing antibodies of the three polio serotypes (1, 2 & 3) were higher in the Northern region compared to those of Ashanti and Greater Accra regions: PV1 = 91.8% (78/85); PV2 = 82.4% (70/85) and PV3 = 77.4% (66/85) ().
Distribution of poliovirus neutralizing antibodies titres by sex, age and region of residence
There was a significant difference in the median PV1 and PV3 (p-value = 0.0514 and 0.0254 respectively) poliovirus neutralizing antibody titre values between males and females. Similarly, there was a statistically significant difference in the median PV1 and PV2 (p-value = 0.0001) titre values among the age groups (<1, 1–4, 5–14 and 15–70). A statistically significant difference was observed in the median poliovirus neutralizing antibody titre values of PV2 in the three study sites (p-value = 0.0046) ().
Table 2. Distribution of respondents’ median neutralizing poliovirus antibody titres.
The strength and direction of the association between age and poliovirus neutralizing antibodies The age of the respondents had a negative relationship on the mean titres of the neutralizing antibodies against the three polio serotypes (). The presence of neutralizing polio antibodies in the sera of respondents decreased with age.
Table 3. Association between age and mean titres of the neutralizing polio antibodies of the three polio serotypes.
Risk factors for low seroprevalence of poliovirus antibodies among respondents
The educational status of mothers played a significant role in the presence of antibodies against poliovirus serotype type 1 & 2 among the respondents. With poliovirus serotype 1, the odds of being seronegative among respondents whose mothers had never attended school was 3.9 times (p < .003) the odds of being seronegative among respondents whose mothers had attended school. A similar picture was found for poliovirus serotype 2 (p < .001) ().
Table 4. Risk factors for low seroprevalence of PV1 antibodies among respondents.
School lameness study
One hundred and twelve schools were visited, from which 34,217 pupils in the selected primary schools were screened. One hundred and eight (0.3%) were found with walking disabilities. Out of the 108 pupils, paralytic polio, (defined as flaccid weakness, muscle atrophy, decreased bone growth in the affected limb, diminished deep tendon reflexes, normal sensation and a history compatible with acute poliomyelitis) accounted for 18.5% (20/108) and upper motor neuron disorders (cerebral palsy, Encephalitis) accounted for 25.9% (28/108) .
The prevalence of residual paralysis of poliomyelitis estimated from the school lameness survey (1–15 years old) was 0.58/1,000 (5.8/10000) [95% CI: 0.33–0.84] ().
Table 5. prevalence of paralytic poliomyelitis with residual paralysis estimated by school (primary, 1–15 years old) lameness surveys in Northern, Ashanti and Greater Accra regions of Ghana, 2016.
The prevalence of residual paralysis in urban and rural districts of the studied regions was not the same. The Bosomtwe district (rural) in the Ashanti region recorded the highest (4/1000) prevalence of paralytic polio and the lowest (0.08/1000) was recorded in the Accra metro, which is urban. Overall, the prevalence of residual paralysis of poliomyelitis in the rural and urban districts were 2.3 and 0.36/1000 respectively.
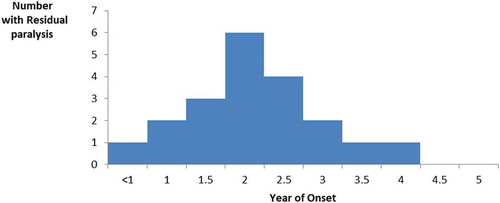
Among the study population, 85% (17/20) of children with residual paralysis from poliomyelitis were in the 10–15-year-old group (). The mean age of the children was 10.65 ± 2.23 years. The majority 60% (12/20) of those with residual paralysis were males. All the parents of the 20 children with residual paralysis were interviewed and they indicated an acute onset of weakness in a previously healthy child occurring before the age of five years. The right leg was the most affected limb (30%; 6/20). Residual paralysis was more in males (55%; 11/20) than in females.
Discussion
It is noted in this study that poliovirus serotypes neutralizing antibodies are 75%-86% of the sera of the respondents. These findings from the study confirm that substantial immunity gaps (differences in the levels of polio neutralizing antibodies) to all three poliovirus serotypes exist in the three regions of Ghana despite intensive efforts to increase immunity levels against polio through polio vaccination. In studies conducted in developed countries: Spain,Citation22 Korea,Citation23 and the USA, neutralising antibodies in the sera of respondents had shown higher seropositivity compared to those in developing countries.Citation24 However, respondents in Maiduguri Nigeria,Citation25-Citation28 recorded lower seroprevalence rates compared to the findings of this study.
