ABSTRACT
Group B streptococcus (GBS) vaccines are currently under development. Data on the natural immunity in diverse age groups will aid establishing the GBS immunization policy. In this study, thirty serum samples were collected from three age groups (neonates/infants, pregnant women, and the elderly) between August 2016 and July 2017. Serotype-specific opsonophagocytic activity (OPA) was assessed using a GBS multiplex opsonophagocytic killing assay (MOPA) against serotypes Ia, III, and V. The mean OPA titers for serotype Ia of the three age groups were not significantly different (p = .156), but tended to be lower in neonates/infants (mean ± standard deviation, 137 ± 278). For serotype III and V, the mean OPA titer of neonates/infants (338 ± 623 and 161 ± 445, respectively) was significantly lower than that of pregnant women (1377 ± 1167 and 9414 ± 6394) and the elderly (1350 ± 1741 and 3669 ± 5597) (p = .002). In conclusion, the lower levels of OPA titers against all tested serotypes in neonates/infants, despite high maternal titers, indicates that intrapartum GBS vaccinations may be required for efficient placental transfer of serotype-specific GBS antibodies with high avidity.
Introduction
Group B streptococcus (Streptococcus agalactiae; GBS) is frequently isolated from the gastrointestinal and genitourinary tracts of healthy adults.Citation1 Because asymptomatic recto-vaginal colonization by GBS is common (10–37%) during women’s reproductive years,Citation2 vaginal colonization by GBS might be associated with neonatal invasive diseases such as sepsis, pneumonia, and meningitis, which affect 1,930 live births per year in the United States alone.Citation3 While current recommendations, including prenatal GBS screening and intrapartum antibiotic prophylaxis, have dramatically reduced early-onset disease (EOD), they have had no effect on late-onset disease (LOD) or late maternal colonization. Alarmingly, the adult GBS infection rate is also on the rise,Citation4 especially in the elderly. For example, in the United States the incidence and mortality of invasive GBS infections in the elderly has increased over three fold over the past five years.Citation3
Administering GBS vaccinations to pregnant women may be an alternate strategy for the prevention of invasive GBS disease in infants, and pregnant women themselves, thereby reducing the risk of premature births and stillbirths.Citation5 It could also be the best strategy for preventing the high disease burden in the elderly.Citation3 Capsular polysaccharide (CPS), the most important virulence factor of GBS, is categorized into 10 distinct polysaccharides (Ia, Ib, II to IX) based on their structural and serological differences.Citation4 More than 86% of EOD and 93% of LOD cases are caused by serotypes Ia, III, and V.Citation4 In adults, serotype V (27.5%) was shown to be the predominant serotype, followed by Ia (24.3%) and III (16.5%).Citation4 Currently, tri- or penta-valent GBS polysaccharide-protein conjugate vaccines (GBS-PCV) containing Ia, III, and V polysaccharides are under development by major pharmaceutical companies.Citation6
Enrolling a large number of pregnant women in clinical trials for the licensure of GBS vaccines is challenging due to the lower baseline incidence of primary endpoint of GBS invasive disease.Citation2,Citation6 Thus, reliable immunogenicity assays are essential for the process of GBS vaccine licensure, as observed with Neisseria meningococcal group C conjugate vaccine approval.Citation7 Previously, we developed a multiplexed, opsonophagocytic assay (MOPA) targeting serotypes Ia, III and V.Citation8 Although the predominant serotype varies by country and population age, serotypes Ia, III, and V are the most prevalent globally. In this study, we measured the levels of baseline functional antibodies against GBS-CPS Ia, III, and V in three targeted groups (neonates/infants, pregnant women, and the elderly) using MOPA. The aim of this study was to obtain indirect information on the protective immunity levels required from GBS vaccination by comparing basal serotype-specific GBS immunity between a non-high-risk group (pregnant women) and high-risk groups (neonates/infants and the elderly).
Materials and methods
Ethics statement
The study was approved by the Korea University Guro hospital (KUGH IRB No. 2016GR0265), and informed written consent was obtained from all participants aged ≥18 years. The institutional review board of the KUGH waived written informed consent for the use of remnant neonatal serum samples after laboratory tests in the department of laboratory medicine. Subject records/information for neonates was anonymized and de-identified prior to analysis.
