ABSTRACT
Healthcare workers (HCWs) are considered high-risk subjects for Hepatitis B Virus (HBV) infection due to occupational exposure to blood and body fluids. Vaccination represents the core strategy for HBV infection prevention. Following our previous publication on this topic, we aimed to assess the effectiveness of booster vaccine doses in eliciting the immunological response in seronegative (<10 mIU/mL) HCWs and students of Careggi Teaching Hospital, Florence (Italy). All subjects received primary vaccination course, and they were tested for serum anti-HBs antibodies. In seronegative subjects, a challenge dose of vaccine was administered and the test was repeated 1 month later. Six hundred and ninety-eight (87.8%) of 795 HCWs and students tested responded to the challenge dose. After this challenge dose, males more often had negative anti-HBs titer compared with females (15.9% vs 10.2%; p < .05). The completion of the second vaccination course was offered to subjects with persistently negative anti-HBs titer. 76.2% (32) of those who accepted the fifth dose, and 3 of the 5 who accepted the sixth dose seroconverted. This report shows the importance to convey a strong message to negative subjects at the initial anti-HBs dosage: accepting all the three additional vaccine doses allows the vast majority of them to obtain protection.
Introduction
Globally, in 2015, an estimated 257 million people, corresponding to 3.5% of the population, were living with chronic Hepatitis B Virus (HBV) infection.Citation1
Left untreated, HBV infection can lead to cirrhosis and hepatocellular carcinoma, which are accountable for about 1 million deaths annually. Safe and effective vaccines have been available since the early 1980s, enabling the prevention of HBV infection and any serious complications from it.Citation2
For this reason, in 1991, the Global Advisory Group to the World Health Organization (WHO) recommended that all countries integrate hepatitis B vaccine into their national immunization programmes by 1997.Citation3,Citation4
Italy was one of the first countries to introduce in 1991 the mandatory universal vaccination against HBV for all newborns and 12-year-old adolescents, with the aim to create 24 generations of immune subjects within the first 12 years of vaccination implementation. In 2004, vaccination of adolescents was stopped, while that of infants was maintained. As a result of the universal vaccination implementation, a significant reduction of new hepatitis B infections has been reported in Italy.Citation5,Citation6
This explains why in Italy virtually all people who are still susceptible or are infected with HBV today are people who were older than 12 years when the hepatitis B vaccine was routinely introduced. Although universal vaccination has dramatically reduced the burden of disease, HBV infection remains an issue for high-risk subjects, such as healthcare workers (HCWs), due to their occupational exposure to blood and body fluids.Citation7
Vaccination represents the core strategy for HBV infection prevention among HCWs, recommended by the Centers for Disease Control and Prevention (CDC) and WHO.Citation8,Citation9 In addition to universal vaccination, the Italian policy also includes a screening of HCWs before employment, done by measuring the serum antibodies against HBsAg (anti-HBs) in order to check the presence of seroprotection.Citation10,Citation11
In subjects with an anti-HBs negative result (<10 mIU/mL), a vaccine challenge dose followed after 1 month by further testing, makes it possible to understand if immunological memory is present (in which case a clear anti-HBs response is detected).If the test is still negative, up to two additional doses of vaccine are administered with the aim of achieving a protective immunological response.Citation12-Citation15
We collected data obtained during the occupational medicine visits of the HCWs and students of health disciplines attending the Careggi Teaching Hospital, (the biggest hospital in the Tuscany Region, with 3.5 million inhabitants) in Florence, Italy. Following our previous publication on this topic, which included only partial dataCitation16 we aimed to assess the effectiveness of booster vaccine doses in eliciting the immunological response in subjects with anti-HBs non-protective levels (<10 mIU/mL) despite the primary vaccination course received in infancy or adolescence.
Materials and methods
Between January 2015 and August 2017 a study including all students of health disciplines and HCWs attending or employed at the Careggi Teaching Hospital (a tertiary adult care center in Italy) was performed. Subjects undergoing preventive visits and periodic controls performed at the Occupational Medicine Service were tested for anti-HBs antibodies as required by the national law.Citation17
Participants were born in Italy between 1 January 1980 and 31 December 1998, and all of them were fully vaccinated during infancy or adolescence. Previous vaccination against HBV was verified in 82.3% of subjects by checking the immunization records provided by participants or the electronic vaccination registry, while in the remaining 17.7% cases, we have relied on the self-reporting. Age and sex were also recorded.
