ABSTRACT
We conducted a clinical trial to assess the safety and putative efficacy of an additional human rabies immune globulin (HRIG; KEDRAB) versus an older product (Comparator, HyperRAB S/D® [Grifols]) and determine whether HRIG interferes with development of endogenous antibodies versus Comparator, when each is given with an active rabies vaccine. This was a prospective, double-blind, single-period, non-inferiority study in which subjects were randomized (1:1) to a single dose (20 IU/kg) of HRIG or Comparator on day 0 and rabies vaccine (RabAvert® [GlaxoSmithKline]; 1 mL of ≥2.5 IU/mL) on days 0, 3, 7, 14, and 28. Anti-rabies antibodies were measured by rapid fluorescent focus inhibition test on day 14, and subjects were followed until day 185. Rabies virus neutralizing antibody (RVNA) titers ≥0.5 IU/mL were considered seroconversion putatively indicative of protection. The non-inferiority criterion was the lower limit of the 90% confidence interval (CI) >–10%, for the between-group difference in the proportion of subjects achieving RVNA ≥0.5 IU/mL. On day 14, 98.3% of 59 subjects in the HRIG group and 100% of 59 in the Comparator group had RVNA ≥0.5 IU/mL (difference between proportions – 1.8%; 90% CI, – 8.2, 3.1; non-inferiority criterion met). One subject in the HRIG group did not meet the seroconversion criteria for anti-rabies antibody, and one subject in the Comparator group showed an anamnestic response, with much higher than expected anti-rabies antibody levels at both baseline and on day 14. Thus, HRIG allows for prophylactic anti-rabies antibody titers and is non-inferior to Comparator, when administered with rabies vaccine.
Introduction
Rabies is a serious viral zoonosis that remains a significant public health problem in many regions of the world.Citation1–Citation5 After entering the central nervous system, the rabies virus causes an acute, progressive encephalomyelitis that is almost always fatal if there is no intervention prior to the emergence of symptoms.Citation6,Citation7 Globally, rabies is responsible for approximately 59,000 deaths annually, with infection from dogs accounting for over 99% of fatal cases.Citation8 In the United States, canine rabies has been largely controlled since the 1970s as a result of routine vaccination of domestic animals and wildlife, and animal control programs. Since then, the vast majority of rabies circulates among wildlife. In 2017, the major reservoir species in the United States were bats (32.2%), raccoons (21.1%), foxes (7%), cats (6.2%), dogs (1.2%), and cattle (0.8%).Citation9 As a result of aggressive control efforts, rabies in humans is extremely rare in the United States. During 2017, samples from 21 persons suspected clinically of having developed rabies were submitted to the U.S. Centers for Disease Control and Prevention (CDC) for diagnostic testing. Two persons (9.5%) were confirmed to have had rabies, and both died.Citation9
Human infection occurs when an infected animal transmits the virus to man via saliva through a bite, a scratch, fluid (blood, saliva) contact with mucous membranes (such as the eyes, nose, or mouth), or licking of a wound.Citation12 Following viral inoculation e.g. at the bite site, viral entry into axonal terminals at the neuromuscular junction is mediated through nicotinic acetylcholine receptors, although neural cell adhesion molecule and p75 neurotrophin receptors may also play a role in neurotrophism and cell-to-cell spread.Citation6,Citation13 From the peripheral nervous system, rabies virus spreads to the central nervous system (CNS) via retrograde fast axonal transport.Citation14 CNS neuropathophysiology includes altered serotonergic/cholinergic signaling, and altered immediate early gene activation patterns, although their role in the pathogenesis of clinical rabies is unclear.Citation15–Citation17 Interestingly, at autopsy in rabies patients, inflammation is generally mild and neurodegeneration is minimal, suggesting that changes in neuronal function rather than neuronal loss or inflammatory effects drive rabies neuropathology.Citation6
Rabies remains a fundamentally incurable disease. Although nearly universally fatal if left untreated, post-exposure prophylaxis (PEP) for individuals with suspected exposure to rabies is uniformly effective when appropriately administered.Citation18 Recommendations for PEP include 1) immediate washing of the wound with soap and water, and irrigation with a virucidal agent; 2) induction of active immunity with vaccine; and 3) providing passive immunity by administering rabies immunoglobulin.Citation19 Rabies immunoglobulin provides rapid passive rabies protection by reducing the local viral burden until a protective response from active immunization is mounted.Citation20–Citation22 The utility of rabies immunoglobulin is supported by results indicating that vaccination alone may not provide protection against rabies in all exposed individuals.Citation21,Citation23,Citation24 When administered according to guidelines, the efficacy of PEP with vaccination plus rabies immunoglobulin, for prevention of death, approaches 100%.Citation18
