ABSTRACT
Herpes Zoster (HZ) presents a considerable public health burden in Italy among people aged ≥50 years. This study aimed to assess the clinical and economic impact of HZ vaccination in the 65 years of age (YOA) cohort in Italy, by comparing the new Adjuvanted Recombinant Zoster Vaccine (RZV) with the currently available Zoster Vaccine Live (ZVL). A static Markov model was developed to follow all 65 YOA subjects from the year of vaccination over their lifetime by comparing three different HZ vaccination strategies: no vaccination, vaccination with ZVL and vaccination with RZV. In the base-case scenario, three 65 YOA cohorts were assumed to be vaccinated within three years, with a vaccine coverage rate of 20%, 35% and 50% at Year 1, 2 and 3 respectively, as recommended by the National Immunization Plan. The three 65 YOA Italian cohorts accounted altogether for 2,290,340 individuals. Of these, it was assumed that 564,178 subjects could be vaccinated with either RZV or ZVL in three years. The vaccination with RZV could prevent an additional total number of 35,834 HZ and 8,131 postherpetic neuralgia (PHN) cases over ZVL, leading to additional total savings of €12.4 million for the national healthcare and social systems. The introduction of RZV can be expected to have higher impact on the burden of HZ disease in the 65 YOA cohort in Italy. The avoided HZ and PHN cases can lead to an associated reduction in economic burden to the healthcare and social systems.
Introduction
Herpes Zoster (HZ), commonly known as shingles, is a debilitating disease caused by reactivation of the Varicella Zoster Virus (VZV) that has been dormant in the spinal and cranial sensory ganglia since a primary infection of varicella, that presents itself as chickenpox during childhood.Citation1,Citation2 HZ is characterized by a usually painful, unilateral vesicular rash, generally limited to a single dermatome, corresponding to the sensory ganglion from which the latent VZV was reactivated.Citation3
In Europe, more than 95% of the adult population show serological signs of a previous VZV infection and are, therefore, at risk of developing HZ.Citation4 The main risk factor for reactivation of VZV is represented by the decline in cell-mediated immunity, so that HZ occurs more frequently in older adults and in individuals who are immunocompromised due to underlying disease or immunosuppressive therapy.Citation5 In Italy, the overall incidence of HZ has been estimated at 6.42 cases per 1,000 person-years among people aged ≥50 years.Citation6
The most common and painful complication of HZ is postherpetic neuralgia (PHN), a chronic neuropathic resilient pain that persists or develops at least 90 days after the acute HZ episode and can continue for months or years.Citation7 PHN occurs in 5–30% of patients, but the proportion of patients experiencing it increases with advancing age.Citation1,Citation2
HZ and PHN generate a considerable clinical and economic burden in healthcare and socio-economic systems. In Italy, around 157,000 new HZ cases are estimated every year, for total annual costs of €41.2 million (M), of which €28.2M are direct costs and €13.0M are indirect costs.Citation8,Citation9
In the US, the vaccination against HZ has become the standard of care for reducing HZ disease burden and complications in older adults,Citation10 while the European Center for Disease Prevention and Control (ECDC) reports its recommendation in 6 European countries by 2017.Citation11 In Italy, the National Immunization Plan 2017–2019 (NIP) recommends and funds HZ vaccination for all individuals 65 years of age (YOA) and for at-risk subjects (i.e., those affected by diabetes mellitus, cardiovascular diseases, chronic obstructive pulmonary disease, and candidate to immunosuppressive treatment) aged ≥50 YOA.Citation12
The Zoster Vaccine Live (ZVL, Zostavax, Merck & Co., Inc.), a one-dose vaccine containing the Oka VZV strain, was the first vaccine to be approved for the HZ prevention in adults ≥50 YOA by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). It was licensed in Italy in 2006.Citation13,Citation14
In clinical trials, ZVL has shown an efficacy in preventing HZ and PHN equal to 51.3% and 66.5% respectively in subjects ≥60 YOA,Citation15 that decreases with increasing age and wanes over time post vaccination,Citation16,Citation17 as also confirmed by the available effectiveness data.Citation18 Thus ZVL is suboptimal in preventing HZ in the older age groups who have a higher HZ risk.
