ABSTRACT
Invasive meningococcal disease (IMD) caused by the bacteria Neisseria meningitidis is rare but potentially fatal. For healthy adolescents, the US Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination with MenACWY and recommends MenB vaccination under shared clinical decision-making (previously “Category B”). The recommendation for MenB vaccination was the first category B recommendation in adolescents, and it is unclear how healthcare providers (HCPs) implement these guidelines. This 2017 web-based survey of US HCPs explored characteristics associated with prescribing or receiving MenB and MenACWY vaccines, HCP knowledge of vaccine recommendations, and real-world practice patterns. Of 529 respondents, 436 prescribed MenB vaccines to their eligible adolescent/young adult patients and 93 prescribed MenACWY vaccines only. MenB vaccine prescribers were more likely to be pediatricians compared with MenACWY vaccine only prescribers, and patients who received MenB vaccines were more likely to be non-Hispanic whites living in shared spaces (eg, college dormitories) than those not receiving the vaccine. Seventy-seven percent of HCPs indicated that they prescribe MenACWY vaccines consistently with ACIP recommendations (to all members of an age group), whereas only 7% indicated that they prescribe MenB vaccines consistently with ACIP recommendations (individual clinical decision making). Patient-related factors, disease-related factors, and guidelines all influenced HCP decisions to prescribe meningococcal vaccines. Providing HCPs with clear guidance on how to initiate discussion of MenB vaccines with patients and their caregivers may aid in fully protecting US adolescents against meningococcal disease caused by 5 of the disease-causing serogroups.
Introduction
Invasive meningococcal disease (IMD) is a rare but serious and unpredictable infection caused by the bacteria Neisseria meningitidis.1,Citation2 The disease is fatal in 10% to 20% of cases, with case fatality rates up to 40% in patients with septicemia; 20% of survivors may experience debilitating long-term sequelae, including hearing loss or cognitive impairment.Citation1
Nearly 80% of cases of IMD in the United States are caused by meningococcal serogroups B, C, W, and Y.Citation3 Two quadrivalent meningococcal vaccines that provide coverage against serogroups A, C, W, and Y (MenACWY) are available in the United States.Citation4,Citation5 In 2005, when a conjugated MenACWY vaccine became available, the Advisory Committee on Immunization Practices (ACIP) recommended routine MenACWY vaccination at age 11 to 12 years.Citation6 At the time, meningococcal disease incidence rates were 0.5 to 1.1 per 100,000 population, and individuals 11 years of age and older accounted for 62% of cases overall, with 75% of these cases attributed to serogroups C, W, and Y. In 2011, as the burden of the disease was still high in young adults aged 16 through 21 years, the ACIP recommended a booster MenACWY dose at age 16 years to protect adolescents through the entire period of increased risk.Citation7 These routine recommendations apply to people within the specified age or risk group and were previously referred to as Category A, although the Centers for Disease Control and Prevention has transitioned to new nomenclature.Citation8–Citation10
The incidence of meningococcal disease has steadily declined in the United States over the last 2 decades, and the reduction in the incidence of meningococcal disease due to serogroups C, W, and Y among adolescents suggests an effect of the MenACWY vaccine program in this age group.Citation11 However, MenACWY vaccines do not protect against meningococcal serogroup B (MenB) disease, which has become the predominant serogroup causing meningococcal disease in recent years in the United States.Citation3 MenB-FHbp (Trumenba®, Pfizer Inc, Philadelphia, PA)Citation12 and MenB-4C (Bexsero®, GSK Vaccines, Srl, Sovicille, Italy)Citation13 are recombinant protein-based MenB vaccines available in the United States for use in persons aged 10 to 25 years. In 2015, although there were only 50 to 60 cases of MenB disease and 5 to 10 related deaths in adolescents and young adults aged 11 to 23 years, more than 80% of MenB cases occurred in individuals aged 16 to 23 years.Citation14 In addition, between 2009 and 2013, several MenB outbreaks had occurred at US colleges. Together with the availability of MenB vaccines, this prompted the ACIP in June 2015 to make a non-routine recommendation for MenB vaccination of healthy 16- to 23-year-olds (16–18 years preferred) under shared clinical decision-making (ie, “Category B” recommendation).Citation14,Citation15 These ACIP recommendations did not categorize college students as at increased risk for MenB disease as incidence estimates from 2009 to 2013, which were considered at that time, among college students aged 18 to 23 years (0.09 per 100,000) were lower than the incidence in all individuals (0.14 per 100,000) and among non-college individuals (0.21 per 100,000) of the same age.Citation14 Another factor in the ACIP decision was that data on breadth and duration of protective coverage with MenB vaccines were not yet available.Citation16
Since the ACIP recommendation for MenB vaccination, further changes in MenB epidemiology have occurred. In 2017, MenB disease was responsible for 38.3% of cases among all age groups.Citation3 Moreover, the percentage of meningococcal disease caused by MenB among adolescents and young adults aged 16‒23 years has increased from 58.3% in 2015 to 69.6% in 2017.Citation3,Citation17 US college students have a 3.5 times greater relative risk for MenB disease versus adolescents not attending college,Citation18 and all college outbreaks since 2011 were caused by MenB.Citation19 In addition, adolescents are the most common carriers of N meningitidis and are more likely to transmit the causative bacteria to others because of age-specific environmental and social behavioral characteristics.Citation20,Citation21
To better understand the implementation of ACIP recommendations for MenB vaccines and their potential effects on vaccine uptake among adolescents, which is a subpopulation with unique immunization delivery challenges,Citation22,Citation23 our study aimed to (1) examine healthcare provider (HCP) characteristics in relation to their prescribing patterns with meningococcal vaccines (MenACWY and MenB), (2) understand real-world decision processes of HCPs and their interpretation of ACIP recommendations, and (3) assess patient characteristics associated with the receipt of MenB vaccines.
