ABSTRACT
The aim of this study was to investigate changes in the incidences of acute otitis media (AOM), recurrent AOM (rAOM) and tympanostomy tube (TT) insertion in children following the introduction of 13-valent pneumococcal conjugate vaccine (PCV13) into the national immunization program (NIP) of Turkey in April 2011. National coverage for the PCV7 was 97% in 2009, 93% in 2010, 96% in 2011 and for the PVC13 was 97% in 2012, 97% in 2013, 96% in 2014, 97% in 2015, 98% in 2016, and 96% in 2017 for Turkish children younger than 12 months of age. A total of 499932 pediatric visits were recorded, and AOM was diagnosed in 23005 (4.6%) children. The incidence of AOM in children ≤5 years of age decreased from 10700/100000 (2011) to 4712/100000 (2017), with a significant decreasing trend (p < .001, r = −0.965). When the mean annual incidences of AOM between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for children ≤5 years of age, the incidence of AOM was found to be decreased by 54% (p = 0.013). The mean incidence of TT insertion was found to be decreased by 65% (p = 0.003) between the transition period of PCV13 and a post-PCV13 period for children ≤5 years of age. On the other hand, rAOM incidence was found to be increased in whole pediatric age groups. Our study showed a significant decrease in the incidences of AOM and TT insertion in children ≤5 years old after implementation of PCV13 in the NIP in Turkey.
Introduction
Acute otitis media (AOM) is the most common diagnosis in sick children visiting outpatient clinics and emergency units and the most common reason for administration of antibiotics. AOM occurs at all ages but is most prevalent between 6 and 24 months of age, after which it begins to decline.Citation1,Citation2 Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis are major bacterial causes of AOM. In the pre-pneumococcal conjugate vaccine (PCV) period, nearly 80% of children up to 3 years of age experienced one or more episodes of AOM (approximately half experienced >3 episodes), and 40% of children had experienced six or more recurrences by the age of 7 years.Citation3 Moreover, AOM may cause extracranial and intracranial complications.Citation4 Tympanostomy tube insertion is most commonly the procedure of choice. In fact, it is by far the most common surgical procedure in children.Citation4
To date, 7-, 10-, and 13-valent formulations of the pneumococcal conjugate vaccine (PCV) have been licensed (PCV7, PCV10, and PCV13).Citation3 PCV7 was included in the NIP of Turkey in November 2008 and was used until late 2011. Then, PCV13 was introduced into the NIP of Turkey in April 2011 with a three-dose schedule at 2, 4 and 6 months of age with a booster dose at 12 months.Citation5 No catch-up program was run for those children unvaccinated with PCV13 age older than 12 months of age. The World Health Organization (WHO) reported national coverage for PCV7 of 97% in 2009, 93% in 2010, and 96% in 2011 and for PCV13 of 97% in 2012, 97% in 2013, 96% in 2014, 97% in 2015, 98% in 2016, and 96% in 2017 for Turkish children younger than 12 months of age.Citation6
Many clinical trials have demonstrated the effectiveness of PCV vaccines in decreasing both invasive and noninvasive diseases among both children and adults.Citation1 However, their impact on AOM appeared to be less consistent, and the magnitude of impact varied between different efficacy and effectiveness studies.Citation7,Citation8 Moreover, the impact of PCV13 vaccination on AOM, recurrent AOM (rAOM) and related complications, including tympanostomy tube (TT) insertion, has not been extensively investigated.
The aim of this study was to investigate the impact of PCV13 vaccination on the incidences of AOM, rAOM and TT insertion in children after the implementation of PCV-13 in the NIP of Turkey with a total period of observation of 7 years.