Study design and sample collection
Serum samples were collected from pregnant women, infants under 3 months of age, and the elderly ≥65 years old, who are the main targets of the GBS vaccine. Between January 2016 and March 2017, subjects who voluntarily agreed to participate in the study were recruited when they visited the outpatient clinic and 30 persons per group were sampled in order of application. Healthy subjects without comorbidities were included. In the case of neonates/infants, 30 cases were collected in the order of sample collection at the laboratory. In the twins, only the first newborn was included in the study. All study samples were obtained from subjects who had not received any antibiotics in the three days prior to blood sampling. All pregnant women underwent vaginal-rectal screening (swab and conventional culture) for GBS colonization at 35–37 weeks (at the time of admission in case of preterm delivery). They were sub-grouped by GBS colonization status, and the capsule types of the colonized GBS isolates were identified by the use of both phenotypic and genotypic assays.Citation9
Multiplexed opsonophagocytic assay (MOPA)
GBS MOPA was conducted as described previously.Citation8 Briefly, 10 µL of the serially diluted serum samples and GBS mixture (streptomycin-resistant serotype Ia, spectinomycin-resistant serotypes III and kanamycin-resistant serotypes V GBS strains) were added to each well of 96-well plates. After 30 min of incubation at room temperature, 42.8 μl of a differentiated HL-60 cell suspension (4.28 × 105 cells per well) and 7.2 μl of baby rabbit complement (BRC; Pel-Freez Biological; Rogers, AR) were added to each well, after which the mixture was incubated for 45 min at 37°C with shaking. Afterward, 10 µL of the final reaction mixture from each well was spotted onto tryptic soy agar containing one of three antibiotics (streptomycin, spectinomycin, or kanamycin). Then, 2,3,4-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) was overlaid onto the spotted plates, after which the plates were incubated overnight at 37°C. The surviving bacterial colonies on the plates were counted and the opsonophagocytic activity (OPA) of the serum samples was determined. The OPA titer was defined as the last dilution of the serum sample reducing colony forming units (CFUs) to 50% of the negative control.
Statistical analysis
All statistical analyzes were performed using SPSS 18.0 (SPSS Korea, Seoul, Republic of Korea). The one-way analysis of variance (ANOVA) is used to determine the statistical differences between the three age groups. A p-value < 0.05 is considered significant.
Results
The mean age of the neonates/infants, pregnant women, and the elderly was 1.3 months (range, 1–3 months), 31.9 years (range, 23–41 years), and 68.8 years (range 65–76 years), respectively. There were 15 premature (gestational age <37 weeks, 53.3%) and 14 full-term (46.7%) newborns in the neonate/infant group. The baseline OPA titers of each age group are shown in and .
Table 1. Comparison of baseline opsonophagocytic activity (OPA) for group B streptococcus (GBS) between risk groups.
Table 2. Comparison of baseline opsonophagocytic activity (OPA) for group B streptococcus (GBS) between risk groups: proportion in each range stratified by the magnitude of OPA titer.
For serotype Ia, the mean OPA titers of the three age groups were not significantly different (p = .596). Notably, relatively low OPA titers were seen in the neonate/infant group compared to the pregnant women and the elderly groups (, )). When the magnitude of the OPA titers were stratified into four ranges (), 70% of neonates/infants, 80% of pregnant women and 83.3% of the elderly had low titers below 100, but there were no significant differences in the distribution of OPA ranges among the three age groups.
Figure 1. Comparison of baseline opsonophagocytic activity (OPA) titers for group B streptococcus (GBS) between three age groups (upper panel, mean ± standard deviation; lower panel, point distribution): (a) serotype Ia, (b) serotype III, and (c) serotype V. NOTE: the same letters (alphabetical superscripts) indicate non-significant differences between groups based on Tukey’s multiple comparison tests.