The ADVIA Centaur_ assay, an antibody-capture microparticle direct chemiluminometric immunoassay, was used to measure the amount of anti- HBs in human serum and plasma. The criteria provided by the producer were applied for the qualitative evaluation of antibodies and antigen detection, according to the specific instruction manual.
Based on anti-HBs concentrations after the primary vaccination series, we identified three categories of subjects in accordance with current International Standards:
with anti-HBs ≥101 mIU/ml, people who were considered “good-responders” to the vaccine (given the still high level of the antibody titer after many years since the primary vaccination series)Citation9,Citation18
with anti-HBs ≥10 mIU/mL, people who were considered protected against HBV infection (since this value is connected with the existence of immunological memory, especially long after vaccination);
with anti-HBs <10 mlU/mL, people who were classified as subjects to be further investigated to understand if they are protected against HBV or not.
In subjects with anti-HBs concentrations <10 mIU/ml, a challenge dose of vaccine was administered and the serological test was performed at 1 month. If an immunological response was obtained (anti-HBs titers ≥10 mIU/ml), no further action was implemented. Conversely, in subjects with persistently negative anti-HBs, the completion of the second vaccination course with two further doses (one immediately and one after four-six months) was proposed in order to try to induce immunological memory in subjects with a primary vaccination failure.Citation12–Citation14
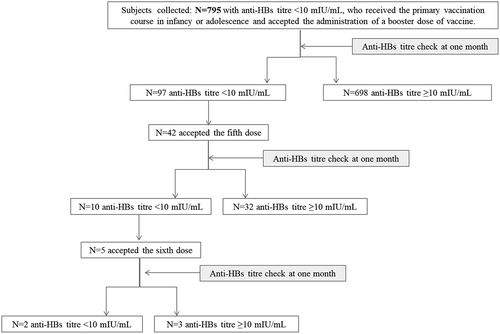
shows the flow chart of the study based on this protocol.
Finally, subjects were classified into two groups based on age at the time of the primary vaccination course: in Group 1 (subjects born between 1980 and 1991) vaccination was performed at 12 years of age, and in Group 2 (born between 1991 and 1998) in the first year of life.
Compulsory vaccination against HBV was implemented in Italy since October 1991; because of which subjects born in 1991 represent a mixed population with regard to the age of administration of vaccination; we assigned those individuals to either vaccination cohort, according to the age in which primary immunization was actually performed.
The descriptive analysis of results was performed using IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY: IBM Corp.). The association between binary variables was evaluated using the simple logistic regression model. The research was carried out in compliance with the Helsinki Declaration.
Results
We have analyzed 795 subjects (512 females and 283 males) with negative anti-HBs titer (<10 mIU/mL), who had received the primary vaccination course (three doses) during infancy or adolescence, and had accepted the administration of a fourth dose of vaccine.
Of them, 146 (18.4%) were vaccinated at 12 years of age (Group 1), and 649 (81,6%) in the first year of life (Group 2). The average intervals between primary vaccination and anti-HBs titers check were: 16.6 years (minimum 12, maximum 25 years) in Group 1 and 20.3 years (minimum 16, maximum 25 years) in Group 2, respectively.
The measurement of the antibody response 1 month after the fourth dose of the vaccine showed that 97 subjects (12.2%) still had anti-HBs titer <10 mIU/mL, 157 (19.7%) between 10 and 100 mIU/mL and 541 (68.1%) had ≥101 mIU/mL ().
Table 1. Distribution of subjects based on anti-HBs titer after 1 month from the fourth dose of vaccine. Group 1 = vaccinated at 12 years of age; Group 2 = vaccinated in the first year of life.
