ABSTRACT
Background
Since 2007, trivalent inactivated influenza vaccine (TIV) has been provided free-of-charge to primary, middle school and high school students in Beijing. However, there have been few school-based studies on influenza vaccine effectiveness (VE). In this report, we estimated influenza VE against laboratory-confirmed influenza illness among school children in Beijing, China during the 2016–2017 influenza season.
Methods
The VE of 2016–2017 TIV against laboratory-confirmed influenza virus infection among school-age children was assessed through a case–control design. Conditional logistic regression was conducted on matched case–control sets to estimate VE. The effect of prior vaccination on current VE was also examined.
Results
All 176 samples tested positive for influenza A virus with the positive rate of 55.5%. The average coverage rate of 2016–2017 TIV among students across the 37 schools was 30.6%. The fully adjusted VE of 2016–2017 TIV against laboratory-confirmed influenza was 69% (95% CI: 51 to 81): 60% (95% CI: −15 to 86) for influenza A(H1N1)pdm09 and 73% (95% CI: 52 to 84) for influenza A(H3N2). The overall VE for receipt of 2015–2016 vaccination only, 2016–2017 vaccination only, and vaccinations in both seasons was 46% (95% CI: −5 to 72), 77% (95% CI: 58 to 87), and 57% (95%CI: 17 to 78), respectively.
Conclusions
Our study during school outbreaks found that VE of 2016–2017 TIV was moderate against influenza A(H3N2) as well as A(H1N1)pdm09 viruses.
Introduction
Schools have been recognized as playing a significant role in the spread of influenza during an epidemic, which is thought to be due to the frequent close contact between a high number of children in the limited space with problematic hygiene practices in the school environment,Citation1–Citation4 the more intense and prolonged viral shedding,Citation5 and little acquired immunity in children.Citation6 School-age children have higher incidence of influenza infection than adults, and are an important link in the transmission of influenza within schools, families, and communities.Citation7–Citation9 The individual and societal burden on both infected children and their family members is substantial.Citation10,Citation11 The study of epidemiological characteristics of influenza outbreaks in ChinaCitation12 showed that primary, middle and high schools were important places affected by influenza outbreaks.
Vaccination is recognized to be the most (cost-)effective way to prevent influenza and its complications, reducing student absenteeism during the influenza season,Citation13 and reducing the overall burden of disease from influenza.Citation14 Studies have demonstrated that high levels of vaccine-induced immunity among school-age children can protect both vaccinated and unvaccinated populations by herd immunity not only in schools but also in the wider community.Citation2,Citation15–Citation17However, there have been relatively few studies examining influenza vaccine effectiveness (VE) in the context of school-based vaccination.
Our study contributes to the discussion on VE among schoolchildren during influenza outbreaks. In order to prevent influenza outbreaks in schools, a school-based annual influenza vaccination campaign had been implemented in Beijing since 2007, providing seasonal-inactivated trivalent influenza vaccines (TIV) free-of-charge for primary, middle and high school students prior to the wintertime influenza seasons.
During the 2016–2017 influenza season in Beijing, influenza A(H1N1)pdm09 and A(H3N2) viruses co-circulated, with predominance of influenza A(H3N2) viruses as in most northern hemisphere countries.Citation18 The aim of this analysis was to estimate the effectiveness of influenza vaccine in preventing influenza illness among school children in Beijing, China during the 2016–2017 influenza season. In addition, it was aimed for to explore whether prior influenza vaccination can influence the protective effect of current season vaccination among school children.
Methods
Study design and participants
Since the school-based annual influenza immunization campaigns across Beijing typically commence in mid-October, with vaccinees considered immune 14 days after vaccination, and increased influenza virus circulation usually begins in early November, school influenza outbreaks between 1 November 2016 to 30 April 2017were eligible for inclusion in the primary VE analysis.
School outbreaks of influenza illness were found through two existing syndromic surveillance systemsCitation19 in Beijing that monitor influenza-like illness (ILI) (measured or self-reported temperature ≥38°C with either cough or sore throat) and febrile illnesses of any etiology (measured or self-reported temperature ≥37.5°C). The definitions for an ILI outbreak and a febrile outbreak were defined as follows:
ILI outbreak: 10 or more epidemiological-linked ILIs identified in a school within 1 week; local CDC staff used a broad definition of potential links, including any opportunities for face-to-face contact in a classroom or any school setting, though (as noted below), most ILI outbreaks were identified within a single classroom or within several adjacent classes.