Results
Participants
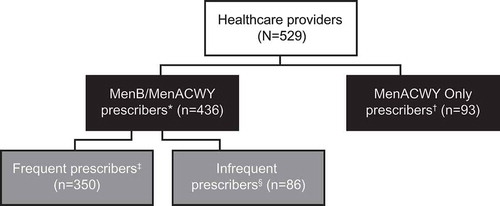
A total of 529 HCPs met the study inclusion criteria and completed the web-based survey. These HCPs were among the 3630 of the 73,350 potential participants who responded to the survey invitation (4.9% response rate). Patient chart reviews of 2832 patients aged 16–23 years were conducted by 453/529 HCPs (85.6%). Of the 529 HCPs, 431 (81.5%) indicated that they prescribed both the MenB and MenACWY vaccines to eligible adolescent or young adult patients, 5 (0.9%) indicated that they prescribed only the MenB vaccine, and 93 (17.6%) indicated that they prescribed only the MenACWY vaccine. For the purpose of this analysis, prescribers of MenB only were combined with prescribers of both MenACWY and MenB to form the MenB/MenACWY prescriber analysis group (n = 436; ). Among MenB/MenACWY prescribers, 350/436 (80.3%) were identified as frequent prescribers (who indicated that they prescribed the MenB vaccine “almost always” or “usually”) and 86/436 (19.7%) were identified as infrequent prescribers (who indicated that they prescribed the MenB vaccine “sometimes,” “upon request,” or “rarely/never”). Of the 2832 patient chart reviews conducted, 2379 were included in the analysis because the patient had received either the MenB vaccine only (n = 349), both the MenB and MenACWY vaccine (n = 1172), or the MenACWY vaccine only (n = 858); 453 charts were excluded because the patients had not received either vaccine. Similar to the HCP analysis, those who received the MenB vaccine only were combined with those who received both MenACWY and MenB vaccines to form the MenB/MenACWY receiver analysis group (n = 1521).
Characteristics of healthcare providers prescribing the meningococcal serogroup B vaccine
According to the bivariate analysis, factors significantly associated with HCP likelihood of prescribing MenB vaccine were sex, age, type of HCP, specialty, number of patients aged 16–23 years typically treated per month, interpretation of ACIP recommendations for MenB vaccines for adolescents, use of private/commercial insurance or Medicaid, and the percentage of their patients (according to the HCP) who understood the difference between the MenB and MenACWY vaccines ().
Table 1. HCP characteristics by whether they prescribe the MenB vaccine.
In the multivariable analysis adjusting for all model covariates (), HCPs with a higher likelihood of prescribing the MenB vaccine (based on a threshold of P < .10) were more likely to be men, pediatricians, or to treat a higher number of patients aged 16–23 years each month compared with those who did not prescribe MenB vaccines. A higher likelihood of prescribing MenB was also associated with having spent more years in the practice, having more patients using student health insurance plans compared with no insurance, having fewer patients on Medicaid versus no insurance, or having a higher number of patients who the HCP perceived as understanding the difference between the MenB and MenACWY vaccines.
Table 2. HCP factors associated with likelihood of prescribing MenB vaccine*.
Characteristics of patients receiving the meningococcal serogroup B vaccine
Factors that significantly varied between patients who had received the MenB vaccine versus those who had received the MenACWY vaccine only were age, ethnicity, student status, living arrangement, insurance coverage, whether the patient had received other vaccines in the past, or whether the patient was treated by a frequent MenB vaccine prescriber (). In the multivariable analysis adjusting for all model covariates (), patients who received the MenB vaccine were more likely to be male, non-Hispanic white (compared with all other race/ethnicity categories), living in a campus dormitory or other shared space, or treated by a frequent MenB prescriber. Patients were more likely to receive the MenB vaccine if they received the human papilloma virus (HPV) vaccine but less likely to receive the MenB vaccine if they had ever received the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine.
Table 3. Patient characteristics by whether they received the MenB vaccine*.
Table 4. Patient factors associated with likelihood of receiving MenB vaccine*.