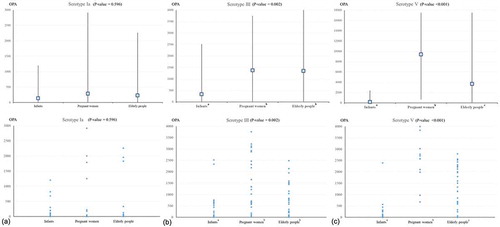
For serotype III, the mean OPA titers were significantly lower for neonates/infants than those in the other two groups (p = .002), but there was no significant difference between the OPA titer of pregnant women and the elderly (, )). Overall, 60% of neonates/infants (n = 18) showed OPA titers below 100; the proportion of OPA titers below 100 was significantly higher in neonates/infants (60%) than in pregnant women (13.3%) or the elderly (13.3%) (p < .001) ().
The OPA titers of serotype V were notably lower in neonates/infants (mean ± standard deviation, 161 ± 445) than in the elderly (3669 ± 5597) or pregnant women (9414 ± 6394), with significant differences between the three age groups (p < .001) (, )). Moreover, 76.7% of neonate/infants had OPA titers below 100, whereas 93.3% of pregnant women and 70% of the elderly had high titers greater than 1,000, showing the significant differences between the groups (p < .001) ()
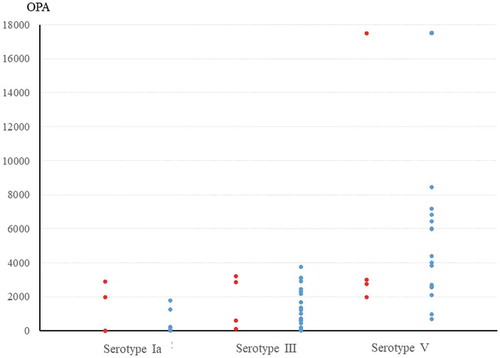
Of the 30 pregnant women who were included in the study, 4 (13.3%) were colonized with GBS on vaginal-rectal screening. There were no significant differences in the OPA titers for each serotype in GBS-colonized and GBS non-colonized pregnant women: serotype Ia (1,230 ± 1,467 versus 139 ± 416, p = .234), III (1,705 ± 1,569 versus 1,327 ± 1,124, p = .556), and V (6,318 ± 7,465 versus 9,890 ± 6,879, p = .346) (). When the magnitude of the OPA titers were stratified into the four ranges, there were no significant differences in the distribution of OPA titer for each serotype in GBS-colonized and GBS non-colonized pregnant women (). Regardless of GBS colonization status, pregnant women had relatively high OPA titers, above 1,000 for serotype III (≥ 50%) and serotype V (≥ 90%). The OPA titers for colonized serotypes were generally high in colonized pregnant women, but did not always correlate with colonization. Case 3 in carried serotypes III and Ⅷ but also showed a relatively high OPA titer, above 1,000, for serotype Ia. The OPA titers for serotype V were consistently high, titers above 1,000, regardless of colonization status ().
Table 3. Comparison of baseline opsonophagocytic activity (OPA) titers between group B streptococcus (GBS)-colonized and non-colonized pregnant women: proportion in each range stratified by the magnitude of OPA titer.
Table 4. Group B streptococcus (GBS) serotypes and opsonophagocytic activity (OPA) titers among four GBS-colonized pregnant women.
Figure 2. Comparison of baseline opsonophagocytic activity (OPA) titers for group B streptococcus (GBS) between GBS-colonized and GBS non-colonized pregnant women at their 35–37 weeks prenatal screening: distribution of OPA titers in each group (red circles, GBS-colonized women and blue circles, GBS non-colonized women).
When we compared OPA titers (mean ± standard deviation) in preterm (N = 16) and full-term (N = 14) neonates/infants, there were no statistically significant differences for serotype Ia (207 ± 365 versus 57 ± 72, p = .127), III (355 ± 599 versus 319 ± 670, p = .878), and V (244 ± 599 versus 65 ± 96, p = .278) (). Prenatal maternal GBS screening was performed in 24 mothers of 30 neonates/infants, and three of them (12.5%) were colonized with GBS. When the OPA titers (mean ± standard deviation) in neonates/infants with GBS-colonized (N = 3) and GBS non-colonized mothers (N = 21) were compared, they were higher in neonates/infants with GBS-colonized mothers than in neonates/infants with GBS non-colonized mothers, though the differences were not statistically significant,: serotype Ia (411 ± 686 versus 115 ± 226, p = .534), III (1,640 ± 1,360 versus 209 ± 292, p = .209), and V (845 ± 1,339 versus 99 ± 168, p = .437). The neonates/infants with GBS-colonized mothers showed remarkably high OPA titers for all three serotypes, except for one preterm neonate/infant (preterm birth at 30 weeks), (Supplementary Table 1).