Febrile outbreak: 10 or more febrile illnesses within a single school classroom within 2 days.
The time period of a school outbreak began with the earliest illness onset date (index case) among cases within an outbreak and ended when no new cases with ILI or fever were found for seven consecutive days (as determined by the investigating team based on daily contact with school teachers and staff). The details of the discovery, investigation, oral pharyngeal (OP) swabs collection and the enrollment procedures have been described previously.Citation17 OP swabs were tested by rRT-PCR in the local CDC collaborating laboratories managed by Beijing CDC using PCR procedures recommended by the WHO Collaborating Center for Reference and Research on Influenza at the Chinese National Influenza Center.
The matched case–control design was used to estimate influenza VE. Cases were students who had ILI or febrile illness connected with a reported school outbreak and were positive for influenza virus. Controls were classmates of cases who were fully asymptomatic (i.e., had no symptoms of fever, cough or sore throat) during the period from the illness onset of the index case to the end of the school outbreak. Students were excluded if they were vaccinated less than 14 days before symptom onset. Four controls matched for class and week were selected for each case. For classes in which the control-to-case ratio <4, we attempted to enroll all controls, and controls were reused to create multiple matched sets (one set for each case) as previously described.Citation20
Data collection
Information on enrolled schools and students was collected using a standardized questionnaire. School information included the type of school (primary, middle, or high school), location (urban or rural area), the total number of students, and number of students who received 2016–2017 TIV. Individual-level information on participants was provided by students and their parents, including age, sex, height, weight, presence of chronic medical conditions, symptoms, date of illness onset and swab collection. Because most students were identified at the start of their illness and no follow-up was conducted, we could not document whether illnesses were medically attended. Documented influenza vaccination records for 2016–2017 and 2015–2016 TIV were collected through Beijing Management System of Information on Immunization Program. Staff administering vaccinations entered influenza vaccination records directly into the electronic registry during each school’s vaccination campaign. Local CDC staff then extracted vaccination records of cases and controls from the registry following each outbreak.
Data analysis
Questionnaire and laboratory data were entered in duplicate using EpiData Software and data were analyzed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA). Median and range values were calculated for continuous variables, and percentages were calculated for categorical variables. Participant characteristics and vaccination status of cases and asymptomatic controls were compared using χ2 tests. Univariable and multivariable conditional logistic regression were used to estimate odds ratios (ORs) with their 95% confidence intervals (CI) comparing cases and controls. Adjusted models included age group, sex, school type, location, body mass index (BMI) and chronic medical conditions.VE was calculated as 100% × [1− OR]. We generated separate VE estimates against positivity for any influenza virus and positivity against influenza A subtypes. To aid in interpretation, we also report VE stratified by school types. We also tested for potential effect modification by prior season’s 2015–2016 TIV. All p values were based on a two-sided test of statistical significance. Significance was accepted at the level of p < .05.
Ethics statement
The study was approved by the institutional review board and human research ethics committee of Beijing CDC. Informed consent was completed by parents by telephone prior to children’s participation. A signed informed consent was required from their parents or legal guardian for students aged <18 years.
Results
Participants characteristics and vaccination status
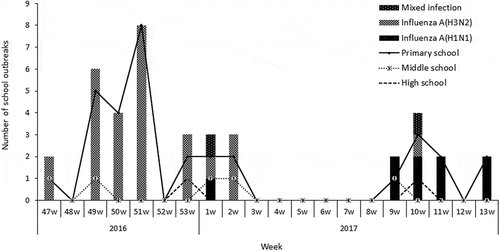
From 1 November 2016 to 30 April 2017, a total of 40 influenza outbreaks in schools were identified in Beijing. According to the inclusion criteria, 39 outbreaks in 37 schools were eligible for this study, with a total of 1826 students (). Among them, most outbreaks were reported in primary schools (32 outbreaks, 82.1%), followed by middle schools (5 outbreaks, 12.8%) and high schools (2 outbreaks, 5.1%). Most outbreaks affected a single school classroom (33, 84.6%), and 6 (15.4%) outbreaks affected two classes. The number of reported cases per outbreak ranged from 5 to 34 (median: 9). The average vaccination coverage rate of 2016–2017 TIV was 30.6% for the 37 outbreak schools, and was 28.4% for all outbreak classes involved. The timing of outbreaks by school type is illustrated in .
Figure 1. Flow chart of subject enrollment in the test-negative case–control design study for estimating influenza vaccine effectiveness among children during school-based outbreaks during the 2016–2017 influenza season in Beijing, China.