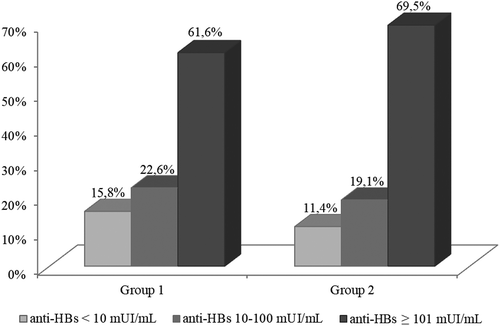
In particular, in Group 1 (vaccinated at 12 years of age) 15.8% (n. 23) of subjects still had anti-HBs titer <10 mIU/mL, 22.6% (n.33) between 10 and 100 mIU/mL and 61.6% (n.90) had ≥101 mIU/mL. In Group 2 (vaccinated in the first year of life), 11.4% (n.74) had anti-HBs titer <10 mIU/mL, 19.1%(n.124) between 10 and 100 mIU/mL and 69.5% (n.451) ≥101 mIU/mL (). We analyzed the relation between the titer of anti-HBs <10 mIU/mL after the fourth dose of vaccine, and the age at the time of the first vaccination course. Although the proportion of subjects with anti-HBs<10 mIU/mL was higher in Group 1 than in Group 2, no significant difference was found (Chi-square = 2.11; p > .05; 95% CI 0.88–2.41).
Figure 2. Proportion of subjects with anti-HBs < 10 mUI/mL, between 10 and 100 mUI/mL and ≥ 101 mUI/mL after the fourth dose of vaccine, broken down by group (Group 1 vaccinated at 12 years of age; Group 2 vaccinated in the first year of life).
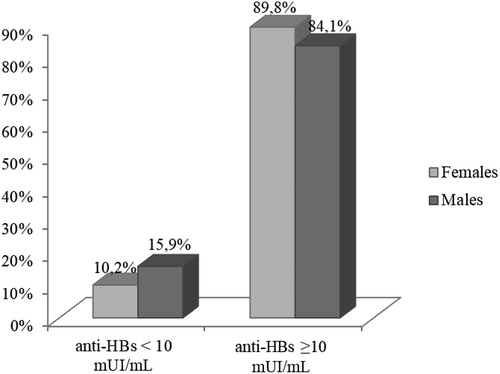
We also evaluated the possible role of sex in the responsiveness to a booster dose of vaccine. shows that 15.9% (n.45) of males still had anti-HBs titer <10 mIU/mL 1 month from the fourth dose of vaccine versus 10.2% (n.52) of females (Chi-square = 5.62; p < .05; 95% CI 0.39–0.92).
Figure 3. Proportion of subjects with anti-HBs <10 mUI/mL and ≥ 10 mUI/mL after the fourth dose of vaccine, broken down by sex.
In order to obtain seroconversion in subjects who still tested anti-HBs<10 after the fourth dose of vaccine, the completion of the second vaccination course was offered to 97 subjects with persistently negative anti-HBs titer. Only 42 of them (43.3%) accepted the fifth dose of vaccine. An anti-HBs titer check 1 month later showed that 76.2% (n. 32) of those receiving the fifth dose seroconverted, while the remaining 23.8% (n.10) still had no immunological response. The sixth dose was accepted by very few subjects (n.5); three of them had seroconverted one month later, while two did not reach an anti-HBs titer≥10 mIU/mL despite the completion of the second course of hepatitis B vaccination.
Discussion
In a recent document of the US Centers for Disease Control and Prevention, serological testing for immunity after routine vaccination is not recommended in universal infant and adolescent hepatitis B vaccination programs given the high protection obtained and the negative cost-effectiveness profile of such practice. This is also true for other countries like Italy.