Furthermore, as a live-attenuated vaccine, ZVL is contraindicated for use in immunosuppressed or immunodeficient individuals in whom administration of ZVL may result in disseminated disease.Citation14
Recently, a new two-dose Adjuvanted Recombinant Zoster Vaccine (RZV, Shingrix, GSK) has been developed and approved in Canada, the US and Japan for the prevention of HZ in adults ≥50 YOA, while in Europe and Australia, the license also includes the prevention of PHN.Citation19–Citation23
RZV is a non-live vaccine that combines the VZV glycoprotein E (gE) with the AS01B, a GSK proprietary Adjuvant System, able to generate VZV-specific, strong, and sustained humoral and cellular immune responses. As a non-live vaccine, there is no contraindication for immunosuppressed patients.Citation22,Citation24
In two phase III clinical trials,Citation24,Citation25 RZV efficacy against HZ was greater than 90% in all age groups studied with little decline over time up to 4 years. The efficacy of RZV in reducing the PHN and other HZ complications was >90% in all subjects aged ≥50 years.
Based on the available immunogenicity, efficacy, safety and cost-effectiveness data for both ZVL and RZV, in October 2017 the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC, US)Citation26 released a preferential recommendation for RZV over ZVL. The ACIP recommended that all the immunocompetent subjects ≥50 YOA should be vaccinated with RZV and the persons who have already received ZVL earlier in life should be revaccinated.Citation26 Furthermore, the National Advisory Committee on Immunization (NACI, Canada) has recently released a strong recommendation for using RZV in adults ≥50 YOA for the prevention of HZ without contraindications.Citation27
Recent analyses demonstrated the superior public health impact of RZV compared with ZVL in Germany and Japan,Citation28,Citation29 as well as the cost-effectiveness of RZV in older adults in USA and Germany.Citation30,Citation31
A previous study has shown that introducing a HZ vaccination program for older adults using ZVL would have a beneficial clinical and health economic impact in Italy,Citation32 while currently there is no such evidence for RZV in the Italian setting.
The aim of the present study is to evaluate the clinical and related economic impact of implementing an RZV vaccination program in comparison to “no vaccination” and to the current vaccination program with ZVL in the 65 YOA cohort in Italy.
Materials and methods
ZOster ecoNomic Analysis model
The ZOster ecoNomic Analysis (ZONA) model was previously developed in MS Excel ()Citation28,Citation29 and adapted to the Italian healthcare setting to estimate the clinical impact, in terms of HZ and PHN cases, complications and HZ-related deaths avoided, and economic impact, considering both direct and indirect costs avoided, of HZ vaccination in the 65 YOA cohort in Italy. It is a static Markov model that follows the cohort of 65 YOA individuals from the year of vaccination over their lifetime and with annual cycle lengths.
Figure 1. Schematic overview of the ZONA model.Citation28,Citation29
HZ: Herpes Zoster; PHN: postherpetic neuralgia. Figure originally published in D. Curran et al. 2017Citation28
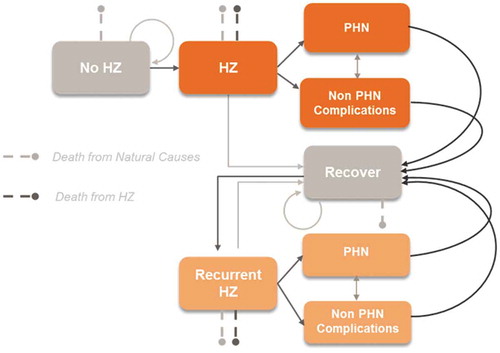
The structure of the model is presented in . Health states considered were healthy, HZ, PHN, non PHN complications, and death, as well as recurrent cases. Transition probabilities were derived from Italy-specific literature and are age-specific.