Materials and methods
This retrospective study was performed at Ataşehir Memorial Hospital and Şişli Memorial Hospital located within two different districts of Istanbul (population > 15 million, approximately 20% of Turkey’s population), Turkey. The cases were children aged 1 month-18 years with a diagnosis of AOM applied in our study hospitals’ pediatric outpatient and ear-nose-throat (ENT) outpatient clinics and emergency room (ER) units from January 1, 2011 to December 31, 2017. They were identified according to the International Statistical Classification of Diseases and Related Health Problems-10th (ICD-10 codes): H.65 nonsuppurative otitis media, H.66 suppurative otitis media, and unspecified otitis media and H.67 otitis media in diseases classified in other codes. We also identified a subgroup of patients who underwent tympanostomy tube placement by using hospital operation room records via hospital patient databases. AOM diagnosis was done either experienced pediatricians and ENT specialists during the study period, also TT insertion decisions and operations were done ENT specialists, we did not evaluate clinic presentation of each patients' rather than we preferred to use ICD-10 codes that was mostly used in studies evaluating impact of PCVs.
Any AOM visit identified within 21 days of a previous AOM visit in the same individual was assumed to be the same episode of diseaseCitation8 and was therefore excluded from the analysis. Any new diagnosis of AOM in the same individual after more than 30 days of previous AOM treatment in the same year was considered as recurrent AOM. For comparison purpose as a control group, we considered incidence of adenovirus positive acute gastroenteritis that is unlikely to be related PCV vaccination during the study period for children under age of 5 years. The study was approved by the Şişli Memorial Hospital Ethical Committee.
Statistical analysis
Data were entered into Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and analyzed using Stata 10.0 Statistics/Data Analysis (StataCorp, Lakeway, Drive, TX, USA). The year 2011–2012 was considered a transition period of PCV13 immunization for children ≤5 years of old age (late 2011 is the first year of PCV-13 in the Turkey NIP), and the year 2016–2017 was considered a full vaccination coverage period for children ≤ 5 years old. To measure the linear correlation between the study period (years) and the incidences of AOM, rAOM, and TT insertion, we used the Pearson correlation coefficient (r). A t-test was performed to determine if there was a difference in the mean incidences of AOM, rAOM and TT insertion between the PCV13 transition period and the post-PCV13 period. A p value <.05 was considered significant.
Results
Between 2011 and 2017, a total of 499932 out-patient visits (mean: 71,418 visits/year; range: 53762–89229 visits/year) were attended the ER, pediatric and ENT outpatient clinics (). Among them, 244153 (49%) out-patient visits (mean: 34,879 visits/year; range: 13308–59443 children/year) were ≤5 years old (). The PCV vaccination status of the study population is shown in with respect to study years and age groups. None of the children aged >5 years received any dose of either PCV7 or PCV13; however, after 2016, nearly all children ≤5 years had received PCV13 ().
Table 1. Incidences of Acute Otitis Media, Recurrent Otitis Media and Tympanostomy Tube Insertion with respect to age groups.
Table 2. Pneumococcal conjugate vaccines immunization status of study population with respect to age groups.
Incidences of AOM, rAOM, and TT insertion
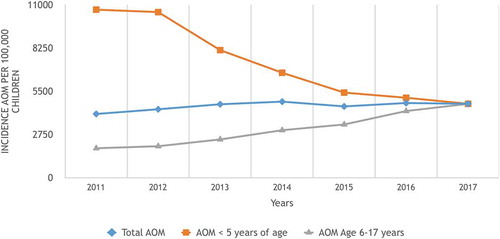
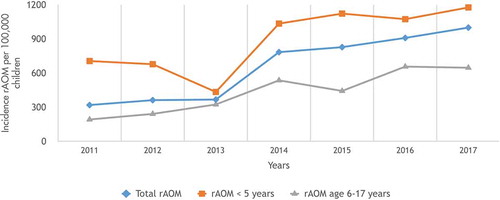
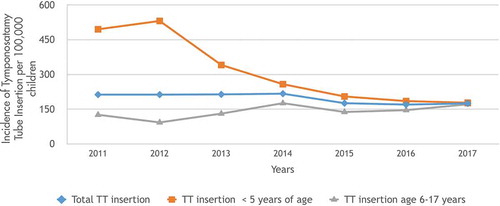
During the study period from 2011 to 2017, a total of 23005 (4.6%) children were diagnosed with AOM (mean: 3286 children/year; range: 2187–4204 children/year) (). Of the study population, rAOM was detected in 3469 (0.7%) children (mean: 495 children/year; range: 172–893 children/year). Most of the rAOM (69%) occurred in children ≤5 years of age (total rAOM = 2407, mean: 343 children/year; range: 94–700 children/year). Nine hundred seventy-four children (0.19%) underwent TT insertion and 623 (64%) of them were ≤5 years of age ().