Previous polio vaccinations and or infections of wild poliovirus (WPV) together with the polio outbreak in 2008 in the Northern region, may account for the levels of polio population immunity or the antibody levels in the three regions of Ghana. These gaps in immunity levels raise concerns of either primary vaccine failure, that is, lack of initial antibody responses where potent vaccines are used or, failure of the cold chain and the subsequent use of non-potent vaccines in the field.Citation29-Citation31 Even though the seropositive rate required for maintaining population immunity to polio has not been universally determined by WHO, studies have demonstrated that the critical vaccination coverage most likely needed to stop any transmission of poliovirus is 80–85% of the population.Citation32
The herd immunity threshold above which one can guarantee the prevention of an outbreak is unclear in Africa and typically for Ghana. However, it has been documented by Sutter,Citation33 that with population immunity levels (polio neutralizing antibody levels) of 66%–80%, polio outbreaks in developed countries can be prevented. In developing countries with suboptimal sanitation and hygiene with the potential of increased poliovirus transmission and greater force of infection, wild poliovirus outbreaks could, however, occur with population immunity levels as high as 94%–97%.Citation33 Therefore, until polio is eradicated globally, many countries including Ghana remain at risk for a poliovirus outbreak.Citation34
Evidence exists that low polio neutralizing antibodies in a population may lead to polio outbreak whilst high polio neutralizing antibodies may interrupt transmission of poliovirus. In an outbreak of wild poliovirus in the Xinjiang Uygur Autonomous Region of China in 2011, a survey indicated that 4.0% of the sample population had no antibodies to the three poliovirus serotypes.Citation35 In the wild polio outbreak in Finland in 1984 and 1985, wild poliovirus type 3 was implicated. Prior to that outbreak in 1982, a seroprevalence survey revealed that only 30% had neutralizing antibodies to type 3 poliovirus.Citation11 Serological studies have shown that the outbreak of polio in Kinshasa and Bandundu in the Democratic Republic of Congo in 2010–2011 was likely due to the immunity gap in PV1.Citation12 In a series of polio outbreak in Northern Nigeria between 2012 and 2013, seroprevalence studies indicated that neutralizing antibody levels among children aged 36–47 months in the study population, was lower than the required levels for poliovirus interruption.Citation8 As long as poliovirus circulation continues anywhere in the world, importations remain a risk and consequently, there remains a limited risk of possible outbreaks among unvaccinated subpopulations. Furthermore, not only wild polioviruses but also cVDPV may spread across countries and reach a susceptible population causing disease, with the possibility of VDPV circulation as long as OPV-based immunization programs are used in high-risk countries like Nigeria and Ghana. It is observed in this study that the level of neutralizing polio antibodies of PV3 was the lowest in the sera of respondents. These results are similar to findings in studies in European countries such as GreeceCitation36 and Germany.Citation37 In similar studies in South Africa (Natal/KwaZulu) and other developing countries (Abidjan, Bombay-India and Cuba) low levels of neutralizing antibodies to PV3 had been documented.Citation38,Citation39 These observations may be explained by a lower potency of poliovirus type 3 antigens in the vaccine. The low level of neutralizing antibodies of PV3 has also been explained by the fact that PV1 antibodies are due to both vaccination and natural immunity, whereas PV2 and PV3 antibodies are mainly due to vaccine-induced immunity.Citation40 There is, therefore, the need for a strategy to boost the immunogenicity of PV3 in the polio eradication programme to avoid any future outbreak of polio involving WPV3. In the second quarter of 2016, the Expanded Programme on Immunization (EPI) in Ghana resolved to switch from the administration of trivalent oral polio vaccine to bi-valent. This was in conformity to the WHO strategic plan on polio eradication.Citation41 This policy direction is on the right path to boost the population immunity levels on PV3.Citation42
The study observed that there is a decline in seroprevalence with age. This is consistent with what had been detected in UruguayCitation43 and South Africa.Citation44 Contrary to this observation, according to WilliamsCitation45 in other studies, neutralizing polio antibodies increased with age. Studies have shown that intestinal immunity to poliovirus wanes over time, therefore individuals could become re-infected and shed poliovirus.Citation46 The older age groups may contribute to wild polio transmission without clinical symptoms, and the World Health Organization has therefore recommended that older individuals get vaccinated as part of the outbreak response.Citation47
This study underscores the importance of maternal education on seroprevalence. A lower seroprevalence of neutralizing antibodies may be results of lower maternal education. These mothers may not understand the need for polio vaccination which may lead to the child not acquiring the needed polio antibodies for protection against the polio virus. This argument of lower maternal education supporting lower seroprevalence has been affirmed by similar findings from a study in Northern Nigeria.Citation8 However, studies in EgyptCitation34 found severe wasting and stunting associated with lower seroprevalence, but these findings were not statistically significant. One of the key players in the reduction of infant and child mortality is women’s education. The higher a woman’s level of education, the more likely it is that she will marry later, play a greater role in decision making and exercise her reproductive rights.