Only two licensed rabies immunoglobulin products were available in the United States prior to 2017: Bayrab/HyperRAB S/D® (Comparator, Rabies Immune Globulin [Human], Grifols Therapeutics Inc., Research Triangle Park, NC, USA)Citation25 and Imogam® (Rabies Immune Globulin [Human], Sanofi Pasteur SA, Lyon, France),Citation26 until the approval of a third product, KEDRAB™ (HRIG, Rabies Immune Globulin [Human], Kedrion Biopharma Inc., Fort Lee, NJ, USA).Citation27 In 2016, HyperRAB S/D represented 96% of the rabies immunoglobulin market in the United States, with the remainder comprising of Imogam.Citation28 Such medicines whose supply are dependent on 3 or fewer manufacturers are particularly vulnerable to drug shortages.Citation29 Correspondingly, in the period from 2001 to 2015, among shortages of any vaccine or immune globulin in the United States, the longest shortage in duration was for rabies immunoglobulin.Citation30 In 2018, HyperRAB S/D was discontinued. As the consequences of untimely or inadequate (e.g. insufficient wound infiltration) administration of rabies immunoglobulin can lead to fatal PEP failure, such shortages are a critical concern in emergency medicine.Citation31,Citation32 Thus, the clinical development of HRIG was undertaken in order to assess the non-inferiority of HRIG compared to the dominant market product and establish its suitability to diversify rabies immunoglobulin supply sources in the United States.
The pharmacokinetics (PK) and safety of this new HRIG had been investigated in 2 phase 1 studies, indicating that it was well tolerated and that recipients achieved an adequate level of rabies virus antibody titers reflective of seroconversion and indicative of protection (≥0.5 IU/mL),Citation33,Citation34 when HRIG was administered in conjunction with active rabies vaccine.Citation27 Virus antibody titer of ≥0.5 IU/mL is used as a correlate of protection since a protective concentration cannot be established in humans. We report here the results of a phase 2/3, single-center, prospective, randomized, double-blind, parallel-group, non-inferiority study for licensure in healthy male and female volunteers ≥18 years old.
Materials and methods
Design
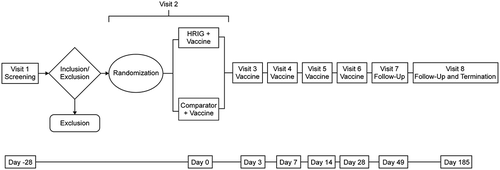
This study (NCT02040090; ) evaluated the safety of the new HRIG versus Comparator, an older marketed product, in a simulated PEP regimen. The objective was to determine whether either preparation interfered with development of endogenous antibodies, when co-administered with an active rabies vaccine (RabAvert®, GlaxoSmithKline, NDC#58160–964). Prior to any study activity, the protocol and Informed Consent Form were approved by RCRC Independent Review Board, Austin TX, USA. All subjects provided informed written consent prior to any study procedures.
Selection of study population
Subjects were healthy male and female volunteers 18 to 75 years of age who reported they had had no prior exposure to rabies epidemic, rabies vaccine, and/or rabies immunoglobulin. Subjects were identified and contacted based on eligibility criteria from an active database maintained by study site.
Treatment
Subjects were randomized (1:1) to receive a single dose (20 IU/kg) of HRIG or Comparator on day 0 and rabies vaccine (1 mL of ≥2.5 IU/mL) on days 0, 3, 7, 14, and 28. HRIG or Comparator was administered as a single dose via intramuscular injection as follows: the first 5 mL of the dose was administered to the left leg lateral muscle; the remainder (up to 5 mL) was administered to the right leg lateral muscle. Additional amounts up to 2.5 mL (for a subject >75 kg but ≤93.75 kg) were administered to the left deltoid muscle. The doses and timing of study treatments were based on the recommendations for rabies PEP at the time of study design.Citation35 The right deltoid muscle was utilized for the administration of the rabies vaccine. The sponsor, principal investigator, and other staff members at the study site who may have had contact with the subject were blinded as to which HRIG product subjects received.
Assessments
Blood and urine collection
Serum samples for determination of rabies virus neutralizing antibody (RVNA) titers were collected on days 0, 3, 7, 14, 28, 49, 185, and/or early discontinuation. Serum samples for immunogenicity markers (complement activation markers C3, C4, and CH50) were collected on days 0 (prior to drug administration), 14, 49, 185, and/or early discontinuation. Samples for hematology, clinical biochemistry, and urinalysis tests were collected at screening, days 7, 28, 49, 185, and/or early discontinuation. Additional samples for hemolysis assessment were collected on days 0, 3, 7, and 14 (and day 28 if results from day 14 were abnormal).