Interpretation of the advisory committee on immunization practices recommendations
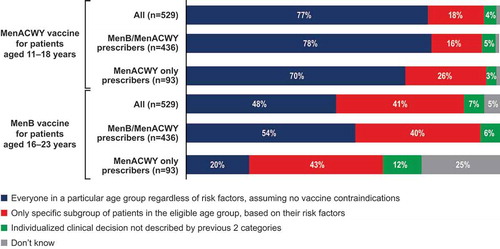
Respondents were asked to indicate their interpretation of ACIP’s recommendations by selecting the decision process they used to determine who should receive the MenACWY and/or the MenB vaccine (). Regarding MenACWY, 77% of all survey respondents interpreted the recommendation as to prescribe to everyone in a particular age group regardless of risk factors, 18% interpreted the recommendation as to prescribe only to specific subgroups of patients based on risk factors, 4% interpreted the recommendation as to prescribe based on individual clinical decision not described by the previous 2 categories, and 1% indicated that they did not know how to interpret the recommendation. For MenB vaccines, the corresponding response rates were 48%, 41%, 7%, and 5%. The percentage of HCPs who interpreted recommendations as to prescribe MenB vaccination based on individualized clinical decision-making was higher among MenACWY only prescribers (12%) compared with MenB/MenACWY prescribers (6%). This difference was significant according to the multivariate analysis (), which showed that MenB/MenACWY prescribers were more likely than MenACWY only prescribers to recommend the MenB vaccine to everyone in a particular age group or subgroup, whereas MenACWY only prescribers were more likely than MenB/MenACWY prescribers to recommend the MenB vaccine based on individual clinical decision-making. In addition, 25% of MenACWY only prescribers indicated that they did not know how to interpret ACIP recommendations for the MenB vaccine, compared with only 0.5% of MenB/MenACWY prescribers.
Factors affecting the decision by healthcare providers to prescribe or not to prescribe the meningococcal vaccines
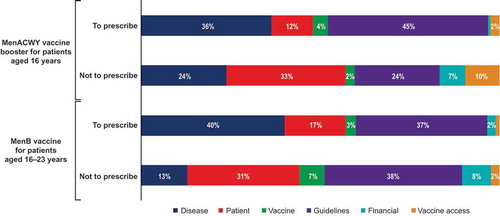
Healthcare providers most frequently ranked guideline considerations and disease-related factors as the most influential reasons regarding their decision to prescribe the MenACWY and MenB vaccines, respectively. In contrast, when HCPs decided not to prescribe the MenACWY or MenB vaccine, they most frequently ranked patient-related factors and guideline considerations, respectively, as most influential (). Compared with the most influential reasons listed above, vaccine-related factors, vaccine access, or patient finances/insurance were less impactful. For both vaccines, financial considerations were more likely to be a top consideration in the decision not to prescribe (ranked highest by 7% for MenACWY and 8% for MenB vaccine) compared with the decision to prescribe (ranked highest by 1% for MenACWY and 2% for MenB vaccine) ().
Practice patterns for the meningococcal serogroup B vaccine
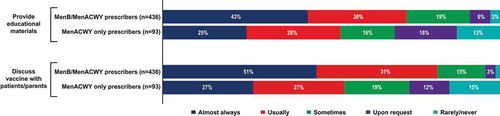
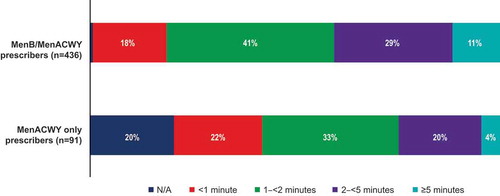
Survey participants were asked about their behavior surrounding discussions with their patients regarding MenB vaccines. Of MenB/MenACWY prescribers, 43% indicated that they almost always provided educational materials and 51% discussed the MenB vaccine with patients or caregivers, in contrast to the respective 25% and 27% of MenACWY only prescribers (). MenB/MenACWY prescribers also typically spent more time discussing the MenB vaccine with patients compared with MenACWY only prescribers, with 81% of MenB/MenACWY prescribers and 57% of MenACWY only prescribers indicating that conversations lasted ≥1 minute ().
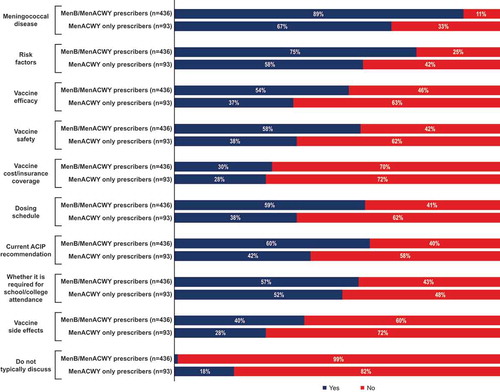
The amount of time spent on discussions about the MenB vaccine was most frequently from 1–<2 minutes, as indicated by 41% of MenB/MenACWY prescribers and 33% of MenACWY only prescribers (). During discussions about the MenB vaccine, the specific topics most frequently raised by all participants were meningococcal disease (discussed by 89% of MenB/MenACWY prescribers and 67% of MenACWY only prescribers) and associated risk factors (discussed by 75% of MenB/MenACWY prescribers and 58% of MenACWY only prescribers; ).
Other topics that were usually discussed were vaccine efficacy, vaccine safety, dosing schedule, ACIP recommendations, and school/college requirements (discussed by 54%‒60% of MenB/MenACWY prescribers and 37%‒52% of MenACWY only prescribers); vaccine costs/insurance and vaccine side effects were discussed less frequently. One percent of MenB/MenACWY prescribers and 18% of MenACWY only prescribers indicated that they do not typically discuss the MenB vaccine with patients or caregivers.