The ZONA model compares three different HZ vaccination strategies: no vaccination (control), vaccination with ZVL, and vaccination with RZV. For each strategy, three cohorts of 65 YOA individuals are assumed to be either vaccinated or not within 3 years with incremental vaccine coverage rates, as recommended by the NIP (i.e., 20% at Year 1, 35% at Year 2, and 50% at Year 3).Citation12 The resulting eligible population is presented in . In the base-case scenario, the compliance to the second dose of RZV was assumed to be 70%. Additionally, for Year 1, two scenario analyses were performed to explore the impact of using alternative RZV second-dose compliance rates, which were assumed to be 50% and 90%, respectively. The same analyses were performed for Years 2 and 3, as presented in the Supplementary Material.
Table 1. Three cohorts of Italian subjects 65 YOA eligible to HZ vaccination according to the NIP.Citation12
Model inputs and assumptions
The model data inputs and assumptions are divided into four sections: demographics, HZ epidemiology, related direct and indirect clinical costs, and vaccine efficacy.
Demographics
A base-case scenario was assumed for each cohort, as summarized in . Three cohorts of Italian subjects 65 YOA for the Years 2017 (Year 1), 2018 (Year 2) and 2019 (Year 3) were retrieved from the Italian Institute of Statistics (ISTAT)Citation33 counting for approximately 749,000, 762,000 and 778,000 individuals, respectively.
Table 2. Base-case epidemiological data inputs.
Table 3. Base-case cost data inputs.
Epidemiological and cost data
Epidemiological data inputs are summarized in . All-cause mortality rates were derived from the ISTAT database,Citation34 while HZ-specific mortality rates were retrieved from the World Health Organization (WHO) database.Citation35
The HZ incidence rates and the relative proportion of PHN were extracted from the study of Coretti et al.Citation8 In addition to PHN, the analysis included the following complications, derived from Alicino et al.:Citation6 ophthalmic HZ (4%), neurological complications (0.7%) and cutaneous complications (0.37%).
Clinical cost data inputs are reported in . Direct and indirect costs of HZ and PHN were derived from the study of Gialloreti et al.Citation9 and were inflated to 2016 prices using the Hospital and Community Health Service (HCHS) index.Citation36
Vaccine efficacy
Vaccine efficacy data points for both RZV and ZVL were previously calculated and validated for a recent similar study performed in Germany by Curran et al.Citation28 and were further validated during two advisory boards with Italian experts in epidemiology, health economics and infectious diseases.
As previously described, vaccine efficacy and waning data (i.e. absolute percentage waning over time of efficacy of RVZ in preventing HZ) for both vaccines were derived from the respective randomized controlled trials and are reported in Table S1 (Supplementary Material).
RZV was developed in a two-dose schedule and, from a public health perspective, it is unlikely that second-dose compliance reaches 100%. Consequently, the efficacy of a single dose of RZV was assessed in a post-hoc analysis of the phase III studies by Curran et al.,Citation28 as reported in Table S2 (Supplementary Material). Moreover, the RZV single-dose efficacy against HZ was conservatively assumed to wane at 10.9% annually, based on the assumptions made by the ACIPCitation26 and as previously published by Le et al.Citation37
Sensitivity analysis
For the Year 1 analysis, a univariate deterministic sensitivity analysis (DSA) was performed with the aim to assess the robustness of the results. The following base-case parameters were varied: HZ incidence rate, PHN proportion, RZV second-dose compliance, vaccine efficacy and waning for both vaccines (ranges are detailed in Table S2 of the Supplementary Material). The corresponding number of HZ cases avoided were recorded and were summarized in a Tornado diagram.
Results
In the base-case scenario, the three cohorts were assumed to be vaccinated with either RZV or ZVL following the NIP recommendations; the public health impact, in terms HZ and PHN cases, complications and deaths avoided, of the HZ vaccination strategies over lifetime is summarized in .
Table 4. Public health impact of RZV and ZVL in three 65 YOA Italian cohorts under base-case assumptions over a lifetime horizon from the date of vaccination.