Diary cards
Diary cards were completed by subjects at home to record adverse events (AEs), concomitant medications, and any additional information deemed relevant by the subjects, for 14 days from the start of treatment (day 0) until day 14 or early discontinuation.
Vital signs, electrocardiograms, and physical examinations
Vital signs were recorded at screening, days 28, 49, 185, and/or early discontinuation. Electrocardiograms (ECGs) were performed at screening, day 185, and/or upon early discontinuation, if applicable. Physical exams were performed at screening, days 49 and 185, and/or upon early discontinuation. Body temperature was recorded before administration of study treatment and at all visits through day 28.
Adverse events
Adverse events were solicited and recorded throughout the study.
Assays
The rapid fluorescent focus inhibition test (RFFIT) was used to determine the total RVNA titer. RFFIT does not distinguish between IgG and IgM, but measures the combined activity of passive (IgG) and active immunity, and is considered the appropriate test to ascertain the effectiveness of rabies vaccination.Citation36,Citation37
Endpoints
Efficacy
The primary endpoint was achievement of an RVNA titer ≥0.5 IU/mL on day 14, as determined by RFFIT. This threshold was chosen based on the World Health Organization recommended minimum anti-rabies antibody titer threshold value (≥0.5 IU/mL), which is considered an adequate measure of seroconversion after vaccination and uniformly thought to provide protection during rabies exposure.Citation33,Citation34 In this non-inferiority trial, the null hypothesis was that the proportion of HRIG + vaccine subjects with anti-rabies concentration ≥0.5 IU/mL on day 14 would not be less than the corresponding proportion of Comparator + vaccine subjects by ≥0.1.
Pharmacokinetics
Secondary endpoints included selected PK parameters: maximum concentration in plasma (Cmax), time to maximum concentration in plasma (tmax), area under the concentration-time curve from 0 to the last observation (AUC0-last), area under the concentration-time curve from 0 to infinity (AUC0-∞), and the plasma half-life (t1/2) of anti-rabies antibody titers, as measured by RFFIT.
Safety
The safety and tolerability of the study treatments were assessed based on vital signs and physical examination findings, ECGs, laboratory findings, and the occurrence of AEs after drug administration. A treatment-emergent adverse event (TEAE) was defined as any AE that occurred on or after the date and time of the first dose of study treatment. Related AEs were considered by the principal investigator to have a relationship (“Related”, “Probable”, “Definite”) to study drug.
Data analysis
Study populations
The as-treated population was defined as all randomized subjects who received at least three vaccine doses and one dose of the HRIG or Comparator on day 0. The safety population included all subjects who were randomized and who received at least one dose of study medication.
Statistical methods
The proportions of subjects in the HRIG and Comparator groups with RVNA titers ≥0.5 IU/mL on day 14 were determined using assessment of proportions and confidence intervals (CIs). The null hypothesis, that the difference between the proportions of subjects in the HRIG versus Comparator groups was ≤ – 0.1, was rejected if the lower bound of an exact 90% binomial CI exceeded 0.1.Citation38 A sample size of 53 in each group provided 80% power to reject the null hypothesis.
Safety and tolerability were assessed descriptively and displayed by arithmetic means and standard deviation (S.D.) for quantitative outcomes and by comparing the differences between day 0 (baseline) and post-dosing days. PK analysis was done by log transformation of the plasma HRIG concentrations, and an asymptotic 90% CI for these values was calculated.
This trial is registered under Clinicaltrials.gov identifier NCT02040090.
Results
Subjects
A total of 118 subjects were randomized and treated with HRIG (59 subjects) or Comparator (59 subjects). Overall, 113 subjects (95.8%) completed the study and 5 subjects (4.2%) terminated early (), most often for an adverse event (2 subjects, both in the HRIG group). All but 5 subjects (4 in the HRIG group and 1 in the Comparator group) received all 5 doses of rabies vaccine. Demographic characteristics were comparable between treatment groups, with the majority of subjects being female (63.6%), white (93.2%), not of Hispanic or Latino ethnicity (97.5%), and with a median age of 47.5 years ().
Figure 2. Subject disposition. HRIG = human rabies immune globulin. *Early termination subjects in Comparator group met the criteria for inclusion in the as-treated population and therefore were not excluded from the analysis.