Table 5. Comparison of baseline opsonophagocytic activity (OPA) titers between preterm and full-term neonates/infants.
Discussion
This is the first immunoassay performed using validated GBS-MOPA, to evaluate naturally acquired functional activity against serotypes Ia, III, and V in diverse age groups.Citation8 Significantly lower OPA titers were seen in the neonates/infants, with the highest disease burden. In adults, OPA titers were higher, especially in pregnant women compared to the elderly. Even though maternal anti-GBS OPA titers were high, transplacental transfer of preexisting maternal antibodies can be quite limited. Similar to the Tdap (tetanus, diphtheria and pertussis) vaccine, GBS vaccination would be required for neonatal protection during each pregnancy. Although prenatal maternal GBS colonization increases the risk of neonatal GBS infection, GBS exposure during the late intrapartum period might induce high serotype-specific anti-GBS immunity in neonates/infants.
A correlation between low maternal serotype-specific capsular antibody concentration and increased risk of invasive GBS disease among newborns was observed,Citation10,Citation11 and the specific concentration of maternal IgG required to protect neonates against invasive EOD was evaluated, mainly through enzyme-linked immunosorbent assays (ELISAs).Citation10,Citation12–Citation15 A matched case-control study conducted by Baker et al.Citation13 reported that a threshold of ≥1 µg/mL in the mother at birth may provide a measure of protection against invasive GBS disease in newborns for serotype Ia, III and V. Lin et al.Citation14,Citation15 had consistent findings, but described a 10-fold higher antibody concentration. Although these studies are not directly comparable due to differences in the methodologies used and the absence of a standardized immunological assay, the proportion of invasive GBS disease was generally lower in cases with serotype-specific capsular antibody titers ≥2 µg/mL compared to the controls in the meta-analysis.Citation10 In a cross-sectional survey, conducted to assess the protective effect of serotype-specific antibodies in the elderly, older adults were found to lack the serotype V CPS-specific serum IgG, and they were more likely to carry serotype V, the leading cause of invasive disease in the elderly.Citation12
Data on opsonophagocytic killing assays with GBS is very limited.Citation16–Citation21 The opsonophagocytic killing assay was conducted to assess the in vitro function of serotype Ia, III or V GBS antibodies elicited in response to the GBS glycoconjugate vaccine in pregnant women,Citation16 neonates,Citation17 healthy non-pregnant adults,Citation18,Citation19,Citation21 and the elderly,Citation20 where each GBS vaccine uniformly promoted opsonophagocytosis against its homologous serotype. Only a few studies were conducted using GBS opsonophagocytic killing assays in a longitudinal cohort of pregnant women. A longitudinal study of 507 pregnant women, by Kwatra et al.Citation22 found that women who were never colonized during pregnancy had higher OPA titers (≥8) in early pregnancy than those who had newly acquired GBS colonization during the study. In a mother/infant paired cohort study, Doare et al.Citation23 proposed a maternally-derived functional antibody threshold, above which no infant colonization was observed. In another cohort study by Kim et al.,Citation24 serotype-specific functional GBS antibodies among Korean infants were evaluated. Only a limited proportion of infants showed detectable OPA titers against serotypes Ia, Ib, and III GBS. In particular, the seropositive rate for serotype Ia was especially low, which is consistent with this study.