The annual incidences of AOM in the whole pediatric age group (0–17 years old) were shown in , with insignificant increasing trends (p = 0.6, r = 0.73). When the mean annual incidences of AOM between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for the whole pediatric age group, the incidence of AOM did not change significantly (4216.5/100000 ± 211/100000 vs 4737/100000 ± 36/100000; p = 0.07, 95%CI: −1173 − 132). However, the incidence of AOM in children ≤5 years of age was found to be significantly decreasing (p < 0.001, r = −0.965) (, ). When the mean annual incidences of AOM between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for children ≤5 years of age, the incidence of AOM was found to be significantly decreased by 54% (10622/100000 ± 110/100,000 vs 4904/100000 ± 272/100000; p = 0.0013, 95%CI: 4823–6611). However, the annual incidences of AOM in children aged 6–17 years were found to be significantly increasing (p < 0.01, r = 0.97) (). When the mean annual incidences of AOM between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for children 6–17 years of age, the incidence of AOM increased significantly (1951/100000 ± 91/100000 vs 4211/100000 ± 57/100000; p = 0.001, 95CI: 1501–3554).
When we evaluated the incidence of rAOM, we revealed that the incidence of rAOM in the whole pediatric age group increased significantly (p = 0.001; r = 0.950) (). Additionally, if the mean incidences of rAOM between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for children in the whole pediatric age group, the incidence of rAOM increased significantly (340/100000 ± 30/100000 vs 954/100000 ± 64/100000; p = 0.006, 95%CI: 397–830). The incidences of rAOM in children ≤5 years of age were found to be significantly increasing with respect to study period years (p = 0.03; r = 0.791) (, ), and mean incidences of rAOM were found to be increased between transition period of PCV13 (years 2011/2012) and a post-PCV13 period (years 2016/2017) for children ≤5 years of age (692/100000 ± 19/100000 vs 1125/100000 ± 72/100000; p = 0.01, 95% CI: 203–663). Moreover, the incidence of rAOM for children 6–17 years of age has been increasing significantly with each passing year (p = 0.001; r = 0.947) (). In addition, the mean incidences of rAOM increased between the transition period of PCV13 (years 2011/2012) and a post-PCV13 period (years 2016/2017) for children 6–17 years of age (216/100000 ± 34/100000 vs 652/100000 ± 7/100000; p = 0.003, 95% CI: 327–543).
When the TT insertion incidences were compared, we revealed that the incidence of TT insertion significantly decreased in the whole pediatric age group (p = 0.018, r = 0.842) () and the mean incidences of TT insertion between the transition period of PCV13 (years 2011/2012) and the post-PCV13 period (years 2016/2017) for the whole pediatric age group decreased significantly (213/100000 ± 1/100000 vs 172/100000 ± 3/100000; p = 0.004, 95%CI:29–51). Furthermore, TT insertion incidence in children ≤5 years of age was significantly decreased from 495/100000 to 178/100000 from 2011 to 2017, respectively (p = 0.002 r = −0.930) (). When the mean incidences of TT insertion between the transition period of PCV13 (years 2011/2012) were compared with those of a post-PCV13 period (years 2016/2017) for children ≤5 years of age, the incidence of TT insertion was found to be decreased by 65% (513/100000 ± 25/100000 vs 181/100000 ± 5/100000; p = 0.003, 95%CI:252–410). However, the TT insertion incidence in children aged 6–17 years did not change significantly with respect to years (p = 0.09, r = 0.678) (). Additionally, the mean incidences of TT insertion between the transition period of PCV13 (years 2011/2012) and a post-PCV13 period (years 2016/2017) for children 6–17 years of age did not change significantly (109/100000 ± 23/100000 vs 158/100000 ± 18/100000; p = 0.14, 95%CI: −29 to 131).