Lameness surveys conducted in a given population is a helpful tool in assessing the poliomyelitis situation in that given community. This study revealed that the prevalence of residual paralysis cases estimated by the school lameness survey was less than one in a thousand (<1/1000) population. This value is quite low in this study compared to the estimate in similar studies in GhanaCitation15,Citation48 and Thailand.Citation21 Oral polio vaccine coverage (OPV3) has increased from as low as 76% in 2003 and 2004 to 94% in 2017 in Ghana. In 2004, Greater Accra, Ashanti and Northern Regions of Ghana recorded 56%, 66% and 93% OPV3 coverage respectively whilst in 2017, 94%, 103%, and 117% were recorded.Citation49 The increase in the oral polio vaccine coverage in these populations in the regions and Ghana as a whole, may account for the drop in the estimated number of residual paralysis in the study population.
Studies have indicated that neutralizing polio antibodies of levels higher than 66–80% is a contributory factor for preventing polio infection.Citation33 In determining the seroprevalence of neutralizing polio antibodies against P1, P2 & P3 in the same study population, the levels of neutralizing polio antibodies of polio serotypes P1, P2 & P3 in the sera of respondents were within 75%-85%.Citation50 These results may provide some biological explanation for the reduction in the observed number of paralytic polio cases in the study population. This finding also corroborates the importance of routine and mass polio vaccinations in developing countries such as Ghana, in which polio is yet to be eradicated.
Over 50% of all poliomyelitis with residual paralysis occurred among children less than three yearsCitation51,Citation52 This was noted in our study and a similar finding in Cameroon.Citation53 The age of onset is also noted in this health condition is clearly different from what is usually encountered in temperate countries, where the disease tends to occur in older age groups and may inflict even elderly people.Citation54 This pattern may indicate individual variation in susceptibility to a disease for different populations or may reflect recall bias in the interviewees. The proclivity of polio infection in the tender age group underscores the importance of observing adequate sanitary conditions in overcrowded homes and communities to facilitate interruption of polio transmission in such places.
The majority (60%) of those with residual paralysis were males. The predominance of cases among males observed in this study has also been reported in the studies conducted in Pondicherry, India and elsewhere.Citation55 Males (boys) are more predisposed to physical activity, which is a risk factor for paralytic polio and even if both sexes were affected at the same time, they are most likely to have a higher incidence of paralytic polio compared to the girls.Citation56
These findings of the study should be considered in the light of limitations. First, there was no immunization history available for adult participants, so it is unclear whether polio sero-immunity was due to past OPV receipt and/or natural immunity. Secondly, this study is hospital-based, a situation which may have resulted in an overestimation of seroprevalence of antibody against poliovirus and selection bias, as the children who are not reached by immunization activities may be less likely to visit hospitals. In the measurement of the prevalence of poliomyelitis, the most accurate technique to measure the prevalence of poliomyelitis is a house-to-house survey. However, such surveys are time-consuming and are costly if carried out independently. Since the demographic and school enrollment data in the study areas suggested that school attendance was high (greater than 90%) most of the information needed to complete the prevalence survey could be obtained from the schools. The results, although not generalizable will give the Ghana Expanded Programme on Immunization (EPI) a fair idea as to the status of immunity in the study population, facilitate innovative strategies to reach the unreached and acquire the needed herd immunity to interrupt the transmission of any future importation of wild poliovirus into the country.