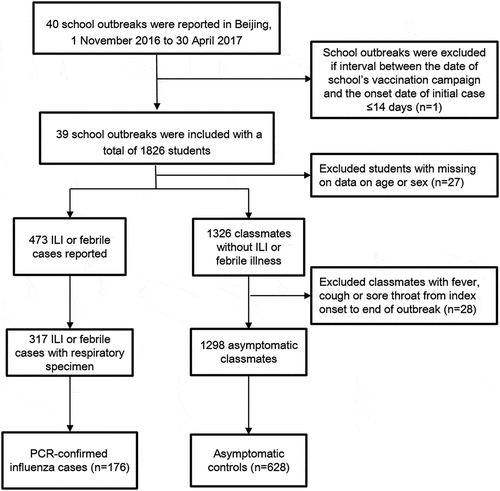
Figure 2. The time distribution of the eligible school influenza outbreaks included in the test-negative case–control study for estimating influenza vaccine effectiveness, 1 November 2016 Figure 1to 30 April 2017.
Three hundred and seventeen of the 473 eligible ILI or febrile illness cases were swabbed and tested. Among them,176 (55.5%) were confirmed with influenza virus infection, including 36 (20.4%) for influenza A(H1N1)pdm09, 136 (77.3%) for influenza A(H3N2) and 4 (2.3%) for influenza A of unknown subtype. Influenza B viruses were not detected in the swabs. Of 1326 classmates without ILI or defined febrile illness at the time of the outbreak investigation, 28 were excluded due to reports of fever, cough or sore throat during the time from the index onset date to the end of the local outbreak. Six hundred and twenty-eight of the remaining students who met the matching criteria were sampled randomly as controls. Age of all students ranged from 6 to 19. Median age of cases (7.5) was similar to controls (7.7).
The rate of influenza vaccination among all students involved was 32.2% (). The vaccination rate of controls (36.5%) was significantly higher than that of cases (17.0%) (P < .001). In the age group 6–10 years, vaccination uptake was much higher among controls (37.4%) than among cases (12.1%), but this was not so in the older age groups. Vaccination rate did not differ by sex, school type, location, BMI and presence of chronic medical conditions ().
Table 1. Participant characteristics for estimates of influenza vaccine effectiveness for 2016–2017 trivalent influenza inactivated vaccine during school outbreaks of influenza in Beijing, 1 November 2016 to 30 April 2017.
Estimation of influenza vaccine effectiveness
The unadjusted and adjusted VE estimates for all influenza viruses and for A(H1N1)pdm09 and A(H3N2) subtypes separately are shown in , stratified by school type. The overall adjusted VE of 2016–2017 TIV against influenza was 69% (95% CI: 51 to 81). Adjusted VE was 60% (95% CI: −15 to 86) for influenza A(H1N1)pdm09, and 73% (95% CI: 52 to 84) for influenza A(H3N2) (). In primary school children, the adjusted VE was 73% (95% CI: 53 to 85).
Table 2. Estimates of vaccine effectiveness (VE) by age group and type of school during outbreaks of influenza in schools in Beijing, 1 November 2016 to 30 April 2017.
VE for combinations of 2016–2017 and 2015–2016 TIV
After including the main effects for 2016–2017 and 2015–2016 TIV in the adjusted model, a significant interaction effect between these vaccine exposures was noted (p < .001), suggesting effect modification by prior vaccination. Therefore, we estimated VE for three TIV combinations with no TIV in either year as the reference. The adjusted overall VE for receipt of 2015–2016 vaccination only, 2016–2017 vaccination only, and vaccinations in both seasons was 46% (95% CI: −5 to 72), 77% (95% CI: 58 to 87), and 57% (95%CI: 17 to 78), respectively ().
Table 3. Effect of previous vaccination on estimates of vaccine effectiveness (VE) in school outbreaks of influenza in Beijing, 1 November 2016 to 30 April 2017.
Discussion
We observed a high influenza VE in children 6–10 years old during outbreaks of influenza in schools, especially against the dominant influenza A(H3N2) subtype. The TIV therefore seemed to have provided protection to vaccinated children.