Discussion
Two meningococcal vaccines, MenB and MenACWY, are needed to fully protect adolescents and young adults against the most common serogroups that cause meningococcal disease in the United States.Citation3–Citation5,Citation12 Based on recent surveillance data, MenB is responsible for most cases – notably 69.6% in 2017 ‒ among US adolescents and young adults aged 16‒23 years.Citation3 The 2 vaccines have different ACIP recommendations: MenACWY vaccination is universally recommended for all individuals aged 11‒18 years,Citation11 whereas MenB vaccination for healthy individuals is recommended in the context of shared clinical decision making.Citation8,Citation16 Our survey of >500 practicing HCPs in the United States revealed challenges in implementing the shared clinical decision-making recommendation with the following 3 key findings: (1) HCPs interpreted the ACIP vaccine recommendations and implemented these recommendations in their clinical practices differently; (2) the characteristics of HCPs who prescribed the MenB vaccine versus those who only prescribed the MenACWY vaccine differed; and (3) the characteristics among the patients reviewed here who received the MenB vaccine versus those who did not varied. These findings may reflect differences in ACIP recommendations for the 2 vaccinesCitation8,Citation11,Citation14 or disparate access to newer vaccines, given that MenB vaccines were licensed and recommended in 2014/2015, whereas MenACWY vaccines were first licensed in 2005.Citation4,Citation12,Citation24
Overall, our findings add to the results from previous survey studies regarding MenB vaccine. In the first national survey of HCPs who treat adolescents, Kempe and colleaguesCitation25 found that among those who discussed the MenB vaccine with patients, 91% prescribed the vaccine. However, among HCPs who “rarely/never” discussed the vaccine with patients, only 11% prescribed the MenB vaccine. Similar to our study, this survey reported differences in HCP’s interpretation of ACIP vaccine recommendations. The authors provided 2 possible interpretations of the category B recommendation: either that the HCP should initiate discussion with patients or caregivers so that they can choose whether to receive the MenB vaccine or that the HCP should decide whether to initiate discussion based on their own understanding of the disease and individual patient risk. The data presented by Kempe and colleagues suggested that the latter interpretation is more common and that HCPs may regard the category B recommendation as “lesser” than the routine or category A recommendation.Citation25 Moreover, there was confusion among HCPs regarding which meningococcal vaccine (ie, MenACWY or MenB) to administer to 11- to 12-year-olds, as well as a misunderstanding about the category A recommendation for MenB vaccination in children aged ≥10 years who are at increased risk of IMD.
Our study suggests a lack of understanding of category B recommendations among HCPs. We show that HCPs who did not prescribe the MenB vaccine were less likely than those who prescribed the vaccine to be aware of MenB guidelines, with 25% of MenACWY only prescribers versus only 0.5% of MenB/MenACWY prescribers responding that they did not know how to prescribe the vaccine based on ACIP recommendations. Moreover, when HCPs were asked to select their strategy for deciding when to prescribe meningococcal vaccines, 77% of respondents correctly interpreted the ACIP category A recommendation for MenACWY vaccine prescription, but only 7% correctly interpreted category B recommendations for MenB.Citation8 The percentage of respondents who thought MenB vaccination was recommended for everyone in a particular age group was similar to the percentage who thought it should be prescribed to specific subgroups based on risk factors.
In another study, Kempe and colleagues reported that 56% and 38% of pediatricians and family physicians surveyed, respectively, were able to correctly define the category B recommendation.Citation26 One reason that these percentages were higher than those reported here may be the difference in how the survey questions were worded. The Kempe study asked respondents to define the recommendation, whereas our study asked HCPs to indicate how they decide whether to prescribe the MenB vaccine in their practice based on ACIP recommendations. It is also possible that those who thought ACIP recommended to prescribe MenB vaccination for “only specific subgroups of patients in the eligible age group, based on their risk factors” may have interpreted college attendance as a risk factor related to individual clinical decision-making. Given this possibility, it is notable that the percentage of respondents who selected either the “at risk” or “individual clinical decision making” interpretation was 48%, similar to the values observed by Kempe and colleagues.Citation26
Also similar to our survey results, factors associated with lower likelihood of prescribing in the Kempe survey included an inability to define the recommendations and the mistaken belief that ACIP category B recommended vaccines were not covered by insurance.Citation26 The most influential factors in HCP decisions to prescribe the MenB vaccine included disease incidence and the efficacy and safety of the MenB vaccine. An overall lack of knowledge about MenB disease or awareness of the MenB vaccine may be a primary reason for not initiating discussion. The authors suggested a need for additional guidance from national organizations (eg, American Academy of Pediatrics [AAP] and the American Academy of Family Physicians [AAFP]) on talking points and how to implement a successful discussion of the MenB vaccine.
In our study, the most important factors influencing HCPs in their decision to prescribe the MenB vaccine were disease-related factors and guideline considerations. This result is consistent with data from Kempe and colleagues,Citation25 indicating that disease outbreaks and disease incidence were the most influential considerations for HCPs. Both the present study and the report from Kempe and colleagues also identified guideline considerations as an important factor in HCP decision not to prescribe the MenB vaccine.Citation25 Identification of guideline considerations as influencing decisions both to prescribe and not to prescribe the vaccine may indicate confusion or disparate interpretations of ACIP’s recommendations among HCPs.