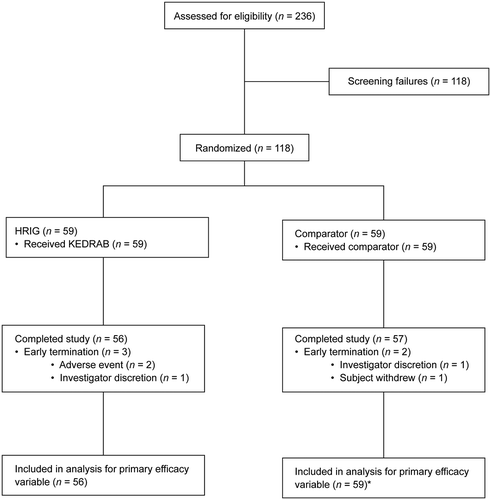
Table 1. Subject characteristicsa.
Efficacy
Overall, 98.2% of subjects in the HRIG group and 100% of those in the Comparator group had an RVNA titer ≥0.5 IU/mL on day 14. The difference between the proportions of subjects achieving this endpoint was – 1.8% (90% CI, – 8.2, 3.0; ). The lower limit of the 90% CI was greater than the pre-specified non-inferiority margin of – 10%, thus demonstrating that the primary endpoint of non-inferiority was achieved.
Table 2. Subjects with geometric mean RVNA ≥0.5 IU/mL on day 14a.
A post-hoc sensitivity analysis, including available data for day 14 for one of the study subjects who discontinued due to an AE, provided results consistent with those from the primary analysis (between-group difference in proportions achieving RVNA ≥0.5 IU/mL = – 1.8%; 90% CI, – 8.1, 3.2).
Pharmacokinetics
The plasma concentration-time profiles following intramuscular injection of HRIG and Comparator appeared similar (), and demonstrated that plasma RVNA concentrations declined in a biphasic manner after the absorption phase was complete. All subjects in both treatment groups had detectable RVNA at day 3, and there were no statistically significant between-group differences in plasma neutralizing antibody PK parameters (). Only the RVNA titers at visit 3 (day 3) were statistically different between the HRIG and Comparator groups. The geometric mean (S.D.) values were 0.18 (0.05) IU/mL and 0.22 (0.05) IU/mL, respectively (p = .0003). Although RVNA titers were still quantifiable on day 185 in all subjects who completed the study, it was not possible to calculate a terminal-phase t1/2 in all subjects because it requires at least 3 quantifiable RVNA titers to be determined from samples collected after the observed tmax. The geometric mean (S.D.) for the terminal-phase t1/2 was 45.9 (1.39) days for HRIG (n = 43) and 50.9 (1.30) days for Comparator (n = 44).
Figure 3. Mean (+S.D.) plasma RVNA concentrations for HRIG and Comparator (each administered with vaccine). S.D. = standard deviation, RVNA = rabies virus neutralizing antibody, HRIG = human rabies immune globulin.
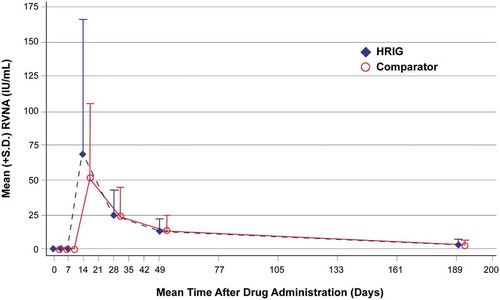
Table 3. RVNA pharmacokinetic parameters for HRIG and comparatora.
Safety
Overall, HRIG was well tolerated with a safety profile similar to that for Comparator (). The most frequently reported TEAEs in the HRIG and Comparator groups, respectively, were injection site pain (49.2% vs. 39.0%), headache (13.6% vs. 15.3%), upper respiratory tract infection (13.6% vs. 13.6%), and myalgia (13.6% vs. 10.2%). The most common drug-related TEAEs in the HRIG and Comparator groups, respectively, were injection site pain (42.4% vs. 28.8%), headache (3.4% vs. 5.1%), and myalgia (1.7% vs. 5.1%; ). While the incidence of injection site pain considered to be related to treatment was numerically higher with HRIG versus Comparator, a post hoc statistical analysis indicated that this difference was not significant (Fisher exact test, p = .178). No deaths occurred during the study. One subject in the HRIG group had a serious TEAE, an intraductal proliferative breast lesion that resulted in discontinuation of study treatment. One additional subject in the HRIG group had a non-serious TEAE of nipple pain that resulted in discontinuation of study treatment. Neither of these TEAEs were considered related to study drug.