Conclusions
This study revealed a moderate level of seroprevalence of neutralizing antibodies to the three polio serotypes with some regional differences. Seropositivity was generally low with increasing age and the mother’s education level may be crucial to seronegativity. The prevalence of paralytic poliomyelitis was low in the study regions, lower rates were found in the urban districts and cases were more in age group less than five years with a higher male preponderance. The EPI, Ghana, may consider young-adult booster-dose of polio vaccine. The EPI can also consider a one-time NID to mop-up build-up susceptible as a result of missed children.
Methods
Study design and setting
The study was made up of two components: hospital-based seroprevalence and school lameness studies. A cross-sectional analytical hospital-based study was conducted in three regions of Ghana. In this seroprevalence study, individuals referred to the laboratory for haematology at the three teaching hospitals were recruited into the study. The respondents were interviewed with a semi-structured questionnaire extracting data on demographic and polio immunization history. The prevalence of residual paralysis from poliomyelitis was determined in a cross-sectional study among primary school pupils in a supplemental lameness survey. Demographic data and history of paralysis were retrieved from respondents by an interviewer-administered questionnaire. Data collection was between April 1st and July 31st, 2016.
The study settings comprised: Northern, Ashanti, and Greater Accra regions which are located in the three ecological zones of Ghana (). These regions are the most populated and have the biggest referral and teaching hospitals in Ghana. Patients attending these hospitals come from a wide catchment area with mixed socioeconomic backgrounds.
Study population
In the hospital-based survey, all children less than five years old and adults referred to the laboratories of the three major referral hospitals (Tamale, Komfo Anokye and Korle-Bu Teaching Hospitals) in the Northern, Ashanti and Greater Accra regions of Ghana from 1st of April to 30th of July, 2016 were screened for participation in this survey. The lameness school survey involved primary school children from one urban and one rural district in the three study sites.
Inclusion and exclusion criteria
All children of consenting parents and adults resident in the three selected regions for the past six months were eligible to participate, except those (a) born or residing outside of Ghana; (b) those with serious acute illnesses requiring hospitalization; (c) those diagnosed or suspected of congenital immunodeficiency disorder or an immediate family member, and (d) those with contraindication to venipuncture.
The lameness survey involved the total enrollment of the selected primary schools in the selected districts. This included pupils of all ages and all classes in the selected primary schools. Pupils in the non-selected primary schools were excluded.
Sample size
The estimated minimum sample size for the hospital survey was 274, however, 307 respondents attending or using the laboratories in the three referral hospitals were recruited into the study. The estimated minimum sample size used for school lameness survey was 32,588 for school children in primary schools. However, 34,217 school children, in the three regions were screened for cases of paralytic poliomyelitis in the school survey. In both studies, the Fishers formula for calculating sample size for populations >10,000 was used.Citation57
where for the hospital survey:
p = 90%, assuming a seroprevalence rate (p) of 90%
is the desired level of precision (= 0.05)
is the critical value for the standard Normal Distribution at
= 5% (1.96)
is the power of the study (= 0.8), the sample size of 274 respondents was obtained.
For the school survey,
, the assumed prevalence of lameness in Ghana (p = .004)
the desired level of precision (= 0.001)
, the critical value for the standard Normal Distribution at
= 5%; (
, the power of the study (
The Sample sizes of both studies were distributed across the three regions by probability proportional to the size of the population of the study regions.
Screening for the hospital-based seroprevalence survey
The selection of participants for the hospital survey required an initial screening for age (<1, 1–4, 5–14, 15–70 years old) at the respective hospitals in each region. After obtaining informed consent and assent from the participants and the mothers or caregivers who had been sent for laboratory investigations, the study procedure commenced. Screening continued until the sample size of each age group was achieved.
Sampling approach- school lameness survey
A two-stage stratified random sampling design was used in each selected region, one urban and one rural district was selected. In each selected district, the district sampling frame was used to allocate the sample size proportionate to the population size and rural and urban difference for each zone (Northern region: 7498, Ashanti region: 14592 and Greater Accra region 12127.Citation58 A total sample size of 34,217 was arrived at for all three study sites. Upon obtaining the desired sample size of school pupils per urban/rural setting, the schools were sampled by simple random sampling. The schools in each urban/rural setting were numbered. The sample elements were selected by rural/urban setting using the Random Number Generator (Calculator). The first randomly selected school was visited and the entire primary school population was screened. This was repeated in the subsequent randomly selected school until the sample size was obtained. Where the school population was more than the desired sample size, the remaining pupils were still screened. In all, 112 schools were visited to obtain the desired sample size.