The protective cutoff values of OPA titers for GBS have not yet been established. Pneumococcus is known to have a protective effect at the relatively low OPA titers of ≥1:8 in pediatric patients;Citation25 however, the protective titer in adults has not been established even in pneumococcus. Given the results of this study and previous longitudinal cohort studies,Citation22–Citation24 it is likely that transfer of GBS protection to neonates may require substantially higher OPA titers in pregnant women. The naturally acquired OPA titer was higher in the elderly than in children in this study, but they may require a higher protective OPA titer, as is seen with pneumococcus.Citation25 It is possible that the higher OPA titer seen in adults compared to neonates/infants is due to repeated exposure to diverse GBS strains, leading to nonspecific OPA against teichoic acid and surface proteins.Citation24 The clinical significance of OPA against these non-capsular antigens is unknown. The protective thresholds of serotype-specific OPA still need to be established and may vary substantially by age group.Citation26
In this study, pregnant women had high OPA titers, whereas neonates/infants showed relatively low titers, highlighting the possibility of ineffective placental transfer of pre-existing maternal antibodies. The variable efficiency for transfer among the IgG subclasses and the importance of timing in gestational week on antibody transfer has been demonstrated.Citation27 The efficiency of antibody transfer increases with gestational age; neonatal Fc receptors might be more highly expressed with advancing gestation.Citation27 Considering the efficiency of transfer and possibility that antibody persistence may be transient,Citation22 the vaccine might need to be given in the third trimester. In contrast, there is evidence supporting vaccination during early pregnancy or before pregnancy. High OPA titers were associated with a lower risk of recto-vaginal acquisition of strains belonging to the homologous serotype,Citation22 resulting in reduced infection of infants during birth,Citation22,Citation28 as well as of the pregnant women themselves.Citation28 In addition, there are reports that the functionality of serotype-specific IgG persists for at least 18 months after immunization.Citation18 There remains uncertainty about the immunization timing that would provide the best protection to newborns.
GBS colonization in Korean pregnant women is increasing. Previously, the GBS carriage rate in Korean pregnant women (2–6%) was estimated to be lower than that in the United States (15–20%).Citation29,Citation30 However, several recent studies have reported an increase in carriage rates since 2005 (6.3–17.3%).Citation31–Citation33 Serotypes III, V, and Ia were the most common in pregnant women (35.2–56.2%, 13.9–24.1%, and 12.1–26.2%, respectively) and infants (44.6–51.0%, 18.4–28.6%, and 14.3–15.3%, respectively).Citation30,Citation32–Citation35 In this study, conducted in South Korea, the GBS-OPA titer of serotype V tended to be higher in adults, including pregnant women, regardless of vaginal colonization. In contrast, the OPA titer of serotype Ia was generally low in all three age groups. This phenomenon could be partly explained by differences in the GBS strains. GBS capsular constituents, apart from surface adherence proteins, could influence cervicovaginal adherence or vaginal persistence.Citation36 In vitro analysis by Lubas et al.Citation37 found significantly lower adherence of serotype Ia GBS strains to the vaginal epithelium. A mouse model study carried out by Patras et al.Citation38 characterized the distinct phenotypes of serotype Ia, III, and V strains. The serotype V strain displayed greater intracellular survival and less cytokine stimulation in cervical cells, and increased persistence in the mouse vaginal tract while serotype Ia was the least adherent overall. These results highlight that serotype V acts differently from serotype Ia and III in its interactions with the host epithelium, which is consistent with its long-term colonization phenotype in vivo. The serotype-specific long-term colonization of GBS might be explained by the differences in their repertoire of cellular adhesins/invasins, their ability to outcompete normal flora, and/or their dialog with the host immune system. Another potential basis may lie in the structural complexity of their capsular polysaccharides. Serotype V possesses a set of 20 unique genes not found in other GBS strains, and is adept at forming biofilms in vitro.Citation38,Citation39 The high OPA titers of serotype V might be explained by previous repeated colonization. In addition, a substantial proportion of immune response isotypes raised to serotype V are IgM and IgA, as well as IgG, so placental transfer could be lower than expected even in with high maternal OPA titers.Citation12,Citation16,Citation20 Careful interpretation is needed to establish the maternal protective threshold for neonates.