Our results also imply that different providers have different interpretations of what is meant by individual clinical decision making. Only about half of MenB/MenACWY prescribers and a quarter of MenACWY only prescribers in our study indicated that they “almost always” provide educational materials and/or discuss the MenB vaccine with patients or their caregivers. This inference is also supported by the finding that guideline considerations were an important consideration for decisions both to prescribe and not to prescribe. Some prescribers may decide they should always discuss the vaccine with patients, whereas some may choose whether or not to initiate discussion based on their own clinical assessment of the vaccine risk-benefit profile.Citation25 Providers might also choose not to discuss the MenB vaccine with their patients if they believe it will not be covered by insurance or that it will take a longer time to explain compared with a category A-recommended vaccine.Citation26
Healthcare providers in our study who prescribed the MenB vaccine to their patients were more likely to be pediatricians and to have spent more time in their current practice. They were also more likely to treat a higher number of adolescent and young adult patients each month and to perceive their patients as understanding the difference between the MenACWY and MenB vaccines. These characteristics altogether suggest that MenB prescribing patterns may be associated with a greater understanding of the disease risks and vaccine benefit in this population through more experience practicing on this particular age cohort. This is consistent with previous evidence suggesting that the more years of practice a pediatrician has, the more likely their patients are to receive the MenB vaccine (although this effect declines with >30 years in practice).Citation27 Our results are also consistent with the survey performed by Kempe and colleagues, in which 73% of pediatricians versus 41% of family physicians surveyed administered the MenB vaccine to their adolescent and young adult patients.Citation25 In 1 study, investigators showed that MenB recipients (aged 16–18 y) were more likely to also be up-to-date on HPV and measles-containing vaccines, which suggests vaccine receipt may depend on the provider.Citation28
Patients included in the chart review who received the MenB vaccine were more likely than those who had not received the vaccine to be non-Hispanic white males. Our study did not assess if these results may have been influenced by potential racial disparities in college enrollment. Although our study was not population-based, the results were consistent with a previous population-based survey of adolescent patients and their caregivers, in which non-Hispanic white patients were more likely to be aware of the MenB vaccine and Hispanic patients were less likely to be aware.Citation29 Another study reported similar findings for the HPV vaccine series, which is more likely to be completed by non-Hispanic white females compared with African American or Hispanic females (although a pooled analysis found that Hispanic females were more likely than non-Hispanic females to initiate the series).Citation30 Notably, more consistent HCP recommendations were identified as an important factor leading to reduced racial disparities in HPV vaccine coverage over time, suggesting that HCP recommendation may be able to play a similar role regarding the MenB vaccination. These factors can also depend on the facility in which patients receive care, with evidence suggesting that teenagers who receive care in community or district health centres are significantly less likely to receive the MenB vaccine.Citation28
Compared with patients who did not receive the MenB vaccine, vaccinated patients in our study were more likely to live in a college dormitory or other shared space, which may reflect an understanding by the HCP or the patient or caregiver that college students are at increased risk of MenB disease. An increased incidence of IMD has been identified among college freshmen living in dormitories,Citation31 and recent data suggest that all college students are at an increased risk of developing IMD compared with nonstudents; this increased risk is entirely driven by MenB disease.Citation32 MenB has been responsible for all college outbreaks since 2011Citation19,Citation33 and is 3.5 times more likely to affect college students compared with nonstudents.Citation18
Individuals in our study who received the MenB vaccine were also more likely to have received the HPV vaccine but less likely to have received the Tdap vaccine. These associations may be related to more complete vaccination records available for those who received the MenB vaccine, given that the MenB vaccine was associated with more HCP and patient familiarity in a previous survey.Citation29 It may also be the case that HCPs included in this survey had more complete records available for HPV compared with Tdap vaccination history. Although both vaccines are recommended by the AAP for administration at age 11‒12 years, in practice adolescents often receive HPV vaccination much later, similarly to ages at which the MenB vaccine would be prescribed.Citation34–Citation36
Adolescent immunization delivery poses unique challenges such as infrequent medical appointments during which many important health topics including vaccination must be covered.Citation23 The estimated MenACWY vaccine coverage of individuals aged 13 to 17 years in 2018 was 86.6% for ≥1 dose and 50.8% for ≥2 doses.Citation37 In contrast, MenB vaccine uptake is much lower, with only an estimated 17.2% of individuals aged 17 years having received ≥1 dose of a multidose vaccine series in 2018Citation37and an estimated <50% complete the series.Citation38 Patients and parents rely on their physicians to guide decisions about vaccination,Citation23,Citation30,Citation39 and pediatricians in turn rely on clear guidance and support from the ACIP regarding how vaccines should be prescribed to their patients.Citation23 Historically, when such guidance has been provided, pediatricians have been demonstrably effective advocates of vaccination and have contributed to achieving high vaccination coverage.Citation23 Our results, consistent with previous reports,Citation25,Citation26 indicate that clarity and additional guidance are needed to help HCPs interpret and implement the category B recommendation for MenB vaccines.