Table 4. Adverse eventsa.
There were no clinically meaningful differences between the HRIG and Comparator groups for changes from baseline in hematology, clinical chemistry, urinalysis, vital signs, ECGs, serology, or immunogenicity during the study. There were no clinically meaningful differences between treatment groups in medical/surgical history or concomitant medication usage. No AEs related to hemolysis or thrombogenicity were observed.
Discussion
The results from this study showed that HRIG was non-inferior to an established product, Comparator, for achieving RVNA ≥0.5 IU/mL on day 14, when each was administered concomitantly with rabies vaccine. The design for this study was based on the Advisory Committee on Immunization Practices guidelinesCitation35,Citation39 for rabies PEP, which recommend that treatment in individuals without prior vaccination consist of both rabies immunoglobulin and vaccine. This protocol provides passive immunity protection in the initial stage of exposure, while the active immune response is just developing and the patient is at risk. Although an endogenous anti-rabies antibody response elicited by active immunization is crucial for effective protection, passive immunization with rabies immunoglobulins may interfere with this active immune response.Citation39 Thus, a key objective of this study was to establish the non-inferiority of the RVNA response after simulated PEP using HRIG + vaccine, as compared to with Comparator + vaccine. The threshold for non-inferiority was achieved by HRIG despite the fact that one subject in the HRIG group demonstrated outlying results in not achieving anti-rabies neutralizing antibody titer ≥0.5 IU/mL on day 14 after administration (but did by day 28). A personal communication with Susan M. Moore, Ph.D., from the reference laboratory (Kansas State University Veterinary Diagnostic Laboratory), revealed that “ … though not common, there are some subjects who fail to reach 0.5 IU/mL by day 14 (1.4%-13%).” Failures to achieve neutralizing antibody titers ≥0.5 IU/mL on day 14 have also been reported in prior studies of rabies vaccines.Citation40–Citation42 One subject who received Comparator had an elevated RVNA level at baseline and RVNA level of 724.1 IU/mL on day 14, which was considerably higher than other subjects and was suggestive of an anamnestic immune response resulting from prior exposure to rabies antigen.Citation43 One subject in the Comparator group withdrew from the study after missing a dose of vaccine on day 28. These subjects met the criteria for inclusion in the as-treated population and therefore were not excluded from the analysis.
Two subjects in the HRIG group withdrew due to adverse events that were not considered related to the study by principal investigator. One subject had a positive mammogram test and ultrasound-guided biopsy of breast mass diagnosed as positive for Grade II ductal carcinoma, after screening but before receiving HRIG on day 0. This subject underwent a breast mass lumpectomy during the study, at which time it was determined to end her participation in the study. Another subject discontinued due to a TEAE of non-serious nipple pain of “moderate” intensity, which was considered “unlikely related” to the study treatment by the principal investigator and which resolved by the end of the study. One subject in the HRIG group prematurely stopped receiving vaccine at the investigator’s discretion due to medication use (prednisone, naproxen, hydrocodone) associated with shoulder pain that was part of the subject’s medication history; no AE was reported in association with this medication use.
Overall, HRIG was well tolerated and had a comparable safety profile to Comparator. Treatment-related local injection site pain was reported more often in the HRIG group (42.4%) compared with the Comparator group (28.8%), but this difference was not statistically significant. There are slight differences in the HRIG and Comparator formulations that may have contributed to the trend toward a higher incidence of injection site pain with HRIG.
The efficacy of HRIG in the present controlled trial is consistent with real-world results for this product. Between January 2006 and December 2015, a total of 1,165,279 vials of 2 mL (each equivalent to 300 IU) and 22,551 vials of 10 mL (each equivalent to 1500 IU) of the product have been sold worldwide. This is sufficient to treat approximately 270,000 individuals, assuming a 70-kg average body weight and the recommended dose of 20 IU/kg. No reports of failure of this product to protect recipients, all of whom were also administered rabies vaccine as recommended, have been received by the manufacturer.