Discussion
To the best of our knowledge, this is the first study in Turkey to assess the impact of PCV13 on incidences of AOM, rAOM and TT insertion in children following PCV13 introduction into the NIP. In this study, we showed a significant impact of PCV13 vaccination on the incidence of AOM in children ≤5 years of age, 7 years after PCV13 introduction in Turkey. Our trend analysis revealed that after the implementation of PCV13 into the NIP of our country in late 2011, with a passing each year, the incidence of AOM has been decreasing significantly with a linear slope in children under 5 years of age. We observed a decrease in the mean incidence of AOM between the transition PCV13 period (years 2011/2012) and the post-PCV13 period (years 2016/2017) of 54%. In order to exclude secular trends, we took incidence of adenovirus positive acute gastroenteritis in children under 5 years of age as a control group. This revealed that incidence rate of adenovirus positive AGE did not change significantly from year 2011 to 2017 (incidence rate of adenovirus positive AGE was 2.4/1000, 2.9/1000, 1.6/1000, 1.4/1000, 1.8/1000, 1.8/1000, 1.8/1000, in year 2011,2012,2013,2014,2015,2016, and 2017, respectively; p = 0.18 r = −0.57). One of the initial studies investigating the impact of PCV7 performed by the Northern California Kaiser Permanente Vaccine Study Center Group reported the efficacy of PCV7 against otitis media visits, episodes, frequent otitis media, and ventilatory tube placement as 8.9%, 7.0%, 9.3%, and 20.1%, respectively.Citation8 Another study conducted by the Finnish Otitis Media Study Group enrolled 1662 infants in a randomized, double-blind efficacy trial of a PCV7 vaccine. The PCV7 vaccine reduced the number of episodes of acute otitis media from any cause by 6%, culture-confirmed pneumococcal episodes by 34%, and the number of episodes due to the serotypes contained in the vaccine by 57%.Citation9 In another study, PCV7 vaccination resulted in a 42% reduction in ambulatory visits and a 41.9% reduction in antibiotic usage for AOM in children under 2 years of age.Citation10 A Swedish national observation study reported a 39% reduction in the incidence of AOM, a 42% reduction in the incidence of AOM-related hospital admissions, an 18% reduction in ventilatory tube insertions and a 15% reduction in myringotomies after pneumococcal vaccination.Citation11 Moreover, many studies have assessed the impact of PCV7 or PCV10 on AOM in different countries in children less than 5 years of age, and those studies revealed a broad range of impact rates ranging from a 13% to 35% reduction.Citation12–Citation15 However, very few studies have reported the postvaccination effect of PCV13 alone on the incidence of AOM in children with respect to time-period follow-up. The most important finding of our study is a high impact (46% reduction) of PCV13 on AOM incidence in children ≤5 years of age. Pichichero et al.Citation16 evaluated the effectiveness of PCV13 for protection against AOM by a prospective observation study. They enrolled 239 children who received a full primary series of PCV13 and compared them with 348 children who received PCV7. They showed 86% relative reduction of serotypes and greatest reduction in serotype 19A.Citation16 Kaplan and colleaguesCitation17 also investigated pneumococcal serotypes isolated from middle ear or mastoid cultures for 3 years after routine implementation of PCV13 (2011–2013) in the USA. They revealed that over the 3-year period, the proportion of isolates included in PCV13 significantly decreased from 50% in 2011 to 29% in 2013.Citation17 None of the above studies estimate the change in AOM incidence.Citation16,Citation17 However, our findings are supported by Lau and colleagues.Citation13 They investigated the effect of PCVs on otitis media using a national primary care database in the United Kingdom. They showed that introduction of PCV7 was associated with a 22% significant reduction in otitis media in children aged <10 years old, with an additional 19% reduction after PCV13 introduction.Citation13 Ben-Shimal and colleaguesCitation18 evaluated the effect of PCVs on the incidence of AOM with respect to PCV periods in children under 3 years old in Southern Israel. They revealed that OM caused by PCV7 serotype + 6A serotype and all-pneumococcal OM rates declined by 65% and 30%, respectively, during the PCV7 period; and during