Estimates of influenza VE among school outbreaks during the influenza season help investigate the preventive benefit of school-based influenza vaccination. In this study, we identified 39 school-based influenza outbreaks from 1 November 2016 to 30 April 2017 in Beijing. The vaccination rate among all students involved was 32.2%, which was significantly higher than the vaccination rate of 9.9% observed among medically attended ILI patients 3–17 years of age during the 2016–2017 season in Beijing.Citation21 Probably due to young non-school-going children not yet being offered free influenza vaccination. We considered this an encouraging finding, which implied that school-located influenza vaccination program has the potential value to improve current suboptimal immunization coverage in Beijing, which is an effective way to raise the vaccination rate in a relatively short period of time.
During the 2016–2017 influenza season, the overall VE in our study (69%, 95% CI: 51 to 81) was higher than that observed among medically attended ILI patients 3–17 years of age (29%, 95%CI: 0 to 49) in Beijing during the same season.Citation21 Analyzed for subtypes, VE against influenza A(H1N1)pdm09 viruses were similar between school children (60%, 95% CI: −15 to 86) and medically attended ILI patients 3–17 years of age (48%, 95% CI: 8 to 71), while VE of influenza A(H3N2) was significantly better among school children (73%, 95% CI: 52 to 84) than among medically attended ILI patients 3–17 years of age (10%, 95% CI: −37 to 40).Citation21 Moreover, this observed VE was also higher than that reported from Europe and North America for the same season, with VE against influenza A(H3N2) of 44.1% among those aged 0–14 years in Europe, 23% among those aged 9–17 years in the US, and 42% among all ages in Canada, respectively.Citation22–Citation24 In Asia, VE against influenza A viruses among those aged 6–15 years in Japan (39%) was also lower than our results.Citation25
During the 2016–2017 influenza season, the majority of the circulating influenza A(H3N2) viruses in Beijing belonged to the variant clade 3C.2a subgroup carrying T131K, R142K and R261Q substitutions, which was considered to be partially responsible for the low VE of influenza A(H3N2) among medically attended ILI patients in the study season.Citation21 Nonetheless, the VE against influenza A(H3N2) viruses in this study did not seem to be weakened due to the genetic variants. Contrarily, the result was relatively good and vaccination provided better protection for children among school outbreaks. A similar outcome was observed during the 2014–2015 season in Beijing. In that season, the circulating influenza A(H3N2) viruses drifted from the vaccine strain, and VE against the influenza A(H3N2) viruses were low or non-significant in most northern hemisphere countries, while our school-based VE evaluation showed a greater protection among school childrenCitation17 than that among medically attended ILI patients 3–17 years of age in Beijing.Citation26 We consider the higher VE observed to be partially attributable to the higher vaccination coverage among schoolchildren. Previous studies have indicated that high vaccine coverageCitation2,Citation27–Citation29 is a critical factor for interrupting transmission of influenza virus effectively. We did not study the effect of vaccination on transmission of influenza virus within schools or between schools and families. However, studies in the US and the UK have demonstrated that in addition to protecting the individual child from influenza virus infection and its complications, vaccinating children (but mostly with live-attenuated influenza vaccine) reduces transmission of influenza virus in the school, family and community.Citation30
Similar to the result of 2014–2015 season, we again found a better VE for those vaccinated in the current season only than those vaccinated in both the current and prior seasons. In recent years, multiple observational studies of repeated annual vaccination had suggested that repeat vaccination may adversely affect vaccine-induced protection.Citation31 However, results from different studies are inconclusive and the mechanisms underlying the potential negative effects of repeat vaccination remain unclear. Therefore, further research is required to understand the immunological basis of repeat vaccination effects.
There are several limitations in this study. First, we acknowledge that some asymptomatic controls may have been symptomless influenza virus carriers because these children had not been tested for influenza, which could result in underestimation of VE. Second, although our study design was appropriate given our focus on non-medically attended illness, it is unclear how our VE estimates compare to those using the more commonly used test-negative design in medical settings. Nonetheless, other studies of children and adults that employed both healthy controls and influenza negative controls reported similar VE estimates using both methods.Citation32,Citation33 Third, although our study design focused on non-medically attended illness was a classical case–control study design for examining risk factors, it is difficult to compare our VE estimates to those obtained from the more commonly used test-negative design targeting medically attended illness. If we hypothesize that the severity of non-medically attended patients was less than that of those seeking medical care and influenza vaccination could reduce the severity, the vaccination rate may be higher among non-medically attended patients. The relatively higher vaccination rate in case group (non-medically attended patients) in our study design might result in underestimating the VE, compared to the test-negative study design. On contrary, as the subjects in the control group of our study design were asymptomatic, the probability of misclassification was lower than the test-negative design which could introduce the positive cases into the negative group due to the sensitivity of the assay. Therefore, the vaccination rate in the control group of our study design would be theoretically higher than that of test-negative study design and the VE of our study design might be over-estimated. Fourth, since most respiratory specimens collected were from symptomatic children who were experiencing mild influenza illness while attending school or being cared for at home, our findings may not be generalizable to those with severe manifestations of disease.