The study efficacy assessment was limited to a surrogate immunogenicity measure since studying clinical efficacy in a placebo-controlled design is ethically unacceptable. That an RVNA level of ≥ 0.5 IU/mL indicates adequate seroconversion, and would putatively prevent rabies infection, is a widely used reference recommended by the World Health Organization.Citation33,Citation34 A small study such as this could not establish or verify a “protective level” of RVNA, as subjects were normal healthy volunteers, not patients who were actually or potentially exposed to rabies virus. Because it would be unacceptable to risk exposing volunteers to a potentially lethal virus, a non-inferiority comparison study in unexposed heathy volunteers with an existing effective product was undertaken. When used in much larger numbers of actual rabies virus exposed patients, it is possible that not all would be protected, although both the study and Comparator products are hyperimmune rabies immunoglobulins standardized to 150 IU/ml potency.Citation39
Prompt appropriate medical care can prevent nearly all cases of rabies.Citation18 The number of PEP treatments given in the United States each year is estimated to be about 40,000 to 50,000.Citation44 During 2017, 4,454 cases of rabies in animals and 2 human rabies cases were reported to the CDC. Neither of the human cases received PEP, and both died.Citation9,Citation45 Because shortages of HRIG have been reported and may limit access to appropriate medical treatment in the acute setting of suspected rabies exposure,Citation22,Citation44–Citation46 the availability of another safe and effective HRIG option has the potential to facilitate PEP, potentially assisting in saving lives.
The two existing 150 IU/mL HRIG products available prior to 2017 were licensed in 1974 and 1984; such formulations have been viewed as standard, interchangeable, and established as an essential component of effective rabies PEP.Citation39 In 2016, the Comparator product studied in this trial accounted for 96% of rabies immune globulin in the United States.Citation28 However, poorly diversified biologic markets are vulnerable to supply disruptions due to manufacturing problems, supply-and-demand pressures, or product discontinuation. Unlike vaccines, shortages of emergency products such as HRIG may require institutional responses up to and including importation of commercial alternatives from abroad, potentially increasing healthcare costs.Citation30 In 2018, the market-leading Comparator product was discontinued. Thus, the establishment of HRIG with demonstrated non-inferiority relative to Comparator, meeting FDA bioequivalence criteria, provides an option for supply continuity for use in life-saving PEP.Citation38
In conclusion, results from this controlled trial indicated that HRIG was non-inferior to Comparator for achievement of RVNA titer ≥0.5 IU/mL on day 14, when each product was administered concomitantly with rabies vaccine. HRIG was well tolerated and had a comparable safety profile to Comparator with no clinically meaningful between-treatment differences in TEAEs, laboratory values, vital signs, and ECGs. HRIG provides an important additional treatment option for PEP in individuals exposed to rabies.
Disclosure of potential conflicts of interest
Mark A. Matson, MD – no conflicts of interest; Eran Schenker, MD – former employee of Kamada Ltd, manufacturer of product investigated; Michal Stein, MD – Employee of Kamada Ltd, manufacturer of product investigated; Vladislava Zamfirova, MD former employee of Kedrion Biopharma Inc., US distributor of product investigated; Huy-Binh Nguyen, PhD - employee of Kedrion Biopharma Inc., US distributor of product investigated. Garrett E. Bergman, MD – former employee of Kedrion Biopharma Inc., US distributor of product investigated.
Additional information
Funding
References
- Fahrion AS, Taylor LH, Torres G, Müller T, Dürr S, Knopf L, de Balogh K, Nel LH, Gordoncillo MJ, Abela-Ridder B. The road to dog rabies control and elimination–what keeps us from moving faster? Front Public Health. 2017;5:103. doi:10.3389/fpubh.2017.00081.
- Zhou H, Vong S, Liu K, Li Y, Mu D, Wang L, Yin W, Yu H, Rupprecht CE Human rabies in China, 1960-2014: a descriptive epidemiological study. PLoS Negl Trop Dis. 2016;10(8):e0004874. doi:10.1371/journal.pntd.0004874.
- Cordeiro RA, Duarte NF, Rolim BN, Soares Júnior FA, Franco ICF, Ferrer LL, Almeida CP, Duarte BH, de Araújo DB, Rocha MFG, et al. The importance of wild canids in the epidemiology of rabies in Northeast Brazil: a retrospective study. Zoonoses Public Health. 2016;63(6):486–93. doi:10.1111/zph.12253.
- Kipanyula MJ Why has canine rabies remained endemic in the Kilosa district of Tanzania? Lessons learnt and the way forward. Infect Dis Poverty. 2015;4:52. doi:10.1186/s40249-015-0085-6.
- Onoja AB, Meseko CA, Tekki SI. Rabies unending malady—Nigeria in perspective. Afr J Med Med Sci. 2014;43:105–09.
- Mahadevan A, Suja MS, Mani RS, Shankar SK Perspectives in diagnosis and treatment of rabies viral encephalitis: insights from pathogenesis. Neurotherapeutics. 2016;13:477–92. doi:10.1007/s13311-016-0452-4.
- Davis BM, Rall GF, Schnell MJ Everything you always wanted to know about rabies virus (but were afraid to ask). Annu Rev Virol. 2015;2(1):451–71. doi:10.1146/annurev-virology-100114-055157.