the PCV13 period, they reported that overall pneumococcal and PCV7 serotype +6A OM episodes declined by 75% and 94%, respectively.Citation18 It is well defined that the colonization of the nasopharynx by S. pneumoniae is the first step in the development of AOM.Citation19 We previously performed a prospective nasopharyngeal carriage surveillance study between September 2011 and September 2013 after PCV13 introduction in our country in Istanbul, and we showed that after the introduction of PCV13 into the NIP, overall (including vaccine type serotypes and non-vaccine type serotypes) nasopharyngeal pneumococcal carriage was decreased to 7%, especially in children ≤5 years of age.Citation20 The decrease in nasopharyngeal pneumococcal carriage in our country after PCV13 introduction is in accordance with the reduction in the incidence of AOM in children ≤5 years of age in the present study. These findings were also shown by Andrade and colleagues,Citation21,Citation22 who showed a 44% reduction in PCV10 serotype nasopharyngeal pneumococcal carriage 8 months after PCV10 introduction in Brazil (21) and then revealed a 43% reduction in all-cause otitis media visits (22). One of the most important observations of our study was the significant decrease in the incidence of TT insertion in the whole pediatric age period, especially in children ≤5 years of age. In parallel with our observations, the Northern California Kaiser Permanente Vaccine Study Center Group reported efficacy of PCV7 against ventilatory tube placement of 20.1%.Citation8 Additionally, a Swedish national observation study reported an 18% reduction in ventilatory tube insertions and a 15% reduction in myringotomies after pneumococcal vaccination.Citation11 However, the Finnish Otitis Media Study Group initially reported that PCV7 efficacy in preventing TT placement was only 4% until 24 months of age.Citation9 However, the same study group reported PCV7 vaccine efficacy in preventing TT placement was 44% in their follow-up study that investigated TT placement history at a single visit at 4–5 years of age.Citation23 Another important observation of our study was the incidence of rAOM in the whole pediatric age group was found to be increased significantly. We also revealed that incidence of AOM in children age 6–17 years of age increased. Several studies have demonstrated that pneumococcal conjugate and polysaccharide vaccination have no beneficial effect on episodes of AOM, otitis media with effusion and reoccurrence of otitis media in children with a history of previous otitis media.Citation24–Citation26 Non-efficacious of pneumococcal vaccines on rAOM could be explained by serotype replacement by non-vaccine type serotypes that occurs when vaccine-induced reductions in the prevalence of vaccine-type serotypes. Serotype replacement were demonstrated in both the nasopharynx and the middle ear after vaccination with PCV7. Secondly, pneumococcal vaccination may also induce replacement by other pathogens involved in middle-ear disease, such as Staphylococcus aureus, H influenzae, or M catarrhalis. Thirdly, recurrent otitis media may be caused not by actual reinfection with pneumococci but by dead bacteria or nonbacterial substances causing ongoing inflammation of the middle-ear mucosa.Citation25 Moreover, it has been demonstrated that children who experience a first AOM episode in early life have a higher risk of recurrences and persistences.Citation27 Generally, first episodes of AOM are often caused by S. pneumoniae but recurrences are often caused by other otopathogens.Citation27 Fortoiner et al. showed that pneumococcal conjugate vaccines postpone the onset and reduces the risk of first AOM episode but have no effect on recurrences of AOM that is supporting the impact of PCV on overall AOM is largely attributable to the prevention of first AOM episode.Citation27 On the other hand, we observed that incidence of AOM was increased in children age 6–17 years of age, this increment may be explained as none of the those children received PCV. Supporting our findings Sasaki et al. reported that the incidence of AOM-related medical visits in pediatric age groups did not decline significantly. Furthermore, in some age groups (age above 5 years) the incidence of AOM-related medical visits increased in Japan after PCV introductionCitation28. But they can not find any reason for this increment like us.