Conclusions
In conclusion, our study based on school influenza outbreaks suggested moderate VE against both influenza A(H1N1)pdm09 and A(H3N2) viruses among school children in Beijing, China during the 2016–2017 season. Students who did not receive the prior season’s vaccine may have enjoyed greater protection than repeated vaccinees.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Bin Nafisah S, Alamery AH, Al Nafesa A, Aleid B, Brazanji NA. School closure during novel influenza: A systematic review. J Infect Public Health. 2018;11(5):657–61. doi:10.1016/j.jiph.2018.01.003.
- Barclay VC, Smieszek T, He J, Cao G, Rainey JJ, Gao H, Uzicanin A, Salathé M, McVernon J. Positive network assortativity of influenza vaccination at a high school: implications for outbreak risk and herd immunity. PLoS One. 2014;9(2):e87042. doi:10.1371/journal.pone.0087042.
- Gemmetto V, Barrat A, Cattuto C. Mitigation of infectious disease at school: targeted class closure vs school closure. BMC Infect Dis. 2014;14:695. doi:10.1186/s12879-014-0695-9.
- Eames KT. The influence of school holiday timing on epidemic impact. Epidemiol Infect. 2014;142(9):1963–71. doi:10.1017/S0950268813002884.
- Li CC, Wang L, Eng HL, You H-L, Chang L-S, Tang K-S, Lin Y-J, Kuo H-C, Lee I-K, Liu J-W, et al. Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis. 2010;16(8):1265–72. doi:10.3201/eid1608.091918.
- Sauerbrei A, Schmidt-Ott R, Hoyer H, Wutzler P. Seroprevalence of influenza A and B in German infants and adolescents. Med Microbiol Immunol. 2009;198(2):93–101. doi:10.1007/s00430-009-0108-7
- Esposito S, Cantarutti L, Molteni CG, Daleno C, Scala A, Tagliabue C, Pelucchi C, Giaquinto C, Principi N. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J Infect. 2011;62(5):379–87. doi:10.1016/j.jinf.2011.02.015.
- Schanzer D, Vachon J, Pelletier L. Age-specific differences in influenza a epidemic curves: do children drive the spread of influenza epidemics? Am J Epidemiol. 2011;174(1):109–17. doi:10.1093/aje/kwr037.
- King JC Jr., Stoddard JJ, Gaglani MJ, Moore KA, Magder L, McClure E, Rubin JD, Englund JA, Neuzil K. Effectiveness of school-based influenza vaccination. New England J Med. 2006;355(24):2523–32. doi:10.1056/NEJMoa055414.
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156(10):986–91. doi:10.1001/archpedi.156.10.986.
- Kiro A, Robinson G, Laks J, Mor Z, Varsano N, Mendelson E, Amitai ZS. Morbidity and the economic burden of influenza in children in Israel--a clinical, virologic and economic review. Harefuah. 2008;147:960–5, 1031.
- Li M, Feng L, Cao Y, Peng Z, Yu H. Epidemiological characteristics of influenza outbreaks in China, 2005–2013. Chin J Epidemiol. 2015;36(7):705–08.
- Hull HF, Ambrose CS. The impact of school-located influenza vaccination programs on student absenteeism: a review of the U.S literature. J SchNurs. 2011;27:34–42.
- Thorrington D, Jit M, Eames K. Targeted vaccination in healthy school children - Can primary school vaccination alone control influenza? Vaccine. 2015;33(41):5415–24. doi:10.1016/j.vaccine.2015.08.031.
- Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ, Leung GM. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. doi:10.1371/journal.pmed.1001527.
- Pannaraj PS, Wang H-L, Rivas H, Wiryawan H, Smit M, Green N, Aldrovandi GM, El Amin AN, Mascola L. School-located influenza vaccination decreases laboratory-confirmed influenza and improves school attendance. Clin Infect Dis. 2014;59(3):325–32. doi:10.1093/cid/ciu340.