Conversely, in specific categories at high risk of HBV infection, such as HCWs, testing for anti-HBs after vaccination is advised.Citation19 This approach allows us to assess the acquisition of immunity to HBV after primary vaccination; indeed, while subjects with anti-HBs levels ≥10 mIU/mL after the primary vaccine series are considered protected and do not necessitate other action, those with anti-HBs <10 mIU/mL require further investigation. In these latter cases, the administration of a challenge dose of vaccine and the serological check at 1 month, allows us to discriminate between the decline of antibody levels occurring after effective immunization, and a failure to respond to the initial vaccination course. In the first case, anti-HBs reaches levels ≥10 mIU/mL after the booster, and subjects are considered protected; while in the second case, anti-HBs titer remains less than 10 mIU/mL, and it is necessary to complete the second vaccination course with two further doses in order to try to obtain an effective response and thus the immunological memory.Citation12–Citation14
Subjects who still test negative for anti-HBs after two complete series of vaccine are regarded as non-responders and should be counseled about precautions to prevent HBV infection and the necessity of prophylaxis in case of exposure to a source patient who is HBsAg-positive or has an unknown HBsAg status.Citation20 The present study integrates data that we have recently presented on long-term immunological memory after the vaccination against HBV.Citation16 In this previous publication, we presented 330 HCWs and students of the health sector with non-protective antibody titers (anti-HBs <10 mlU/mL) after the primary vaccination course, who received a challenge dose of vaccine in order to elicit an anamnestic response. The measurement of the antibody levels 1 month after this further dose showed that 11.2% (n.37) still had anti-HBs titer <10 mIU/mL and they were regarded as primary vaccination failures; a significantly higher proportion of them were vaccinated during adolescence (p < .001).
In this paper, we analyze the response to challenge doses of vaccine in a larger group of HCWs and students (n. 795) who were anti-HBs negative after the primary vaccination course received in infancy or adolescence. Similar to the data presented previously, the measurement of the antibody response at 1 month showed that the 87.8% of subjects (n.698) responded to the challenge showing an anti-HBs titer >10 mIU/mL, confirming that in most cases there was an initial protective immunological response, and that the immune memory remains intact for at least 25 years after the primary vaccination series. However, we cannot rule out the possibility that some subjects who responded to the fourth dose were originally non-responder to the three -dose basic immunization course, and that the fourth dose was instrumental in eliciting an anti-HBs titer >10 and, thus, immunological memory.
Moreover, as shown in our study mentioned above, the proportion of subjects with negative anti-HBs titer after the booster (overall 12.2%) was higher in subjects immunized during adolescence (15.8%) rather than those vaccinated in infancy (11.4%), but in this larger sample, the difference was not statistically significant.
Data reported in literature about the ability of a challenge dose to elicit an anamnestic response in subjects found to have anti-HBs levels <10 mIU/mL many years after the primary vaccination shows a wide range (60–97%).Citation20 Wang et al.Citation21 analyzed 103 adolescents with anti-HBs <10 mIU/ml who received a primary series of hepatitis B vaccination as infants: after a booster dose they achieved the threshold of ≥10 mIU/mL of anti-HBs in 84% subjects.
A lower failure to respond to a challenge dose of vaccine is reported in the study by Bagheri-Jamebozorgi et al.Citation22 where a booster dose in individuals vaccinated in infancy induced an anamnestic response in 97.1% of cases.
Similarly, the study by Dini et al.Citation23 describes the lack of antibody response after the challenge in about 5% of cases; furthermore, no difference was found between subjects vaccinated in infancy and those vaccinated in adolescence in terms of probability of anamnestic response.
Likewise, Zanetti et al.Citation24,Citation25 report an anamnestic response in more than 95% of subjects, but in this case, individuals with anti-HBs titer <10 mIU/mL are predominantly those vaccinated in infancy (36%) rather than those vaccinated at adolescent age (11%). The higher proportion of subjects who tested negative among those vaccinated as infants in some studies compared to others might partly be explained by a higher proportion of individuals immunized with the Hexavac hexavalent vaccine, which was withdrawn in 2005 due to a progressive decrease of potency of the hepatitis B component.
In the scientific literature, many factors (e.g. male sex, smoking, obesity, chronic medical conditions, immune suppression, etc.) were found to be associated with a lower immunological response to the vaccine against HBV.Citation14,Citation26,Citation27 We evaluated the possible role of gender: after the challenge dose of vaccine, males more often had anti-HBs titer <10 mIU/mL compared with females (15.9% vs 10.2%; p < .05). Unfortunately, we were unable to provide information about other factors that potentially influence the immunogenicity of the vaccine, since they are not routinely recorded on the Occupational Medicine Service data-base.