This study had several limitations. First, the study population does not include the entire population of the country’s children, but Istanbul (nearly 20% of whole country’s population) is the largest city of our country Turkey. In Istanbul, more than 100 hospitals exist including state and private hospitals. Ataşehir Memorial Hospital located in Asia region of Istanbul and Şişli Memorial Hospital located in European region of Istanbul. Both hospitals are large hospitals that were especially working with private health insurance system. We think that our study hospitals may be representative of the Istanbul population, and we can get an idea regarding our country profile and may give a data about the impact of PCV13 on AOM. Second, we had no pre-PCV period cohort with which to compare our study population; therefore, we compared our results with those of the PCV transition period, but our data consisted of at least 7 years post-PCV13 follow-up. Our findings were as follows: during the 7-year period following the introduction of PCV13 (2 years of the PCV7 period + 7 years of the PCV13 period), there was a significant reduction in the incidences of AOM and TT insertion in children vaccinated with PCV13 under 5 years of age.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, Takata GS. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA. 2010;304:2161. doi:10.1001/jama.2010.1651.
- Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875. doi:10.1001/jama.279.11.875.
- Skinner JM, Indrawati L, Cannon J, Blue J, Winters M, Macnair J, Pujar N, Manger W, Zhang Y, Antonello J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15- CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine. 2011;29:8870–76. doi:10.1016/j.vaccine.2011.09.078.
- Bluestone CD. Clinical course, complications and sequelae of acute otitis media. Pediatr Infect Dis J. 2000;19(5 Suppl):S37. doi:10.1097/00006454-200005001-00007.
- Ceyhan M. Konjuge Pnömokok Aşılarında Son Gelişmeler: 13-Valanlı Konjuge Pnömokok Aşısı. J Pediatr Inf. 2011;5:68–73. doi:10.5152/ced.2011.25.
- World Health Organization (WHO). Immunization summary. http://apps.who.int/gho/data/node.main.PCV3n?lang=en
- Vojtek I, Nordgren M, Hoet B. Impact of pneumococcal conjugate vaccines on otitis media: A review of measurement and interpretation challenges. Int J Pediatr Otorhinolaryngol. 2017;100:174–82. doi:10.1016/j.ijporl.2017.07.009.
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95.
- Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, Kohberger R, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–09. doi:10.1056/NEJM200102083440602.
- Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121:253–60. doi:10.1542/peds.2007-0619.
- Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36:1027–31. doi:10.1097/INF.0000000000001654.
- Fortunato F, Marinelli D, Cappelli MG, Cozza V, Prato R. Impact of pneumococcal conjugate universal vaccination on pneumococcal disease in Italian children. J Immunol Res. 2015;2015:206757. doi:10.1155/2015/206757. Epub 2015 Aug 16.
- Lau WC, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, Sharland M, Wong IC, Hsia Y. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33:5072–79. doi:10.1016/j.vaccine.2015.08.022.
- Suarez V, Michel F, Toscano CM, Bierrenbach AL, Gonzales M, Alencar AP, Ruiz Matus C, Andrus JK, de Oliveira LH. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: time series analyses. Vaccine. 2016;34:4738–43. doi:10.1016/j.vaccine.2016.07.027.
- Wals PD, Carbon M, Sévin E, Deceuninck G, Ouakki M. Reduced physician claims for otitis media after implementation of pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2009;28:e271–5. doi:10.1097/INF.0b013e3181bad212.