Data collection technique and tools – hospital-based study
The technique for data collection was an interview (face to face) using a semi-structured questionnaire as the tool. The study team was comprised of a physician, nurses, laboratory scientists, and field assistants. The study physician explained the purpose of the study to the parents or caregivers and the adult respondents. After obtaining informed consent from all respondents a standardized questionnaire was administered to them through a face-to-face interview. For the children, less than five years, vaccination history on routine immunization were extracted from their child health records books, clinic records. Parents or caregivers’ recall of doses of vaccines the child had received was acceptable if the parent or caregiver could specify the dose given. Supplemental vaccinations (vaccinations given during national or sub-national immunization days), were obtained through oral histories given by the parent or caregiver.
Blood collection procedure-hospital based study
Two-five (2–5) ml of venous blood was collected through venipuncture into a vacutainer tube by a phlebotomist. Blood sera were separated within six hours at the hospitals and stored at −20 °C in a deep freezer. After the data collection, blood samples (sera) were stored in airtight tubes and transported to the Noguchi Memorial Institute for Medical Research for laboratory analysis in a reverse cold chain at a temperature of +2 to +8 °C. Sera were tested in triplicate for levels of neutralizing antibody titers against poliovirus types 1, 2 and 3, respectively, using modified micro-neutralization assays.
Quality control measures consisted of training research assistants, pretesting the questionnaire and procedures, as well as quality checks of data.
Micro-neutralization test for polio antibodies
Antibodies against poliovirus types 1, 2 and 3 were determined by a microneutralization assay with prototype Sabin strains, according to the WHO guidelines,Citation59 which measured the ability of a human serum sample to neutralise the infectivity and cytopathic effect of each of the three types of poliovirus on cell cultures in vitro. Antibody titers of ≥8 were considered protective.
Data collection technique and tools- school lameness survey
Permission was sought from the Ministry of Education, the Ghana Education Service, and selected schools’ authorities. The survey method was adapted from LA Force’s method of school lameness survey with some modifications.Citation60
On reaching each class in a selected primary school, the teachers were primarily asked if there was any child with walking disability or any kind of weaknesses in the limbs in the class present or absent from school on that day. After that, all children in each class were asked to walk past the survey team, and children with walking disabilities or lamed were identified. The team then sought permission from the class teachers and the headmaster of the school to invite all such pupils to come to the school with their parents the following day. This included all children who were lamed and absent from school on the day of the visit. These children were also made to walk past the survey team for assessment. Clinical and epidemiological data of the children were obtained from the parents using a semi-structured questionnaire by trained medical officers and research assistants. The questionnaire elicited information on gender, date, and place of birth, date of onset of paralysis, residence or place of onset of paralysis, the character of paralysis, history of onset of paralysis and sensation.
The child was further examined clinically in a sitting position. The muscle tone was determined in both legs by passive range of motion. The muscle mass was determined by physical examination and palpation. With an aid, knee jerks deep tendon reflexes were observed and subsequently, sensation, by the ability to distinguish sharp and blunt ends of a pin. Finally, the degree of disability was estimated. Based on the information from the parents and children and the necessary physical examinations, the child’s lameness was attributed to one of these etiological factors: residual paralysis from poliomyelitis, congenital defects, upper motor neuron disorders (e.g., cerebral palsy), trauma due to road traffic accident, post-infection complications such as osteomyelitis or a septic joint, Guillain-Barré syndrome and traumatic neuritis.
Training of research assistants and pretesting of the questionnaire in a primary school in the Ashanti region (Asawasi L/A primary school) and procedures were performed to ensure that quality and relevant data were collected from the field.
Data analysis
Categorical and continuous data were cleaned, coded and entered into Microsoft Excel. Data were then exported to STATA version 13 for analysis. A descriptive analysis of polio neutralizing antibodies was performed by person and place. Univariate analyses were expressed as frequency distributions, percentages, and mean± SD as appropriate. Seroprevalence of poliovirus neutralizing antibodies serotypes 1, 2 and 3 were determined as the proportion of poliovirus neutralizing antibodies among the blood (serum) samples. Wilcoxon rank sum test was used to compare differences in median titres by sex and Kruskal–Wallis test was used to compare by age and residence. The strength and direction of the association between age and poliovirus neutralizing antibodies were determined by simple correlation analysis with a non-parametric, Spearman Rank correlation test for statistical significance. Binary logistic regression models were used to determine factors significantly associated with seroprevalence with independent variables as sex, education status, age, and residence. P < .05 was considered significant. In the school lameness survey, the prevalence of lameness (residual paralysis) was determined by the proportion of children with flaccid paralysis and intact sensation among the total number of children screened.