In our previous publication, we reported only four people who accepted the fifth vaccine dose.Citation16 This extension of the study allows us to present a larger number of subjects who continued the proposed second vaccination course, thus adding relevant information on the foreseeable gain of each additional dose to overcome the non-responder status: in particular, 42 subjects who were still seronegative after the challenge dose (fourth dose after the basic vaccination course), accepted the fifth dose. The anti-HBs titer check at 1 month showed that 76.2% of them responded, implying that insistence in administering further doses after the fourth is of crucial importance to overcome the non-responder status in high-risk subjects like HCWs. Unfortunately, the sixth dose was accepted by very few subjects (n.5); however, three of them reached an anti-HBs titer ≥10 mIU/mL. Although no definitive conclusion can be drawn regarding the percentage gain of the sixth dose in fifth dose–non responders due to the very low numbers, if we assume that 60% is the average gain, and apply the other average response rates after the fourth and fifth doses, it would be possible to forecast a theoretical chance of obtaining protective immunity to HBV in 98.8% of those testing negative at the initial anti-HBs check, who accepted three additional doses. All this shows the importance of conveying a strong message to negative students and HCWs at the initial anti-HBs dosage: accepting all three proposed additional doses of HB vaccine in case they repeatedly test negative after the fourth and even the fifth dose, allows the vast majority of them to obtain protection against hepatitis B, which remains one of the major occupational hazards for those working in the healthcare sector.
Contrary to this evidence and consistent with previously reported data, the adherence to revaccination among HCWs and students is poor (only 43.3% and 50% of them accepted the fourth and fifth dose of vaccine, respectively). Our study does not aim to investigate the reasons for this low adhesion to the second full vaccination course. In the last years, many articles were published about vaccination barriers in HCWs, and the main concerns detected were a misperception of risk and doubts about the effectiveness and safety of vaccine.Citation28–Citation31 To face this issue, in collaboration with the Occupational Medicine Service of the Careggi Hospital, we are planning some actions, in addition to the counseling currently performed, to promote the re-vaccination against HBV in non-seroprotected HCWs and students, such as the distribution of pamphlets and poster publishing. This additional communication strategy aims to improve the awareness among HCWs; in fact, educational interventions to overcome misconceptions and mistrust about vaccinations are described as essential determinants of HCWs attitude toward vaccination.Citation32 In the future, we will evaluate how much these interventions will have been effective.
Limitations of the study: The main limitations of the study are described below.
As mentioned in the methods section, previous vaccination against HBV in enrolled subjects was verified mainly by checking the immunization records provided by participants or the electronic vaccination registry. In 17.7% of the studied subjects, no such documentation was available, and we have relied on the self-reporting; this aspect could lead to recall bias. However self-reporting for the same vaccinations (including influenza, pneumococcal polysaccharide, hepatitis A, hepatitis B, and Human Papilloma Virus) among adults has shown to be sensitive and specific.Citation33
Another limitation is related to the fact that no information was collected about specific conditions and behaviors of participants involved in reducing the immunogenicity of HBV vaccine over time (e.g. tabagism, obesity, chronic liver or renal diseases, diabetes mellitus or human immunodeficiency virus infection).Citation34
Finally, we identify as a limitation the fact that HBV serological tests to detect natural infections (i.e. total anti-HBc) were not performed among the study population. For vaccinated subjects who have received HBV vaccine series during infancy or adolescence, the subsequent documentation of an anti-HBs ≥10 mIU/mL is not required by the Italian national vaccination program; we, therefore, cannot exclude the possibility of acquisition of the HBV infection in non-responders to the basic vaccination course. Few studies reported that some cases of acute hepatitis B and chronic HBV infection can be expected in unvaccinated persons and among vaccine non-responders.Citation20 It is also true that in Italy the incidence rate of hepatitis B has progressively dropped over the last 30 years, reducing the risk of infection acquisition (in 2016 the incidence was 0.6 per 100,000 inhabitants according to the data monitored by the Italian National surveillance system).Citation35
In summary, since the data presented in this paper are very reassuring about the possibility to finally elicit immunological memory, strong communication efforts should be pursued to convince all seronegative negative students and HCWs about the importance of accepting the vaccination with the second full immunization course.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank Marco Postiglione for his contribution to the linguistic revision.