- Pichichero M, Kaur R, Scott DA, Gruber WC, Trammel J, Almudevar A, Center KJ. Effectiveness of 13- valent pneumococcal conjugate vaccination for protection against acute otitis media caused by streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2:561–68. doi:10.1016/S2352-4642(18)30168-8.
- al; SL K, KJ C, WJ B, Ling-Lin P, JR R, JS B, TQ T, JA H, TR P, Gurtman A, et al. Multicenter surveillance of streptococcus pneumoniae isolates from middle ear and mastoid cultures in the 13-valent pneumococcal conjugate vaccine era. Clin Infect Dis. 2015;60:1339–45. doi:10.1093/cid/civ067.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63:611–18. doi:10.1093/cid/ciw347.
- Syrjänen RK, Herva EE, Mäkelä PH, Puhakka HJ, Auranen KJ, Takala AK, Kilpi TM. The value of nasopharyngeal culture in predicting the etiology of acute otitis media in children less than two years of age. Pediatr Infect Dis J. 2006;25:1032–36. doi:10.1097/01.inf.0000241097.37428.1d.
- Soysal A, Karabağ-Yılmaz E, Kepenekli E, Karaaslan A, Cagan E, Atıcı S, Atınkanat-Gelmez G, Boran P, Merdan S, Hasdemir U. The impact of a pneumococcal conjugate vaccination program on the nasopharyngeal carriage, serotype distribution and antimicrobial resistance of streptococcus pneumoniae among healthy children in Turkey. Vaccine. 2016;34:3894–900. doi:10.1016/j.vaccine.2016.05.043.
- Andrade AL, Ternes YM, Vieira MA, Moreira WG, Lamaro-Cardoso J, Kipnis A, Cardoso MR, Brandileone MC, Moura I, Pimenta FC. Direct effect of 10-valent conjugate pneumococcal vaccination on pneumococcal carriage in children Brazil. PLoS One. 2014;9:e98128. doi:10.1371/journal.pone.0098128.
- Sartori AL, Minamisava R, Bierrenbach AL, Toscano CM, Afonso ET, Morais-Neto OL, Antunes JLF, Cristo EB, Andrade AL. Reduction in all-cause otitis media-related outpatient visits in children after PCV10 introduction in Brazil. PLoS One. 2017;12:e0179222. doi:10.1371/journal.pone.0179222.
- Palmu AA, Verho J, Jokinen J, Karma P, Kilpi TM. The seven - valent pneumococcal conjugate vaccine reduces tympanostomy tube placement in children. Pediatr Infect Dis J. 2004;23:732–38. doi:10.1097/01.inf.0000133049.30299.5d.
- Le TM, Rovers MM, Veenhoven RH, Sanders EA, Schilder AG. Effect of pneumococcal vaccination on otitis media with effusion in children older than 1 year. Eur J Pediatr. 2007;166:1049–52. doi:10.1007/s00431-006-0379-6.
- van Heerbeek N, Straetemans M, Wiertsema SP, Ingels KJ, Rijkers GT, Schilder AG, Sanders EA, Zielhuis GA. Effect of combined pneumococcal conjugate and polysaccharide vaccination on recurrent otitis media with effusion. Pediatrics. 2006;117:603–08. doi:10.1542/peds.2005-0940.
- Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, IJzerman E, Hermans P, de Groot R, Zegers B, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003;361:2189–95. doi:10.1016/S0140-6736(03)13772-5.
- Fortanier AC, Venekamp RP, Hoes AW, Schilder AGM. Does pneumococcal conjugate vaccination affect onset and risk of first acute otitis media and recurrences? A primary care-based cohort study. Vaccine. 2019;37(11):1528–32. doi:10.1016/j.vaccine.2019.01.064.
- Sasaki A, Kunimoto M, Takeno S, Sumiya T, Ishino T, Sugino H, Hirakawa K. Influence of pneumococcal conjugate vaccines on acute otitis media in Japan. Auris Nasus Larynx. 2018;45:718–21. doi:10.1016/j.anl.2017.10.006.