The ZONA model estimated that vaccinating 20% of the first 65 YOA cohort (at Year 1) with RZV would prevent 11,880 HZ cases compared to no vaccination, while 5,215 HZ cases would be avoided if ZVL was used ()). A two-fold increase in HZ and PHN cases avoidance was demonstrated when vaccinating the further two 65 YOA Italian cohorts at Year 2 and 3 with RZV compared to ZVL. Assuming a coverage rate of 35% at Year 2, it was estimated that RZV would reduce the number of HZ cases by 21,148, compared with 9,284 using ZVL (Supplementary material table S3). At Year 3, vaccinating 50% of the 65 YOA Italian cohort with RZV would avoid 17,306 more HZ cases compared to ZVL.
Figure 2. HZ (a) and PHN (b) cases avoided with RZV vs. no vaccination and ZVL vs. no vaccination under base-case assumptions and by year of vaccination.
HZ: Herpes Zoster; PHN: postherpetic neuralgia; RZV: Adjuvanted Recombinant Zoster Vaccine; ZVL: Zoster Vaccine Live.
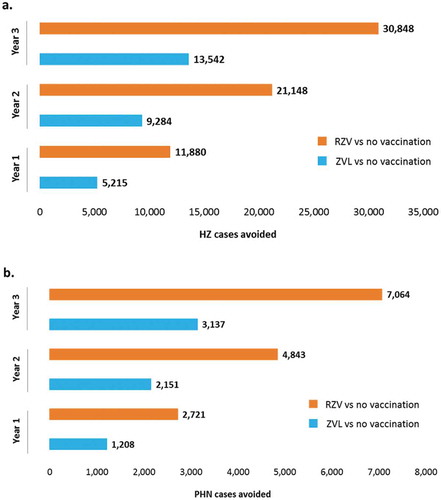
Similarly, our model predicted that, compared to no vaccination, vaccinating the 65 YOA Italian cohort with RZV would prevent 2,721, 4,843 and 7,064 PHN cases in the three years respectively, compared to 1,208, 2,151 and 3,137 respectively for ZVL ()).
Compared to no vaccination, in the base-case scenario, the ZONA model predicted clinical direct costs savings related to HZ, PHN and other complications of €2.8M, €4.9M and €7.2M when vaccinating the 65 YOA Italian cohort with RZV, and of €1.3M, €2.4M and €3.5M using ZVL, at Year 1, 2 and 3, respectively ()). Indirect costs avoided by preventing HZ, PHN and other complications in the 65 YOA Italian cohort with either RZV or ZVL are also presented in ) and highlight a two-fold additional saving when using RZV compared to ZVL.
Figure 3. Direct (a) and indirect (b) costs avoided with RZV vs. no vaccination and ZVL vs. no vaccination under base-case assumptions and by year of vaccination.
RZV: Adjuvanted Recombinant Zoster Vaccine; ZVL: Zoster Vaccine Live.
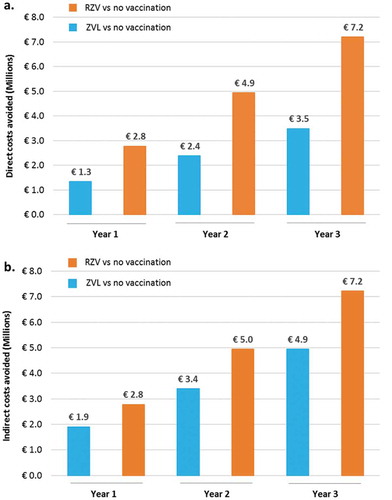
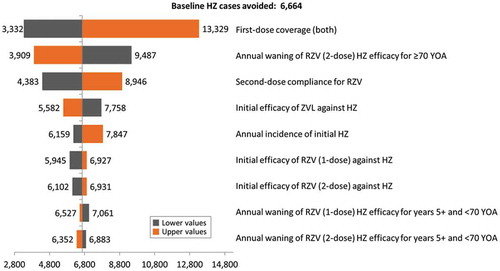
Figure 4. Tornado diagram representing HZ cases avoided with RZV vs. ZVL at year 1 of vaccination.
HZ: Herpes Zoster; YOA: years of age; RZV: Adjuvanted Recombinant Zoster Vaccine; ZVL: Zoster Vaccine Live.