There are some limitations in this study. First, the relatively small sample size may have biased the results. Nevertheless, this is the first immunoassay using validated GBS-MOPA, and the results should be useful as reference data to establish thresholds for protection in the further clinical trials. Second, pregnant women could not be matched with their neonates/infants because the IRB did not permit the use of cord blood samples. Thus, this study could not provide any information on the transplacental transfer of maternal anti-GBS immunity. However, the OPA titers were consistently high in the pregnant women, even compared to healthy old adults, so it can be inferred that most healthy pregnant women would have similarly high OPA titers. Third, ELISA data was not available in this study because a standardized assay was not established. Fourth, because this study was conducted at a tertiary hospital, more than 50% (16 among 30) of the neonates/infants were preterm newborns with low birth weights. Although there was no statistically significant difference between preterm and full-term neonates/infants, it is possible that the OPA titers in this study are lower than those in healthy full-term neonates/infants. Finally, although GBS-MOPA was validated in a single center,Citation8 this method needs to be further validated and standardized in collaboration with other institutions.Citation40,Citation41
In conclusion, this study indicates that GBS OPA titers differ by serotype and between age groups. Despite the high serotype-specific OPA titers in pregnant women, these titers were consistently low in neonates/infants. GBS vaccines should be developed to ensure sufficient transplacental delivery of serotype-specific GBS antibodies irrespective of preexisting maternal immunity, and this needs to be confirmed by clinical trials. Although invasive GBS infection has increased in elderly people, most healthy elderly showed high levels of immunity against GBS in this study. GBS vaccination may not recommended for healthy older adults. Basal immunity in elderly people with comorbidities (diabetes, liver cirrhosis, malignancy, etc.) might differ from that in healthy older adults, so further assessment is required. The results in this study might be used as reference data in further vaccine clinical trials. Additional studies are required to assess the correlation between OPA and ELISA titers.
Disclosure of potential conflicts of interest
The authors disclosed no conflicts if interest.
Supplemental Material
Download MS Word (31.6 KB)Supplemental Material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2019.1688036.
Additional information
Funding
References
- Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database Syst Rev. 2014;6:Cd007467. doi:10.1002/14651858.CD007467.pb4. PMID:24915629.
- Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-Ter Meulen A, Dull PM. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31(Suppl 4):D52–57. doi:10.1016/j.vaccine.2013.02.029. PMID:23973347.
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, Group B streptococcus. 2016. [accessed 2018 Aug 31]. https://www.cdc.gov/abcs/reports-findings/survreports/gbs16.html
- Song JY, Lim JH, Lim S, Yong Z, Seo HS. Progress toward a group B streptococcal vaccine. Hum Vaccin Immunother. 2018;14(11):2669–81. doi:10.1080/21645515.2018.1493326. PMID:29995578.
- Edwards MS, Gonik B. Preventing the broad spectrum of perinatal morbidity and mortality through group B streptococcal vaccination. Vaccine. 2013;31(Suppl 4):D66–71. doi:10.1016/j.vaccine.2012.11.046. PMID:23200934.
- Kobayashi M, Schrag SJ, Alderson MR, Madhi SA, Baker CJ, Sobanjo-Ter Meulen A, Kaslow DC, Smith PG, Moorthy VS, Vekemans J. WHO consultation on group B Streptococcus vaccine development: report from a meeting held on 27–28 April 2016. Vaccine. 2016. doi:10.1016/j.vaccine.2016.12.029. PMID:28017431.
- Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51(9):717–22. doi:10.1099/0022-1317-51-9-717. PMID:12358061.
- Choi MJ, Noh JY, Cheong HJ, Kim WJ, Lin SM, Zhi Y, Lim JH, Lim S, Seo HS, Song JY. Development of a multiplexed opsonophagocytic killing assay (MOPA) for group B Streptococcus. Hum Vaccin Immunother. 2018;14(1):67–73. doi:10.1080/21645515.2017.1377379. PMID:28933634.
- Brigtsen AK, Dedi L, Melby KK, Holberg-Petersen M, Radtke A, Lyng RV, Andresen LL, Jacobsen AF, Fugelseth D, Whitelaw A. Comparison of PCR and serotyping of Group B Streptococcus in pregnant women: the Oslo GBS-study. J Microbiol Methods. 2015;108:31–35. doi:10.1016/j.mimet.2014.11.001. PMID:25447890.
- Dangor Z, Kwatra G, Izu A, Lala SG, Madhi SA. Review on the association of Group B Streptococcus capsular antibody and protection against invasive disease in infants. Expert Rev Vaccines. 2015;14(1):135–49. doi:10.1586/14760584.2014.953939. PMID:25242617.
- Edwards MS, Nizet V, Baker CJ. Group B streptococcal infections. In: Remington JS, Klien JO, Baker CJ, Wilson CB, editors. Infectious diseases of the fetus and newborn infant. 6th ed. Philadelphia: WB Saunders Co; 2006. p. 1091–141..