- Zhang L, Yang P, Thompson MG, Pan Y, Ma C, Wu S, Sun Y, Zhang M, Duan W, Wang Q. Influenza vaccine effectiveness in preventing influenza illness among children during school-based outbreaks in the 2014–2015 season in Beijing, China. Pediatr Infect Dis J. 2017;36(3):e69–e75. doi:10.1097/INF.0000000000001434.
- WHO. Recommended composition of influenza virus vaccines for use in the 2017–2018 northern hemisphere influenza season. Wkly Epidemiol Rec. 2017 Mar 17;92(11):117–28.
- Pilot E, Schwarz C, Wang L, Krafft T. Syndromic surveillance: a tool for enhanced detection of disease outbreak in China. In: Wang WY, Krafft T, Rosenberg M, Pilot E, editors. Chapter 19, Health and environmental change in urban Areas. Beijing, China: China Environment Press; 2014. ISBN: 978-7-5111-1940-7.
- Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Stat Sci. 1996;11(1):35–53. doi:10.1214/ss/1032209663.
- Wu S, Pan Y, Zhang X, Zhang L, Duan W, Ma C, Zhang Y, Zhang M, Sun Y, Yang P, et al. Influenza vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings: A test-negative case-control study in Beijing, China, 2016/17 season. Vaccine. 2018;36(38):5774–80. doi:10.1016/j.vaccine.2018.07.077.
- Kissling E, Rondy M. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I-MOVE multicentre case control studies at primary care and hospital levels in Europe. Euro Surveill. 2017 Feb 16;22(7). doi:10.2807/1560-7917.ES.2017.22.7.30464.
- Flannery B, Chung JR, Thaker SN, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, et al. Interim estimates of 2016–17 seasonal influenza vaccine effectiveness - United States, February 2017. MMWR. 2017;66(6):167–71. doi:10.15585/mmwr.mm6606a3.
- Skowronski DM, Chambers C, Sabaiduc S, Dickinson JA, Winter A-L, De Serres G, Drews SJ, Jassem A, Gubbay JB, Charest H, et al. Interim estimates of 2016/17 vaccine effectiveness against influenza A(H3N2), Canada, January 2017. Euro Surveill. 2017;22(6). doi:10.2807/1560-7917.ES.2017.22.6.30460.
- Shinjoh M, Sugaya N, Yamaguchi Y, Iibuchi N, Kamimaki I, Goto A, Kobayashi H, Kobayashi Y, Shibata M, Tamaoka S, et al. Inactivated influenza vaccine effectiveness and an analysis of repeated vaccination for children during the 2016/17 season. Vaccine. 2018;36(37):5510–18. doi:10.1016/j.vaccine.2018.07.065.
- Ma C, Pan Y, Zhang L, Zhang Y, Wu S, Sun Y, Duan W, Zhang M, Wang Q, Yand P. Influenza vaccine effectiveness against medically attended influenza illness in Beijing, China, 2014/15 season. Hum VaccinImmunother. 2017;13(10):2379–84.
- Nichol KL. Improving influenza vaccination rates among adults. Cleve Clin J Med. 2006;73(11):1009–15. doi:10.3949/ccjm.73.11.1009.
- Holm MV, Blank PR, Szucs TD. Developments in influenza vaccination coverage in England, Scotland and Wales covering five consecutive seasons from 2001 to 2006. Vaccine. 2007;25(46):7931–38. doi:10.1016/j.vaccine.2007.09.022.
- Basta NE, Chao DL, Halloran ME, Matrajt L, Longini IM. Strategies for pandemic and seasonal influenza vaccination of schoolchildren in the United States. Am J Epidemiol. 2009;170(6):679–86. doi:10.1093/aje/kwp237.
- Pebody RG, Sinnathamby MA, Warburton F, Andrews N, Boddington NL, Zhao H, Yonova I, Ellis J, Tessier E, Donati M, et al. Uptake and impact of vaccinating primary school-age children against influenza: experiences of a live attenuated influenza vaccine programme, England, 2015/16. Euro Surveill. 2018;23(25). doi:10.2807/1560-7917.ES.2018.23.25.1700496.
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):1–14. doi:10.1080/14760584.2017.1334554.
- Thompson MG, Li D-K,Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, Bozeman S, Reynolds SB, Odouli R, Henninger ML, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis. 2014;58:449–57. doi:10.1093/cid/cit750.
- Ferdinands JM, Olsho LEW,Agan AA, Bhat N, Sullivan RM, Hall M, Mourani PM, Thompson M, Randolph AG. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US Children, 2010–2012. J Infect Dis. 2014;210:674–683. doi:10.1093/infdis/jiu185.