Public health impact analysis of RZV and ZVL in three 65 YOA Italian cohorts under base-case assumptions over a lifetime horizon from the date of vaccination revealed that the vaccination with RZV could prevent an additional total number of 35,834 HZ and 8,131 PHN cases over ZVL. This approximately corresponds to a 56% reduction in these outcomes, amounting to a reduction of €12.4M in direct clinical and indirect costs over the three years ().
Vaccination of the first cohort with RZV (at Year 1, 20% coverage) even considering the worst-case scenario in terms of second-dose compliance (50%), would still prevent 4,383 HZ and 968 PHN cases more than with ZVL, leading to higher direct and indirect costs savings (). Similar findings were found for Years 2 and 3, as reported in Tables S3 and S4, respectively (Supplementary Material).
Table 5. Public health impact of RZV and ZVL in the 65 YOA Italian cohort at Year 1, assuming a coverage rate of 20% over a lifetime horizon from the date of vaccination and different RZV second-dose compliance rates.
The results of the DSA for the 65 YOA Italian cohort at Year 1 are summarized in the Tornado diagram presented in and highlight that in all scenarios, the vaccination with RZV prevents more HZ cases than the vaccination with ZVL. Outcomes of this analysis were most sensitive to the vaccine coverage rates for both vaccines and the RZV second-dose compliance rates.
Discussion
The aim of this study was to evaluate the potential clinical and economic impact of HZ vaccination (as avoided HZ and PHN cases, complications, HZ-related deaths and related clinical cost savings) in Italian cohort of 65 YOA subjects, as recommended by the NIP, using the new RZV in comparison to no vaccination and to the vaccination with ZVL. The NIP in Italy recommends and funds HZ vaccination for all individuals 65 YOA and for at-risk patients aged ≥50 YOA. This study specifically focuses on the cohort of all individuals 65YOA with the aim to evaluate the potential clinical and economic impact of HZ vaccination, introducing RZV compared to the currently available ZVL.
By using an existing and validated static Markov model that follows all subjects 65 YOA from the year of vaccination over their lifetime and with annual cycle lengths,Citation28,Citation29 this public health impact analysis demonstrated the value of both vaccines in reducing the burden of HZ and the related direct clinical and indirect costs for the national healthcare and social systems.
The two main assumptions that led the model to predict superior public health impact in the 65 YOA Italian cohort of RZV compared to ZVL were the higher efficacy and the projections that this efficacy would be sustained over time. Following the NIP recommendations and vaccinating with RZV 20% of the 65 YOA cohort at Year 1, 35% at Year 2, and 50% at Year 3,Citation12 a total number of 35,834 HZ and 8,131 PHN cases over ZVL would be prevented. The RZV impact on case avoidance would lead to a substantial favorable economic impact in the management of HZ costs, amounting to an additional reduction of €12.4M in direct clinical and indirect costs over the three years.
A gradual increase of RZV second-dose compliance rates would lead to a higher impact on the number of HZ cases avoided by using RZV rather than ZVL, ranging from approximately 35,834 cases avoided with a 70% compliance rate to 48,102 with a 90% compliance rate, translating in €16.2M additional direct clinical and indirect costs avoided.
The results of this study are consistent with findings previously reported in other countries, demonstrating the higher public health impact of RZV for the prevention of HZ and PHN in older adults compared to no vaccination or vaccination with ZVL.Citation28,Citation29 Moreover, the ZONA model provided public health impact results that are in line with those obtained in cost-effectiveness analyses of HZ vaccination in older adults performed in the US and Germany.Citation30,Citation31 The latter is in turn consistent with studies that used other independent models.Citation26,Citation37 The results of these studies proved the higher public health impact of RZV compared to ZVL, which has been further recognized and transformed into a recommendation for the use of RZV in the US,Citation26 CanadaCitation27 and Germany.Citation38
The robustness of the ZONA model was supported by several other publications,Citation28–Citation31 and data input and assumptions were confirmed by Italian experts in epidemiology, health economics and infectious diseases in 2 Advisory Boards. The major strength of the present study is that its scenarios were developed based on the current NIP recommendations by the Italian Ministry of Health, thus allowing a concrete contextualization of the results obtained. Moreover, this study included numerous local data sources that ensured an accurate estimation of the potential public health impact of HZ vaccination in the Italian setting. A full health economic analysis is planned, which includes the cost-effectiveness and a complete sensitivity analysis of introducing RZV in the Italian setting.