- Edwards MS, Rench MA, Palazzi DL, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis. 2005;40(3):352–57. doi:10.1086/426820. PMID:15668856.
- Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SL, Kasper DL, Platt R. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis. 2014;209(5):781–88. doi:10.1093/infdis/jit549. PMID:24133184.
- Lin FY, Philips JB 3rd, Azimi PH, Weisman LE, Clark P, Rhoads GG, Regan J, Concepcion NF, Frasch CE, Troendle J, et al. Level of maternal antibody required to protect neonates against early-onset disease caused by group B Streptococcus type Ia: a multicenter, seroepidemiology study. J Infect Dis. 2001;184(8):1022–28. doi:10.1086/323350. PMID:11574917.
- Lin FY, Weisman LE, Azimi PH, Philips JB 3rd, Clark P, Regan J, Rhoads GG, Frasch CE, Gray BM, Troendle J, et al. Level of maternal IgG anti-group B streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. J Infect Dis. 2004;190(5):928–34. doi:10.1086/422756. PMID:15295698.
- Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Edwards MS, Kasper DL. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J Infect Dis. 2004;189(6):1103–12. doi:10.1086/382193. PMID:14999615.
- Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21(24):3468–72. doi:10.1016/S0264-410X(03)00353-0. PMID:12850362.
- Edwards MS, Lane HJ, Hillier SL, Rench MA, Baker CJ. Persistence of functional antibodies to group B streptococcal capsular polysaccharides following immunization with glycoconjugate vaccines. Vaccine. 2012;30(28):4123–26. doi:10.1016/j.vaccine.2012.04.048. PMID:22537994.
- Baker CJ, Paoletti LC, Wessels MR, Guttormsen HK, Rench MA, Hickman ME, Kasper DL. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999;179(1):142–50. doi:10.1086/314574. PMID:9841833
- Palazzi DL, Rench MA, Edwards MS, Baker CJ. Use of type V group B streptococcal conjugate vaccine in adults 65–85 years old. J Infect Dis. 2004;190(3):558–64. doi:10.1086/422010. PMID:15243932.
- Baker CJ, Rench MA, Paoletti LC, Edwards MS. Dose-response to type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine in healthy adults. Vaccine. 2007;25(1):55–63. doi:10.1016/j.vaccine.2006.07.018. PMID:16919857.
- Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol Infect. 2015;21(6):568.e513–521. doi:10.1016/j.cmi.2015.01.030. PMID:25680313.
- Le Doare K, Faal A, Jaiteh M, Sarfo F, Taylor S, Warburton F, Humphries H, Birt J, Jarju S, Darboe S, et al. Association between functional antibody against Group B Streptococcus and maternal and infant colonization in a Gambian cohort. Vaccine. 2017;35(22):2970–78. doi:10.1016/j.vaccine.2017.04.013. PMID:28449969.
- Kim HW, Lee JH, Cho HK, Lee H, Seo HS, Lee S, Kim KH. Opsonophagocytic antibodies to serotype Ia, Ib, and III Group B streptococcus among Korean infants and in intravenous immunoglobulin products. J Korean Med Sci. 2017;32(5):737–43. doi:10.3346/jkms.2017.32.5.737. PMID:28378545.
- Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013;19(3):412–25. doi:10.1007/s10156-013-0601-1. PMID:23657429.
- Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, Kowalsky L, Tyrell G, Baker CJ. Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis. 2001;184(3):285–91. doi:10.1086/322029. PMID:11443553.
- Calvert A, Jones CE. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr Opin Infect Dis. 2017;30(3):268–73. doi:10.1097/qco.0000000000000372. PMID:28362650.
- Chiarot E, Spagnuolo A, Maccari S, Naimo E, Acquaviva A, Cecchi R, Galletti B, Fabbrini M, Mori E, Ruggiero P, et al. Protective effect of Group B Streptococcus type-III polysaccharide conjugates against maternal colonization, ascending infection and neonatal transmission in rodent models. Sci Rep. 2018;8(1):2593. doi:10.1038/s41598-018-20609-5. PMID:29416049.