Authors’ contributions
Conceptualization and design: JKLO, JO, EAA, PA. Data collection and Statistical analysis: JKLO. Drafting of the manuscript: JKLO, JO, EAA, PA, MP were responsible for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical considerations
Ethical clearance was obtained from the Ghana Health Service Ethics Committee (GHS-ERC 14/11/15) and the Institutional Review Board of Noguchi Memorial Institute for Medical Research (NMIMR- IRB CPN IORG 0000908). Participants voluntarily participated in the study and they (or caregivers) signed written informed consents.
Additional information
Funding
References
- Roberts L. Infectious disease. Polio outbreak breaks the rules. Science. 2010;330(6012):1730–31. doi:10.1126/science.330.6012.1730.
- WHO. [accessed 2019 Jan]. http://polioeradication.org/polio-today/polio-now/.
- Ghana Health Service (GHS). Disease Surveillance Division. Annual report. 2017.
- Reinheimer C, Friedrichs IMK, Rabenau HF, Doerr WH. Deficiency of immunity to poliovirus type 3: a lurking danger. BMC Infect Dis. 2012;12:685–89.
- Philippa MB, White JG. Prevalence of antibody to poliovirus in England and Wales 1984-6. Br Med J (Clin Res Ed). 1986 Nov 1;293(6555):1153–55.
- Frantzidou F, Diza E, Halkia D, Antoniadis A. A seroprevalence study of poliovirus antibody in the population of northern Greece. Copyright by the European society of clinical microbiology and infectious diseases. CMI. 2004;11:63–82.
- Giwa FJ, Olayinka AT, Ogunshola FT. Seroprevalence of poliovirus antibodies amongst children in Zaria, Northern Nigeria. Vaccine. 2012 Nov 6;30(48):6759–65. doi:10.1016/j.vaccine.2012.09.023.
- Iliyasu Z, Nwaze E, Verma H, Mustapha AO, Weldegebriel G, Gasasira A, Wannemuehler KA, Pallansch MA, Gajida AU, Pate M, et al. Survey of poliovirus antibodies in Kano, Northern Nigeria. Vaccine. 2014;32:1414–20. doi:10.1016/j.vaccine.2013.08.060.
- Habib MA, Soofi S, Ali N, Sutter RW, Pallansch MH, Qureshi H, Akhtar T, Molodecky NA, Okayasu H, Zulfiqar A, et al. A study evaluating poliovirus antibodies and risk factors associated with polio seropositivity in low socioeconomic areas of Pakistan. Vaccine. 2013 Apr 8;31(15):1987–93. doi:10.1016/j.vaccine.2013.02.003.
- HaiBo W, Hui C, Zheng RD. Seroprevalence of antipolo antibodies among children. J ASM. 2013;Org. (20).
- Lapinleimu K. Elimination of poliomyelitis in Finland. Rev Infect Dis. 1984;6:S457–S460.
- Alleman MM, Wannemuehler KA, Weldon WC, Kabuayi JP, Ekofo F, Edidi S, Mulumba A, Mbule A, Ntumbannji RN, Coulibaly T, et al. Factors contributing to outbreaks of wild poliovirus type 1 infection involving persons aged ≥15 years in the Democratic Republic of the Congo, 2010–2011, informed by a pre-outbreak poliovirus immunity assessment. J Infect Dis. 2014 Nov 1;210(Suppl 1):S62–S73. doi:10.1093/infdis/jiu282.
- de Miranda MP, Gomes MC, de Andrade HR. Seroprevalence of antibodies to poliovirus in individuals living in Portugal, 2002. Euro Surveill. 2007 Sep;12(9):E070913.4.
- Calderon-Margalit R1, Sofer D, Gefen D, Lewis M, Shulman L, Mendelson E, Swartz TA, Shohat T. Immune status to poliovirus among immigrant workers in Israel. Prev Med. 2005 Jun;40(6):685–89. doi:10.1016/j.ypmed.2004.09.008.
- Nicholas DD, Kratzer JH, Ofosu-Amaah S, Belcher DW. Is poliomyelitis a serious problem in developing countries? The Danfa experience. Br Med J. 1977;1:1009–12. doi:10.1136/bmj.1.6067.1009.