However, this study has some limitations. First, as long-term RZV waning rates are not yet available, we have used the same assumptions as in the impact analysis performed for Germany,Citation28 in which both the vaccine efficacy waning rates and upper and lower bounds were validated with a group of international experts. Furthermore, the RZV single-dose efficacy against HZ was conservatively assumed to wane at 10.9% annually, based on the assumptions made by the ACIPCitation26 and as previously published by Le et al.Citation37
A second limitation of this analysis is the current lack of data on RZV effectiveness in the real-world setting. Third, the RZV second-dose compliance had to be assumed as data recorded in both the ZOE-50 and ZOE-70 studies (~95%) were not applicable in the real-world setting; therefore, scenario analyses were performed to assess the impact of its variation from 50% to 90%. Nevertheless, even in the worst-case scenario we considered in this analysis (50%), vaccinating with RZV would still prevent more HZ and PHN cases than ZVL, leading to higher direct clinical and indirect costs savings.
Currently, there is no price available for RZV in Italy. Therefore, this study focused on the potential clinical benefits and associated cost avoidance due to prevention by vaccination and does not take into account investment costs. A full assessment of the cost effectiveness of HZ vaccination will not be possible until RZV price becomes available.
Thirdly, the NIP recommends vaccinating both the 65YOA cohort and ≥50 YOA with an at-risk condition. Since there is no robust data available for these at-risk conditions we were unable to do a full analysis to demonstrate the overall impact of HZ vaccination within the NIP setting. For future research it would be an added benefit to have such data available. A full health economic analysis is planned, which includes the cost-effectiveness and a complete sensitivity analysis of introducing RZV in the Italian setting.
Based on this analysis, it can be expected that RZV would have a higher clinical impact compared to ZVL on the burden of HZ disease in Italy when introduced into the NIP. The avoided HZ and PHN cases would be associated with a reduction in healthcare resource utilization and associated costs. This evidence may help policy makers and clinicians to make a more informed decision about HZ vaccination.
Highlights/Focus on patient section
What is the context?
Herpes Zoster (HZ), also known as shingles, mostly occurs in adults aged ≥50 years and with a weakened immune system. HZ is characterized by a painful rash that can be followed by a long-lasting pain, a complication defined as postherpetic neuralgia (PHN). In Italy, this disease affects about 6/1,000 person-years among adults aged ≥50 years, translating in around 157,000 new HZ cases per year. Due to the high number of cases and treatment associated, HZ has a high impact on the Italian healthcare and socio-economic systems.
HZ can be prevented through vaccination, that is now recommended by the 2017–2019 Italian National Immunization Plan for everyone aged 65 years and for subjects aged ≥50 years at risk for presence of chronic obstructive pulmonary disease, diabetes, cardiovascular disease or because candidate to immunosuppressive therapy. A one-dose Zoster Vaccine Live (ZVL) is already available in Italy but recently a new two-dose Adjuvanted Recombinant Zoster Vaccine (RZV) has been approved in Europe.
What is new?
While the impact of the ZVL was already assessed in Italy, this is the first evaluation of the potential public health impact (in terms of avoided HZ and PHN cases and clinical costs) of the new RZV in Italy using a well-validated mathematical model that compared three different strategies:
no vaccination
vaccination with ZVL
vaccination with RZV.
In our projections, vaccinating 3 cohorts of individuals aged 65 years with RZV in Italy would have a greater impact on reducing the burden of HZ, with 35,834 HZ and 8,131 PHN additional cases avoided, compared with the vaccination with ZVL.
What is the impact?
HZ vaccination offers the potential to substantially reduce the HZ burden in Italy. Our model predicts that RZV prevents more HZ and PHN cases than ZVL, with also a major reduction of disease-related costs thus translating into a higher positive impact for the Italian healthcare system.