- Lee K, Shin JW, Chong Y, Mikamo H. Trends in serotypes and antimicrobial susceptibility of group B streptococci isolated in Korea. J Infect Chemother. 2000;6(2):93–97. doi:10.1007/PL00012158.
- Kang HM, Lee HJ, Lee H, Jo DS, Lee HS, Kim TS, Shin JH, Yun KW, Lee B, Choi EH. Genotype characterization of group B streptococcus isolated from infants with invasive diseases in South Korea. Pediatr Infect Dis J. 2017;36(10):e242–e247. doi:10.1097/INF.0000000000001531.
- Kim DH, Min BJ, Jung EJ, Byun JM, Jeong DH, Lee KB, Sung MS, Kim KT, Kim YN. Prevalence of group B streptococcus colonization in pregnant women in a tertiary care center in Korea. Obstet Gynecol Sci. 2018;61(5):575–83. doi:10.5468/ogs.2018.61.5.575.
- Lee HT, Kim SY, Park PW, Ahn JY, Kim KH, Seo JY, Jeong JH, Kwoun WJ, Seo YH. Detection and genomic analysis of genital group B streptococcus in pregnant Korean women. J Obstet Gynecol Res. 2019;45(1):69–77. doi:10.1111/jog.13810.
- Hong J-S, Choi CW, Park K-U, Kim SN, Lee HJ, Lee HR, Choi EH, Park KH, Suh CS, Kim BI. Genital group B Streptococcus carrier rate and serotype distribution in Korean pregnant women: implications for group B streptococcal disease in Korean neonates. J Perinat Med. 2010;38(4):373–77. doi:10.1515/jpm.2010.050.
- Seo YS, Srinivasan U, Oh K-Y, Shin J-H, Chae JD, Kim MY, Yang JH, Yoon H-R, Miller B, DeBusscher J, et al. Changing molecular epidemiology of group B streptococcus in Korea. J Korean Med Sci. 2010;25(6):817–23. doi:10.3346/jkms.2010.25.6.817.
- Yoon IA, Jo DS, Cho EY, Choi EH, Lee HJ, Lee HJ. Clinical significance of serotype V among infants with invasive group B streptococcal infections in South Korea. Int J Infect Dis. 2015;38:136–40. doi:10.1016/j.ijid.2015.05.017.
- Patras KA, Nizet V. Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and preventative approaches. Front Pediatr. 2018;6(27):1–17. doi:10.3389/fped.2018.00027. PMID:29520354.
- Bodaszewska-Lubas M, Brzychczy-Wloch M, Adamski P, Gosiewski T, Strus M, Heczko PB. Adherence of group B streptococci to human rectal and vaginal epithelial cell lines in relation to capsular polysaccharides as well as alpha-like protein genes - pilot study. Pol J Microbiol. 2013;62(1):85–90. PMID:23829083.
- Patras KA, Rosler B, Thoman ML, Doran KS. Characterization of host immunity during persistent vaginal colonization by group B streptococcus. Mucosal Immunol. 2015;8(6):1339–48. doi:10.1038/mi.2015.23. PMID:25850655.
- Rinaudo CD, Rosini R, Galeotti CL, Berti F, Necchi F, Reguzzi V, Ghezzo C, Telford JL, Grandi G, Maione D. Specific involvement of pilus type 2a in biofilm formation in group B streptococcus. PLoS One. 2010;5(2):e9216. doi:10.1371/journal.pone.0009216. PMID:20169161.
- Vekemans J, Crofts J, Baker CJ, Goldblatt D, Heath PT, Madhi SA, Le Doare K, Andrews N, Pollard AJ, Saha SK, et al. The role of immune correlates of protection on the pathway to licensure, policy decision and use of group B Streptococcus vaccines for maternal immunization: considerations from World Health Organization consultations. Vaccine. 2019;37(24):3190–98. doi:10.1016/j.vaccine.2019.04.039.
- Le Doare K, Kampmann B, Vekemans J, Heath PT, Goldblatt D, Nahm MH, Baker C, Edwards MS, Kwatra G, Andrews N, et al. Serocorrelates of protection against infant group B streptococcus disease. Lancet Infect Dis. 2019;19(5):e162–e172. doi:10.1016/S1473-3099(18)30659-5.