- Ofosu-Amaah S, Kratzer J, Nicholas DD. Is poliomyelitis a serious problem in developing countries? Lameness in Ghanaian schools. Br Med J. 1977;1:1012–14. doi:10.1136/bmj.1.6067.1012.
- World Health Organization. Expanded programme on immunization-Burma. WHO Weekly Epidemiol Rec. 1977;52:145–46.
- World Health Organization. Survey for residual paralysis-Egypt. WHO Weekly Epidemiol Rec. 1977;52:269–71.
- World Health Organization. Expanded programme on immunization- Philippines. WHO Weekly Epidemiol Rec. 1978;53:144–46.
- World Health Organization. Expanded programme on immunization, poliomyelitis in Indonesia. WHO Weekly Epidemiol Rec. 1979;54:177–78.
- World Health Organization. Expanded programme on immunization, poliomyelitis in Thailand. WHO Weekly Epidemiol Rec. 1979;54:202–03.
- Pacho N, Amela C, De Ory F. Age-specific seroprevalence of poliomyelitis, diphtheria and tetanus antibodies in Spain. Epidemiol Infect. 2002;129:535–41. doi:10.1017/S0950268802007781.
- Jee YM, Cheon DS, Kim KS, Lee SH, Yoon JD, Lee SW, Go U, Yang BK, Ki MR, Choi BY, et al. A seroprevalence study of poliovirus antibody among primary school children in Korea. Epidemiol Infect. 2004;132:351–55. doi:10.1017/s0950268804002171.
- Wallace T, Gregory S, Aaron T, William C, Oberste SM. Seroprevalence of poliovirus antibodies in the United States population, 2009–2010. BMC Public Health. 2016;16:72. doi:10.1186/s12889-016-3386-1.
- Baba MM, Haruna BA, Ogunmola O, Ambe JP, Shidali NN, Oderinde B, Marcello A, Talle M. A survey for neutralizing antibodies to the three types of poliovirus among children in Maiduguri, Nigeria. J Med Virol. 2012 Apr;84(4):691–96. doi:10.1002/jmv.23228.
- Ghana Health Service (GHS). a polio-free country. Disease Surveillance Division Report. 2015.
- Centers for Disease Control and Prevention. Imported wild poliovirus causing poliomyelitis - Bulgaria. MMWR Morb Mortal Wkly Rep. 2001;50(46):1033–35.
- Centers for Disease Control and Prevention. Resurgence of wild poliovirus type 1 transmission and consequences of importation - 21 countries, 2002–2005. MMWR Morb Mortal Wkly Rep. 2006;55(6):145–50.
- Grassly NC, Fraser C, Wenger J. New strategies for the eradication of polio India. Science. 2006;314:1150–53. doi:10.1126/science.1130388.
- Grassly NC, Wenger J, Durrani S. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369:1356–62. doi:10.1016/S0140-6736(07)60531-5.
- Centers for Disease Control. Public health dispatch. An outbreak of poliomyelitis-dominican republic and haiti. MMWR. 2000;49:1049–103.
- Anderson RM. The concept of herd immunity and the design of community-based immunization programmes. Vaccine. 1992;10:928–35.
- Sutter RW, Kew O, Cochi SL. Poliovirus vaccines—live. In: Plotkin SA, Orenstein WA, editors. Vaccines. Vol. ch26. 4th ed. Philadelphia (PA): W.B.Saunders; 2004.
- El-Sayed N, Al-Jorf S, Hennessey KA, Salama M, Watkins MA, Abdel Wahab JA, Pallansch MA, Gary H, Wahdan MH, Sutter RW. Survey of poliovirus antibodies during the final stage of polio eradication in Egypt. Vaccine. 2007;25(27):5062–70. doi:10.1016/j.vaccine.2007.04.022.
- HaiBo W, Cui H, Zheng RD. Seroprevalence of antipolo antibodies among children. J ASM. 2013;Org. (20):33.
- Frantzidou F, Diza E, Halkia D, Antoniadis A. A seroprevalence study of poliovirus antibody in the population of Northern Greece. Clin Microbiol Infect. 2005;11:68–71. doi:10.1111/j.1469-0691.2004.00998.x.
- Diedrich S, Hermann C, Eckart S. Immunity status against poliomyelitis in Germany: determination of cut-off values in International Units. BMC Infect Dis. 2002;2:2. doi:10.1186/1471-2334-2-2.