One possible limitation of our study is that a total of 3630 HCPs from the Lightspeed/All Global panel responded to 73,350 e-mail invitations, representing a response rate of 4.9%. We did not have access to a membership list from a medical society (eg, AAP, AMA, AAFP) or their administrative support to remind members to complete the survey and help improve the response rate. Therefore, we used a large, vetted physician panel to obtain a number of responses that enabled reliable conclusions to be drawn. This approach has been used by other peer-reviewed studies, including as an example a published chart review survey of physicians treating patients with plaque psoriasis who were recruited with the same panel used here.Citation40 We also minimized potential selection bias through the study design by directing HCPs to select the most recent patient charts that met the clearly outlined criteria. Additionally, analytical techniques such as using multiple regression were used to control for potential confounders and differences in baseline characteristics between MenB versus non-MenB groups. The outcomes from this chart review study were similar to outcomes observed in previous studies.Citation40 Another possible limitation is that HCPs may have based responses about whether or not they prescribed the MenB vaccine on recollection alone, which may have been subject to bias. Additionally, HCPs who did not prescribe any meningococcal vaccines were excluded from the survey; thus, the fraction of HCPs who prescribe MenB vaccines out of all US HCPs (including those who do not prescribe meningococcal vaccines) may be smaller than the fraction in this study. Lastly, inferences about characteristics associated with MenB vaccination could only be made regarding patients included in the study, rather than the adolescent population as a whole. The total number of patients treated by each HCP was unknown because charts were not randomly selected; rather, they were selected by the HCPs to meet specific quotas of patients who had or had not received the MenB vaccine.
Conclusion
Our study adds to a growing body of evidence demonstrating a need to help HCPs better understand and implement the category B ACIP recommendation for individual clinical decision-making regarding MenB vaccination.Citation25,Citation26 Providing clear guidance, including regarding how to initiate discussion of MenB vaccines with patients and their caregivers as suggested by the AAP and ACIP,Citation41 may help ensure that US adolescents are fully protected against meningococcal disease caused by all 5 serogroups.
Methods
Participants
A web-based survey was conducted between August and October of 2017 among HCPs, including primary care providers, nurse practitioners, and physician assistants. HCPs were recruited through the full, double opt-in Lightspeed/All Global panelCitation42 of >55,600 US HCPs with American Medical Association (AMA) membership. Each participant was expected to complete the survey in <50 minutes.
Eligible participants were primary care providers with confirmed certification and ≥3 years of experience as a pediatrician, family practitioner, or internal medicine specialist, as well as nurse practitioners and physician assistants who worked in 1 of the above specialties. Eligible HCPs also spent ≥50% of their time in direct patient care, treated ≥30 patients aged 16–23 years each month, recommended MenACWY and/or MenB vaccination to their adolescent or young adult patients (regardless of whether the vaccine was administered in the office), and consented to study participation.
Healthcare providers who self-identified as prescribers of the MenB vaccine for their eligible patients were asked to review 4‒8 charts of recently seen patients, of which up to 4 charts were for patients who had received a MenB vaccination within the previous 6 months and up to 4 charts were for patients who had not received a MenB vaccination within the previous 6 months. Eligible patients identified by chart review were aged 16–23 years during a wellness visit within the previous 6 months (at which time they were not pregnant or breastfeeding), had no severe or life-threatening vaccine allergies, and had received ≥1 childhood vaccination.
Healthcare providers who completed the survey and reviewed at least 4 patient charts received an honorarium of $75 plus $15 per additional patient chart. The study was reviewed and approved by the Pearl Institutional Review BoardCitation43 (IRB Study No.: 17-KANT-153; 29 East McCarty St., Ste. 100, Indianapolis, IN 46225), an independent IRB registered with the Office for Human Research Protections and the US Food and Drug Administration that is fully accredited by the Association for the Accreditation of Human Research Protection Programs. Pearl IRB found that the research met the requirements for a waiver of documentation of consent under 45 CFR 46.101(b)(4).
Survey and chart review
The survey collected information on HCP characteristics; interpretations of ACIP recommendations for adolescent vaccines including the HPV vaccine, the MenB and MenACWY vaccines, and the Tdap vaccine; vaccination practice patterns; and factors influencing their decision whether or not to prescribe a MenB and/or MenACWY vaccine. Requested HCP characteristics included sex, age, type of HCP, medical specialty, practice setting (office/clinic, hospital, university/medical school, government/VA, or other), practice location, years in practice, number of patients aged 16–23 years typically treated per month, types of healthcare coverage plans accepted, and time spent in direct patient care.
Healthcare providers were asked to interpret ACIP recommendations for MenB and MenACWY vaccines by selecting 1 of the following 4 choices regarding how the vaccine should be prescribed: (1) to everyone in a particular age group, regardless of risk factors, assuming no vaccine contraindications, (2) only to specific subgroups of patients in the eligible age group, based on their risk factors, (3) based on individualized clinical decision not described by the previous 2 categories, or (4) do not know. HCPs were also asked for their frequency of providing educational materials for, discussing, and recommending MenB and/or MenACWY vaccines, with answers ranging from “almost always” to “rarely/never.” Time spent discussing the vaccine with patients was requested, as well as the specific topics discussed (ie, meningococcal disease, risk factors, vaccine efficacy, vaccine safety, vaccine side effects, vaccine cost/insurance coverage, dosing schedule, ACIP recommendations, and school/college vaccination requirements).
Healthcare providers were asked to rank the following factors from the most impactful to the least impactful on their decision to prescribe or not to prescribe each vaccine (MenB and MenACWY): disease-related factors (eg, level of risk to the community or patient, past experience), patient-related factors (eg, age eligibility, request or lack thereof by the patient or the patient’s school, living arrangements, travel plans), vaccine-related factors (eg, efficacy, side effects), guideline considerations (from ACIP or AAP), financial considerations (cost of vaccine or vaccination), or vaccine access considerations (availability at the practice or at local pharmacies).