- World Health Organization. Expert consultation on rabies; 2013 [accessed 2017 Dec 21]. http://apps.who.int/iris/bitstream/10665/85346/1/9789240690943_eng.pdf
- Ma X, Monroe BP, Cleaton JM. Rabies surveillance in the United States during 2017. J Am Vet Med Assoc. 2018;253(12):1555–68. doi:10.2460/javma.253.12.1555.
- Di Quinzio M, McCarthy A Rabies risk among travellers. CMAJ. 2008;178(5):567. doi:10.1503/cmaj.071443.
- Gluska S, Zahavi EE, Chein M, Gradus T, Bauer A, Finke S, Perlson E, Schnell MJ Rabies virus hijacks and accelerates the p75NTR retrograde axonal transport machinery. PLoS Pathog. 2014;10(8):e1004348. doi:10.1371/journal.ppat.1004348.
- Gluska S, Finke S, Perlson E. Receptor-mediated increase in rabies virus axonal transport. Neural Regen Res. 2015;10(6):883–84. doi:10.4103/1673-5374.158337.
- Fu ZF, Weihe E, Zheng YM. Differential effects of rabies and borna disease viruses on immediate-early and late-response gene expression in brain tissues. J Virol. 1993;67:6674–81.
- Tsiang H Neuronal function impairment in rabies-infected rat brain. J Gen Virol. 1982;61(pt 2):277–81. doi:10.1099/0022-1317-61-2-277.
- Bouzamondo E, Ladgana A, Tsiang H. Alteration of potassium-evoked 5-HT release from virus-infected rat cortical synaptosomes. Neuroreport. 1993;4(5):555–58. doi:10.1097/00001756-199305000-00023.
- Ravish HS, Chandana K, Pradeep KD, Iswarya S, Rachana AR. Safety, immunogenicity and clinical efficacy of post exposure prophylaxis in confirmed rabies exposures. Glob Vaccines Immunol. 2016;1(3):56–59. doi:10.15761/GVI.1000116.
- Centers for Disease Control and Prevention. What care will I receive? [updated 2016; accessed 2017 Dec 14]. www.cdc.gov/rabies/medical_care/index.html.
- Madhusudana SN, Ashwin BY, Sudarshan S Feasibility of reducing rabies immunoglobulin dosage for passive immunization against rabies: results of in vitro and in vivo studies. Hum Vaccin Immunother. 2013;9(9):1914–17. doi:10.4161/hv.25431.
- Gadekar RD, Domple VK, Inamdar IF, Aswar NR, Doibale MK. Same dog bite and different outcome in two cases—case report. J Clin Diagn Res. 2014;8(6):JD01–2. doi:10.7860/JCDR/2014/9017.4468.
- Bharti OK, Madhusudana SN, Gaunta PL, Belludi AY. Local infiltration of rabies immunoglobulins without systemic intramuscular administration: an alternative cost effective approach for passive immunization against rabies. Hum Vaccin Immunother. 2016;12(3):837–42. doi:10.1080/21645515.2015.1085142.
- Zhang Y, Zhang S, Li W, Hu Y, Zhao J, Liu F, Lin H, Liu Y, Wang L, Xu S, et al. A novel rabies vaccine based-on toll-like receptor 3 (TLR3) agonist PIKA adjuvant exhibiting excellent safety and efficacy in animal studies. Virology. 2016;489:165–72. doi:10.1016/j.virol.2015.10.029.
- Parviz S, Chotani R, McCormick J. Rabies deaths in Pakistan: results of ineffective post-exposure treatment. Int J Infect Dis. 2004;8(6):346–52. doi:10.1016/j.ijid.2004.02.008.
- HyperRAB (Rabies Immune Globulin [Human]) prescribing information. Research Triangle Park: Grifols Therapeutics Inc.; 2012 Sep.
- Imogam (Rabies Immune Globulin [Human]) prescribing information. Lyon (France): Sanofi Pasteur SA; 2014 Oct.
- KEDRAB (Rabies Immune Globulin [Human]) prescribing information. Fort Lee (NJ): Kedrion Biopharma Inc.; 2017 Aug.
- The Plasma Proteins Market in the United States 2016. Orange (Connecticut): The Marketing Research Bureau, Inc.; 2017 July.