Disclosure of potential conflicts of interest
AV reports personal fees from the GSK group of companies and from Sanofi Pasteur MSD, outside the submitted work. SB, SD and EF report no conflict of interest. CC, DC, IL, APi, RT and DVO are employees of the GSK group of companies. CC, DC and RT hold shares in the GSK group of companies. APu was a temporary employee of the GSK group of companies during the conduct of the study.
Trademark
Shingrix is a trademark owned by or licensed to the GSK group of companies.
Zostavax is a trademark from Merck Sharp & Dohme Corp.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download Zip (1.1 MB)Acknowledgments
Authors thank Lijoy Varghese, Stephane Lorenc and Mohamed Neine for their involvement in the modeling.
Authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and publications coordination, on behalf of GSK. Stephanie Garcia coordinated manuscript development and editorial support.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(sup3):21–26. doi:10.1586/erv.10.30.
- Cohen JI. Herpes Zoster. N Engl J Med. 2013;369(3):255–63. doi:10.1056/NEJMcp1302674.
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–81.
- Bollaerts K, Riera-Montes M, Heininger U, Hens N, Souverain A, Verstraeten T, Hartwig S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect. 2017;145(13):2666–77. doi:10.1017/S0950268817001546.
- Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008;26(3):675–697, viii. doi:10.1016/j.ncl.2008.03.011.
- Alicino C, Trucchi C, Paganino C, Barberis I, Boccalini S, Martinelli D, Pellizzari B, Bechini A, Orsi A, Bonanni P, et al. Incidence of herpes zoster and post-herpetic neuralgia in Italy: results from a 3-years population-based study. Hum Vaccin Immunother. 2017;13(2):399–404. doi:10.1080/21645515.2017.1264834.
- Schmader K, Gnann JW Jr., Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008;197(Suppl 2(Supplement_2)):S207–215. doi:10.1086/522152.
- Coretti S, Codella P, Romano F, Ruggeri M, Cicchetti A. Cost-effectiveness analysis of Herpes Zoster vaccination in Italian elderly persons. Int J Technol Assess Health Care. 2016;32(4):233–40. doi:10.1017/S0266462316000337.
- Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, Di Marzo R, Volpi A. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10(1):230. doi:10.1186/1471-2334-10-230.
- Symoniak MR, Farrokh P, Gandhi MA, Slish JC. Herpes zoster subunit vaccine for the prevention of herpes zoster. Am J Health Syst Pharm. 2018;75(12):861–69. doi:10.2146/ajhp170399.
- European Centre for Disease Prevention and Control (ECDC). Vaccine scheduler - Herpes Zoster: recommended vaccinations. [accessed 2018 Oct 19]. https://vaccine-chedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=51&SelectedCountryIdByDisease=−1
- Gazetta Ufficiale della Republica Italiana. Piano Nazionale Prevenzione Vaccinale 2017–2018 (PNPV). 2017 [accessed 2018 Jul 23]. http://www.gazzettaufficiale.it/eli/id/2017/02/18/17A01195/sg
- Food and Drug Administration. Approval letter - Zostavax. 2018 Mar 21 [accessed 2018 Jul 23]. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm602293.pdf.
- Merck & Co. Inc. Zostavax (zoster vaccine live) prescribing information. [accessed 2017 Jun 15]. www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent Herpes Zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi:10.1056/NEJMoa051016.
- Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney, D.J., Betts, R., Gelb, L., Guatelli, J.C., and Harbecke, R., for the Shingles Prevention Study G. Long-term persistence of Zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–09. doi:10.1093/cid/ciu918.
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al. Persistence of the efficacy of Zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55(10):1320–28. doi:10.1093/cid/cis638.
- Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, Bialek S, Hechter RC, Jacobsen SJ. Declining effectiveness of Herpes Zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016;213(12):1872–75. doi:10.1093/infdis/jiw047.