- Akoua-Koffi G, Thonnon J, Kouassi-Renaud M, Dosso M, Ehouman A. Post-vaccination anti-poliomyelitis seroprevalence in an urban setting in Abidjan. Bull Soc Pathol Exot. 1995;88:117–20.
- Deshpande JM, Kamat JR, Rao VK, Nadkarni SS, Kher AS, Saigaokar SD. Prevalence of antibodies and enteroviruses excreted by healthy children in Bombay. Indian J Med Res. 1995;101:50–54.
- Bassioni LE, Barakat I, Nasr E, de Gourville EM, Hovi T, Blomqvist S, Burns C, Stenvik M, Gary H, Kew OM, et al. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am J Epidemiol. 2003;158(8). doi:10.1093/aje/kwg202.
- World Health Organization. Progress towards global interruption of wild poliovirus transmission. Wkly Epidemiol Rec. 2012;87:195–200.
- Ghana Health Service (GHS). Expanded Programme on Immunization. Half Year Report.2016.
- Pírez MC, Olivera I, Diabarboure H, Montano A, Barañano R, Badía F, Bonnet MC. Seroprevalence of anti-polio antibodies in a population 7 months to 39 years of age in Uruguay: implications for future polio vaccination strategies. Vaccine. 2009;27:2689–94. doi:10.1016/j.vaccine.2009.02.042.
- Schoub BD, Blackburn NK, McAnerney JM. Seroprevalence to polio in personnel at a virology institute. J Infect. 2001;43(2):128–31. doi:10.1053/jinf.2001.0867.
- Williams JO, David-West TS. Poliovirus antibody in children from a paediatric hospital in Ibadan, Nigeria. Rev Roum Virol. 1990;41:129–32.
- Grassly NC. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis. 2012;205(10):1554–61. doi:10.1093/infdis/jis213.
- World Health Organization. Polio. 2013 [accessed 2018 Jul]. http://www.who.int/mediacentre/factsheets/fs114/en/index.html.
- Ofosu-Amaah S, Kratzer J, Nicholas DD. Is poliomyelitis a serious problem in developing countries?” Lameness in Ghanaian schools. Br Med J. 1977;1:1012–14. doi:10.1136/bmj.1.6067.1012.
- Ghana Health Service (GHS). Expanded programme on immunization. Annual Report. 2018.
- Opare JKL, Odoom JK, Akweongo P, Afari EA. lameness among individuals in three regions of Ghana. 2017. unpublished.
- Boche R. La poliomyelite au Cameroun. Revue d’epidemiologie, medecinesociale et santepublique. 1973;21:79.
- Guyer B. The seroepidemiology of poliovirus in Yaounde, Cameroon: a survey following one year of immunization. J Trop Pediatr. 1976;27:140–43. doi:10.1093/tropej/27.3.140.
- Heymann L, Virginia DFM, Lichnevski GKM, Flaubert M. Estimation of incidence of poliomyelitis by three survey methods in different regions of the United Republic of Cameroon. Bull World Health Organ. 1983;61(3):501–07. World Health Organization.
- Collingham KE, Pollock TM, Roebuck MO. Paralytic poliomyelitis in England and Wales 1976-7. Lancet. 1978 May 6;1(8071):976–77. doi:10.1016/s0140-6736(78)90360-4.
- Rotti SB, Satpathy SK, Mehta SP. Prevalence of paralytic poliomyelitis in Pondicherry, South India. Epidemioll Commun Health. 1982;36:279–81. doi:10.1136/jech.36.4.279.
- Horstmann D. Acute poliomyelitis relation of physical activity at the time of onset to the course of the disease. J Am Med Assoc. 1950;142(4):236–41. doi:10.1001/jama.1950.02910220016004.
- Cochran WG. Sampling techniques. 3rd ed. New York (NY): John Wiley and Sons; 1977.
- Ghana Education Service (GES). Enrolment in Schools. Education Management Information System (EMIS) Ghana. 2016. http://www.ghanaeducationdata.com.
- WHO. Manual the virological investigation of polio. Global programme for vaccines and immunization. Expanded programme on immunization. Geneva: WHO; 1997.
- Laforce FM, Lichnevski MS, Keja J, Henderson RH. Clinical survey techniques to estimate prevalence and annual incidence of poliomyelitis in developing countries. Bull World Health Organ. 1980;58:609–20.