For chart reviews, patient demographics (eg, age, sex, race/ethnicity), employment status, living arrangement, insurance coverage, and vaccination history were requested. If the patient received the MenB vaccine, HCPs were asked if they discussed the vaccine with the patient or received a request from the patient, and also to indicate the specific reasons that the patient received the vaccine (eg, increased risk, travel plans, school/college requirements).
Analysis
On the basis of a survey question that asked HCPs whether they prescribed or recommended the MenB and/or MenACWY vaccine to their eligible adolescent or young adult patients (regardless of whether the vaccine was administered in the office or elsewhere), HCPs were divided into the following 2 groups: MenB/MenACWY prescribers (HCPs who recommended either the MenB vaccine only or who recommended both MenB and MenACWY vaccines) or MenACWY only prescribers (HCPs who recommended the MenACWY vaccine only). Bivariate analyses of HCP characteristics associated with being a MenB/MenACWY prescriber versus a MenACWY only prescriber were conducted to identify factors that differed significantly between the 2 groups. On the basis of which vaccines they had received within the past 6 months, patients included in chart reviews were separated into the following 3 groups: those who had received the MenB vaccine (whether or not they had also received the MenACWY vaccine), those who had received the MenACWY vaccine only, and those who had not received a meningococcal vaccine. Bivariate analyses were conducted to compare patient characteristics between those who had received the MenB vaccine and those who had received the MenACWY vaccine only.
Variables identified to be significant (P < .05) in the bivariate analyses and other variables of theoretical importance were entered into multivariable logistic regression models. These models were designed to evaluate significance and relative associations among potential predictors of vaccination while simultaneously controlling for other potential predictors. Backward elimination was used to select variables significantly associated with each outcome; the threshold for inclusion in the models was an alpha level of 0.10. Because prescribers and nonprescribers of each vaccine responded to different sets of questions, the most impactful factors on the decision to prescribe or not to prescribe MenB or MenACWY vaccines were only analyzed descriptively.
Disclosure of potential conflicts of interest
AS and LH are employees of Pfizer and may hold stock or stock options. AD serves on advisory boards for Merck, Pfizer, and Sanofi Pasteur and as a consultant to Pfizer related to MenB vaccination. AG, LL, and VL were employees of Kantar Health at the time the study was conducted, which was a paid contractor by Pfizer, and conducted the survey.
Acknowledgment
Editorial/medical writing support was provided by Anna Stern, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), and was funded by Pfizer Inc. Editorial/medical writing support was also provided by Uma Dasam and Urmila Rao, PhD, of Indegene Lifesystems Pvt Ltd, for initial draft development and was funded by Pfizer Inc.
Additional information
Funding
References
- Martinón-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2 suppl):S12–20. doi:10.1016/j.jadohealth.2016.03.041.
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease-United States, 1996–2015. Clin Infect Dis. 2018;66(8):1276–81. doi:10.1093/cid/cix993.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report; 2017 [accessed 2019 May 10] https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf.
- Menactra® (Meningococcal [Groups A, C, Y and W-135] polysaccharide diphtheria toxoid conjugate vaccine). Swiftwater (PA): Full Prescribing Information, Sanofi Pasteur Inc.; 2018.
- Menveo® (Meningococcal [Groups A, C, Y and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine). Full prescribing information, GSK vaccines S.r.l. Sovicille (Italy); 2017.
- Bilukha OO, Rosenstein N. National center for infectious diseases, centers for disease control and prevention. Prevention and control of meningococcal disease. recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54:1–21.
- Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines — Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60(3):72–76.
- Ahmed F. US Advisory Committee on Immunization Practices (ACIP) handbook for developing evidence-based recommendations version 1.2; 2013.
- Carr W. ACIP evidence-based recommendations work group proposal. Paper presented at: ACIP Meeting; 2018 February 21–22; Atlanta, GA.
- Lee G, Carr W, Reingold A, Hunter P, Lee G, Temte J, Campos-Outcalt D, Rubin L, O’Leary S, Savoy M, Group AE-BRW, Group AEBRW. Updated framework for development of evidence-based recommendations by the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2018;67(45):1271–72. doi:10.15585/mmwr.mm6745a4.
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR–2):1–28. doi: rr6202a1[pii].
- Trumenba® (meningococcal group B vaccine). Full prescribing information. Philadelphia (PA): Wyeth Pharmaceuticals Inc (a subsidiary of Pfizer Inc); 2018.
- Bexsero (meningococcal group B vaccine). Full prescribing information. Sovicille (SI) (Italy): GSK Vaccines, Srl; 2018.
- MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–76. doi:10.15585/mmwr.mm6441a3.
- Romero J, Cohn A. Welcome and Introductions. Advisory Committee on Immunization Practices (ACIP). [accessed 2019 September 10]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Welcome-Romero-Cohn-508.pdf.
- MacNeil J. Considerations for use of serogroup B meningococcal (MenB) vaccines in adolescents. [accessed 2016 April 4]. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/mening-03-macneil.pdf.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report; 2015 [accessed 2019 May 10]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf.
- Mbaeyi SA, Joseph SJ, Blain A, Wang X, Hariri S, MacNeil JR. Meningococcal disease among college-aged young adults: 2014–2016. Pediatrics. 2019;143(1). doi:10.1542/peds.2018-2130.
- Marshall GS, Dempsey AF, Srivastava A, Isturiz RE. US college students are at increased risk for serogroup B meningococcal disease. J Pediatric Infect Dis Soc. 2019. doi:10.1093/jpids/piz024.