References
- World Health Organization (WHO). Global hepatitis report 2017. WHO web-site. [accessed 2019 Apr 10]. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med PMID: 8988900. 1997;336:196–204. doi:10.1056/NEJM199701163360307.
- World Health Organization (WHO). Expanded programme on immunization global advisory group. Weekly Epidemiol Rec 1992;3:11–16.
- Van Damme P, Kane M, Meheus A. Integration of hepatitis B vaccination into national immunisation programmes. Viral hepatitis prevention board. BMJ. 1997;314:1033–36. PMID: 9112852. doi:10.1136/bmj.314.7086.1033.
- Boccalini S, Pellegrino E, Tiscione E, Pesavento G, Bechini A, Levi M, Rapi S, Mercurio S, Mannelli F, Peruzzi M, et al. Sero-epidemiology of hepatitis B markers in the population of Tuscany, Central Italy, 20 years after the implementation of universal vaccination. Hum Vaccin Immunother. 2013;9(3):636–41. PMID: 23354158. doi:10.4161/hv.23259.
- Stroffolini T, Mele A, Tosti ME, Gallo G, Balocchini E, Ragni P, Santonastasi F, Marzolini A, Ciccozzi M, Moiraghi A. The impact of the hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J Hepatol. 2000;33:980–85. PMID: 11131462.
- Rice BD, Tomkins SE, Ncube FM. Sharp truth: health care workers remain at risk of bloodborne infection. Occup Med (Lond) PMID: 25663385. 2015;65(3):210–14. doi:10.1093/occmed/kqu206.
- World Health Organization (WHO). Global health sector strategy on viral hepatitis 2016–2021. 2016. WHO web-site. [accessed 2019 Apr 10]. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua.
- Centers for disease control and prevention. Immunization of health-care personnel: recommendations of the advisory committee on immunization practices (ACIP). MMWR. 2011;60(RR–7):1–45. PMID: 22108587. Available at https://www.cdc.gov/mmwr/pdf/rr/rr6007.pdf
- Law n. 165, 27 May 1991. Obbligatorietà della vaccinazione contro l’epatite virale B. Gazz. Uff., n. 127, 1°giugno 1991.
- Italian Ministry of Health. Ministerial Circular n.20, 4 October 1991. Disposizioni relative all’applicazione della legge 27 maggio 1991, n.165. Gazz.Uff. n. 251, 25 ottobre 1991.
- Bonanni P, Bonaccorsi G. Vaccination against hepatitis B in health care workers. Vaccine PMID:11257366. 2001;19(17–19):2389–94. doi:10.1016/S0264-410X(00)00460-6.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. In: Hamborsky J, Kroger A, Wolfe S, editors. Chapter 10, Hepatitis B. 13th. Washington (DC): Public Health Foundation; 2015. p. 149–174. Available at. https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html
- Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J PrevMed PMID:9651632. 1998;15:1–8. doi:10.1016/S0749-3797(98)00003-8.
- Italian Ministry of Health. Piano Nazionale Prevenzione Vaccinale (PNPV) 2017–2019. Gazz. Uff. n. 41,l 18 febbraio 2017. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf
- Bini C, Grazzini M, Chellini M, Mucci N, Arcangeli G, Tiscione E, Bonanni P. Is hepatitis B vaccination performed at infant and adolescent age able to provide long-termimmunological memory? An observational study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother PMID: 29106317. 2018;14(2):450–55. doi:10.1080/21645515.2017.1398297.
- Law n. 81, 9 April 2008. Testo Unico sulla salute e sicurezza sul lavoro. Gazz. Uff. n. 101, 30 aprile 2008.
- Westmoreland D, Player V, Heap DC, Hammond A. Immunization against hepatitis B–what can we expect? Results of a survey of antibody response to immunization in persons ‘at risk’ of occupational exposure to hepatitis B. Epidemiol Infect PMID:2140795. 1990;104(3):499–509. doi:10.1017/S0950268800047506.