- Food and Drug Administration. Approval Letter - SHINGRIX, 2017. 2017 Oct 20 [accessed 2018 Jul 23]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581750.pdf
- Health Canada. Shingrix product monograph including patient medication information. 2017 [accessed 2018 Dec 6]. https://pdf.hres.ca/dpd_pm/00041658.PDF
- Pharmaceuticals and Medical Devices Agency. New drugs approved in FY 2017. 2018 [accessed 2018 Oct 16]. http://www.pmda.go.jp/files/000224507.pdf#page=10
- Committee for Medicinal Products for Human Use (EMA - CHMP). Summary of opinion, Shingrix (EMA/CHMP/811150/2017). 2018 [accessed 2018 Oct 16]. https://www.ema.europa.eu/documents/smop-initial/chmp-summary-positive-opinion-shingrix_en.pdf
- Australian Government - Department of health: therapeutic good administration. SHINGRIX recombinant Varicella Zoster virus glycoprotein E antigen vaccine 50 micrograms powder vial and suspension vial for suspension for injection. [accessed 2018 Oct 19]. https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=5E9F90B4A76B269ACA258304004238C9&agid=(PrintDetailsPublic)&actionid=1
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted Herpes Zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of Herpes Zoster vaccines. Morb Mortal Wkly Rep. 2018;67(3):103–08. doi:10.15585/mmwr.mm6703a5.
- National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS): updated recommendations on the use of Herpes Zoster vaccines. 2018 [accessed 2018 Oct 16]. https://www.canada.ca/en/services/health/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines.html
- Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, von Krempelhuber A, Anastassopoulou A. Assessment of the potential public health impact of Herpes Zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13(10):2213–21. doi:10.1080/21645515.2017.1345399.
- Watanabe D, Mizukami A, Holl K, Curran D, Van Oorschot D, Varghese L, Shiragami M. The potential public health impact of herpes zoster vaccination of people aged ≥ 50 years in Japan: results of a Markov model analysis. Dermatol Ther (Heidelb). 2018;8(2):269–84. doi:10.1007/s13555-018-0236-3.
- Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, Hicks K, Lee B, Yawn B. Cost-effectiveness of an Adjuvanted Recombinant Zoster Vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. doi:10.1016/j.vaccine.2018.07.005.
- Van Oorschot D, Anastassopoulou A, Poulsen Nautrup B, Varghese L, von Krempelhuber A, Neine M, Lorenc S, Curran D. Cost-effectiveness of the recombinant zoster vaccine in the German population aged ≥60 years old. Hum Vaccin Immunother. 2018:1–11. doi:10.1080/21645515.2018.1509645.
- Boccalini S, Alicino C, Martinelli D, Bechini A, Tiscione E, Pellizzari B, Prato R, Icardi G, Iannazzo S, Bonanni P. Clinical and economic impact of herpes zoster vaccination in elderly in Italy. Hum Vaccin Immunother. 2017;13(2):405–11. doi:10.1080/21645515.2017.1264832.
- Istat - Istituto Nazionale di Statistica. Statistiche demografiche. [accessed 2018 Jul 23]. http://demo.istat.it/pop2017/index.html
- Istat - Istituto Nazionale di Statistica. Tavole di mortalità. [accessed 2018 Jul 23]. http://dati.istat.it/Index.aspx?DataSetCode=DCIS_MORTALITA1.
- World Health Organization. WHO database for deaths related to HZ for Italy (ICD10 – B02 codes). [accessed 2017 Nov 10]. http://data.euro.who.int/dmdb/
- Curtis L, Burns A. Unit costs of health and social care 2016. Canterbury (UK): Personal Social Services Research Unit, University of Kent; 2016.
- Le P, Rothberg MB. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med. 2018;178(2):248–58. doi:10.1001/jamainternmed.2017.7431.
- Robert Koch Institut. Mitteilung der Ständigen Impfkommission (STIKO) beim RKI. Wissenschaftliche Begründung zur Empfehlung einer Impfung mit dem Herpes zoster-subunit-Totimpfstoff. Epidemiol Bull 2018;50:541–70.
- Ministero della Salute. Tariffe delle prestazioni di assistenza ospedaliera per acuti, per tipo di ricovero (Euro). 2018 [accessed 2018 Jul 23]. http://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=28/01/2013&redaz=13A00528&artp=1&art=1&subart=1&subart1=10&vers=1&prog=001