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12(6):950–57. doi:10.3201/eid1206.051297.
- Burman C, Serra L, Nuttens C, Presa J, Balmer P, York L. Meningococcal disease in adolescents and young adults: a review of the rationale for prevention through vaccination. Hum Vaccin Immunother. 2019;15(2):459–69.
- Vetter V, Baxter R, Denizer G, Safadi MA, Silfverdal SA, Vyse A, Borrow R. Routinely vaccinating adolescents against meningococcus: targeting transmission & disease. Expert Rev Vaccines. 2016;15(5):641–58. doi:10.1586/14760584.2016.1130628.
- Brady MT. Strength and clarity of vaccine recommendations influence providers’ practice. Pediatrics. 2018;142(3). [Epubahead of print]. doi:10.1542/peds.2018-1633.
- Bexsero® (Meningococcal Group B Vaccine). Full prescribing information. Siena (Italy): GSK Vaccines, Srl; 2018.
- Kempe A, Allison MA, MacNeil JR, O’Leary ST, Crane LA, Beaty BL, Hurley LP, Brtnikova M, Lindley MC, Albert AP. Adoption of serogroup B meningococcal vaccine recommendations. Pediatrics. 2018;142(3):e20180344. doi:10.1542/peds.2018-0344.
- Kempe A, Allison MA, MacNeil JR, O’Leary ST, Crane LA, Beaty BL, Hurley LP, Brtnikova M, Lindley MC, Liang JL, et al. Knowledge and attitudes regarding category B ACIP recommendations among primary care providers for children. Acad Pediatr. 2018;18(7):763–68. doi:10.1016/j.acap.2018.04.005.
- Watkins E, Feemster K. Factors associated with uptake of meningococcus B vaccination after an ACIP category B recommendation. Open Forum Infect Dis. 2018;5(Suppl 1):S737. doi:10.1093/ofid/ofy210.2113.
- Bart SM, Eberhart M, Feemster K. Impact of a category B recommendation: meningococcal B (MenB) vaccine uptake among adolescents in Philadelphia county. Toronto (Canada): Pediatric Academic Societies; 2018.
- Srivastava A, Dempsey A, Galitsky A, Tibbets K, Huang L Socio-economic inequities in parental awareness and utilization of meningococcal serogroup B vaccines. Paper presented at: Pediatric Academic Societies; 2018 May 5–8; Toronto, Canada.
- Burdette AM, Webb NS, Hill TD, Jokinen-Gordon H. Race-specific trends in HPV vaccinations and provider recommendations: persistent disparities or social progress? Public Health. 2017;142:167–76. doi:10.1016/j.puhe.2016.07.009.
- Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA. 2001;286(6):688–93. doi:10.1001/jama.286.6.688.
- CDC. Enhanced meningococcal disease surveillance report; 2016. [accessed 2019 May 10]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.
- Soeters HM, McNamara LA, Blain AE, Whaley M, MacNeil JR, Hariri S, Mbaeyi SA. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25(3):434–40. doi:10.3201/eid2503.181574.
- Centers for Disease Control and Prevention. 2019 recommended immunizations for children 7–18 years old; [accessed 2019 June 20]. https://www.cdc.gov/vaccines/schedules/downloads/teen/parent-version-schedule-7-18yrs.pdf.
- Lin X, Shrader L, Rodgers L, Stokley S, Markowitz LE. Increasing human papillomavirus vaccination at the recommended age. Vaccine. 2019;37(5):686–89. doi:10.1016/j.vaccine.2018.12.023.
- Bednarczyk RA, Ellingson MK, Omer SB. Human papillomavirus vaccination before 13 and 15 years of age: analysis of national immunization survey teen data. J Infect Dis. 2019. doi:10.1093/infdis/jiy682.
- Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, Singleton JA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–23. doi:10.15585/mmwr.mm6833a2.
- Centers for Disease Control and Prevention. February 2019 ACIP Meeting - Meningococcal Vaccines; Atlanta, GA [accessed 2019 April 29]. https://www.youtube.com/watch?v=6ZmOh1SLLKQ.
- C.S. Mott Children’s Hospital. Mott poll report: parents not keeping up with teen vaccines; [accessed 2018 August 8]. https://mottpoll.org/sites/default/files/documents/071717_teenvaccines.pdf.
- Zhang M, Goren A, Lee S, DiBonaventura MD, Olson WH. Characterizing patients with psoriasis on injectable biologics adalimumab, etanercept, and ustekinumab: A chart review study. J Dermatolog Treat. 2016;27(4):339–45. doi:10.3109/09546634.2015.1118427.
- Marshall GS, Tan L. Understanding the category B recommendation for serogroup B meningococcal vaccine. Pediatrics. 2017;139(5):e20163484. doi:10.1542/peds.2016-3484.
- Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States 2019. [accessed 2019 March 5] https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- Iro MA, Snape MD, Voysey M, Jawad S, Finn A, Heath PT, Bona G, Esposito S, Diez-Domingo J, Prymula R, et al. Persistence of bactericidal antibodies following booster vaccination with 4CMenB at 12, 18 or 24 months and immunogenicity of a fifth dose administered at 4 years of age-a phase 3 extension to a randomised controlled trial. Vaccine. 2017;35(2):395–402. doi:10.1016/j.vaccine.2016.11.009.