- Schillie S, Vellozzi C, Reingold A. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep PMID: 29939980. 2018;67(1):1–31. doi:10.15585/mmwr.rr6701a1.
- Centers for disease control and prevention. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR. 2013;62(RR–10):1–19. [accessed 2019 Apr 10]. https://www.cdc.gov/mmwr/pdf/rr/rr6210.pdf.
- Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Li XE, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother PMID: 27874311. 2017;13(4):909–15. doi:10.1080/21645515.2016.1250990.
- Bagheri-Jamebozorgi M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT, Nejad-Ghaderi M, Jamalizadeh A, Shokri F, Jafarzadeh A. The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin Immunother PMID: 25483689. 2014;10(12):3731–36. doi:10.4161/hv.34393.
- Dini G, Toletone A, Barberis I, Debarbieri N, Massa E, Paganino C, Bersi F, Montecucco A, Alicino C, Durando P. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: a cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum Vaccin Immunother PMID: 27925503. 2017;13(2):440–44. doi:10.1080/21645515.2017.1264788.
- Zanetti AR, Mariano A, Romano L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366(9494):1379–84. PMID: 16226616. doi:10.1016/S0140-6736(05)67568-X.
- Zanetti A, Desole MG, Romanò L, d’Alessandro A, Conversano M, Ferrera G, Panico MG, Tomasi A, Zoppi G, Zuliani M, et al. Safety and immune response to a challenge dose of hepatitis B vaccine in healthy children primed 10years earlier with hexavalent vaccines in a 3, 5, 11-month schedule: an open-label, controlled, multicentre trial in Italy. Vaccine PMID: 28624307. 2017;35:4034–40. doi:10.1016/j.vaccine.2017.05.047.
- Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol PMID: 16372285. 2006;78(2):169–77. doi:10.1002/jmv.20524.
- Clemens R, Sänger R, Kruppenbacher J, Hobel W, Stanbury W, Bock H, Jilg W. Booster immunization of low-and non-responders after a standard three dose hepatitis B vaccine schedule—results of a post-marketing surveillance. Vaccine. 1997;15(4):349–52. PMID: 9141203.
- Petek D, Kamnik-Jug K. Motivators and barriers to vaccination of health professionals against seasonal influenza in primary healthcare. BMC Health Serv Res. 2018;18(1):853. doi:10.1186/s12913-018-3659-8.
- Lorenc T, Marshall D, Wright K, Sutcliffe K, Sowden A. Seasonal influenza vaccination of healthcare workers: systematic review of qualitative evidence. BMC Health Serv Res. 2017;17(1):732. doi:10.1186/s12913-017-2703-4.
- Topuridze M, Butsashvili M, Kamkamidze G, Kajaia M, Morse D, McNutt LA. Barriers to hepatitis B vaccine coverage among healthcare workers in the Republic of Georgia: an international perspective. Infect Control Hosp Epidemiol. 2010 Feb;31(2):158–64. doi:10.1086/649795.
- Kisic-Tepavcevic D, Kanazir M, Gazibara T, Maric G, Makismovic N, Loncarevic G, Pekmezovic T. Predictors of hepatitis B vaccination status in healthcare workers in Belgrade, Serbia, December 2015. Euro Surveill. 2017;22:30515. doi:10.2807/1560-7917.
- Maltezou HC, Theodoridou K, Ledda C, Rapisarda V, Theodoridou M. Vaccination of healthcare workers: is mandatory vaccination needed? Expert Rev Vaccines. 2019 Jan;18(1):5–13. doi:10.1080/14760584.2019.1552141.
- Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T, Jackson JM, Crain AL, Euler G. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine. 2013;31:3928–35. doi:10.1016/j.vaccine.2013.06.041.
- Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–16. doi:10.1016/j.jhep.2009.01.002.
- Italian integrated surveillance system for acute viral hepatitis SEIEVA [Internet]. [accessed 2019 Apr 10]. http://www.iss.it/seieva/.