- US Government Accountability Office. Report to Congressional Committees: Drug shortages-certain factors are strongly associated with this persistent public health challenge (2016). [accessed 2019 July 16]. www.gao.gov/assets/680/678281.pdf
- Ziesenitz V, Mazer-Amirshahi M, Zocchi M, Fox ER, May LS. U.S. vaccine and immune globulin product shortages, 2001-15. Am J Health Syst Pharm. 2017;74(22):1879–86. doi:10.2146/ajhp170066.
- Wilde H, Sirikawin S, Sabcharoen A, Kingnate D, Tantawichien T, Harischandra PAL, Chaiyabutr N, de Silva DGH, Fernando L, Liyanage JB, et al. Failure of postexposure treatment of rabies in children. Clin Infect Dis 1996;22(2):228–32. doi:10.1093/clinids/22.2.228.
- Wilde H, Sirikanin S, Sabcharoen A. Failure of postexposure treatment in Thailand. Vaccine. 1989;7:49–57. doi:10.1016/0264-410x(89)90010-8.
- World Health Organization. Rabies vaccines: WHO position paper – April 2018. Weekly Epidemiological Rec. 2018;93:201–20.
- SAGE Working Group on Rabies vaccines and immunoglobulins and the World Health Organization (WHO) Secretariat. Background paper: Proposed revision of the policy on rabies vaccines and rabies immunoglobulins. [Accessed 2019 July 18]. https://www.who.int/immunization/sage/meetings/2017/october/1_Background_paper_WG_RABIES_final.pdf
- Advisory Committee on Immunization Practices. Human rabies prevention—United States, 1999. MMWR. 1999;48:1–21.
- Bahloul C, Taieb D, Kaabi B, Diouani MF, Ben Hadjahmed S, Chtourou Y, Imen B’Chir B, Dellagi K Comparative evaluation of specific ELISA and RFFIT antibody assays in the assessment of dog immunity against rabies. Epidemiol Infect. 2005;133(4):749–57. doi:10.1017/s095026880500381x.
- Moore SM, Hanlon CA Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis. 2010;4(3):e595. doi:10.1371/journal.pntd.0000595.
- Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Statistical approaches to establishing bioequivalence (2001). U.S. Department of Health and Human Services (2001). [Accessed 2019 July 8]. https://www.fda.gov/media/70958/download
- Advisory Committee on Immunization Practices. Human rabies prevention—United States, 2008. MMWR. 2008;57:1–28.
- Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Zhao Y, Claudius M A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults. Hum Vaccin Immunother. 2014;10(10):2805–12. doi:10.4161/21645515.2014.972773.
- Li R, Li Y, Wen S, Wen H, Nong Y, Mo Z, Xie F, Pellegrini M. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccin Immunother. 2015;11(2):435–42. doi:10.4161/21645515.2014.994460.
- Cramer JP, Jelinek T, Paulke-Korinek M, Reisinger EC, Dieckmann S, Alberer M, Bühler S, Bosse D, Meyer S, Fragapane E, et al. One-year immunogenicity kinetics and safety of a purified chick embryo cell rabies vaccine and an inactivated Vero cell-derived Japanese encephalitis vaccine administered concomitantly according to a new, 1-week, accelerated primary series. J Travel Med. 2016;23(3):pii: taw011.
- Malerczyk C, Briggs DJ, Dreesen DW, Banzhoff A Duration of immunity: an anamnestic response 14 years after rabies vaccination with purified chick embryo cell rabies vaccine. J Travel Med. 2007;14(1):63–64. doi:10.1111/j.1708-8305.2006.00097.x.
- Centers for Disease Control and Prevention. Cost of rabies prevention [updated 2015; accessed 2017 Dec 15]. www.cdc.gov/rabies/location/usa/cost.html
- Ma X, Monroe BP, Cleaton JM, Orciari LA, Yager P, Li Y, Kirby JD, Blanton JD, Petersen BW, Wallace RM. Rabies surveillance in the United States during 2016. J Am Vet Med Assoc. 2018;252(8):945–57. doi:10.2460/javma.252.8.945.
- Bourhy H, Goudal M, Mailles A, Sadkowska-Todys M, Dacheux L, Zeller H. Is there a need for anti-rabies vaccine and immunoglobulins rationing in Europe? Euro Surveill. 2009;14(13):pii:19166.
- Uwanyiligira M, Landry P, Genton B, de Valliere S Rabies postexposure prophylaxis in routine practice in view of the new Centers for Disease Control and Prevention and World Health Organization recommendations. Clin Infect Dis. 2012;55(2):201–05. doi:10.1093/cid/cis384.
- Food and Drug Administration. CBER-regulated products: resolved shortages [updated 2017; accessed 2017 Dec 11]. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shortages/ucm351943.htm