ABSTRACT
One of the main challenges in early clinical research with respiratory syncytial virus (RSV) live-attenuated vaccines (LAVs) is to assess immunogenicity in healthy adults. Healthy adults will have preexisting levels of serum neutralizing antibodies that could prematurely neutralize the LAV and underestimate the potential effect of the vaccine on the immune system. Data on prevalence and distribution of virus neutralizing titers (VNTs) in healthy adults is limited and there is no absolute threshold for protection against RSV-infection that can serve as an eligibility criterion in early phase trials. We assessed the RSV-specific serum VNT in healthy adults outside the Dutch RSV-Season in two clinical studies performed in 2017 (exploratory study, n = 100) and 2018 (first-in-human LAV-study, n = 190) using the same neutralizing assay. Our findings show that the prevalence and distribution of serum VNT was overall consistent in the two clinical studies. Log2 VNTs were normally distributed, distributions of VNTs were similar and there was no statistical difference in mean log2 VNT for both studies (p = .3). Serum VNTs were comparable during the 6 months of screening in the FIH LAV-study. Our findings will help to determine a cutoff serum VNT to be used as an eligibility criterion in future early phase clinical trials.
Introduction
Respiratory Syncytial Virus (RSV) usually causes mild upper respiratory tract infections in healthy adults. However, it can cause severe acute lower respiratory infections (ALRIs) in infants, elderly subjects and immunocompromised adultsCitation1–Citation3 RSV-associated ALRI is a major cause of pediatric mortality worldwide.Citation4 Immunoprophylaxis with neutralizing monoclonal antibodies (Palivizumab) is used in high-risk infants; however, treatment is relatively expensive and thus its use is limited to high-income countries. Consequently, there is a high need for an active immunization strategy to reduce mortality and the high disease burden of RSV infections.Citation4,Citation5 However, an effective RSV vaccine is yet to be licensed despite considerable research and development efforts. Fortunately, new promising candidate RSV vaccines are currently in development.Citation6 A large proportion of these vaccines use a live-attenuated vaccine (LAV) concept.Citation7 RSV LAVs have several benefits: LAVs have the potential to induce a broad and durable humoral and cellular immune response, can be administered intranasally and are considered to be safe because they do not seem to cause vaccine-enhanced RSV disease in naïve recipients.Citation8,Citation9
One of the main challenges in early clinical research with RSV LAVs is to assess immunogenicity in first-in-human (FIH) trials. A commonly used immunogenicity endpoint in these trials is the neutralizing activity of serum expressed as the fold change in virus neutralizing titer (VNT) determined by RSV neutralization assays.Citation6 For obvious safety reasons FIH vaccine studies are performed in healthy (non-naïve) adult volunteers. Because all healthy adults have been previously exposed to RSV, they will have acquired serum neutralizing antibodies. The potential effects of the LAV on the immune system could be underestimated when the LAV is prematurely neutralized by high levels of circulating neutralizing antibodies. Eligibility criteria based on serum VNTs in healthy adults are frequently used in RSV-controlled human infection model (CHIM) studies to increase the chance of successful infection after inoculation with a wild-type RSV strain.Citation10–Citation15 Likewise, using low preexisting serum VNTs as an eligibility criterion in LAV clinical trials would be a rational approach to improve the chance of observing an immune response in healthy adults. However, the use of a VNT cutoff value will impact inclusion rates because healthy adults will have varying preexisting serum VNTs.Citation14
There is currently insufficient data available on the prevalence and distribution of the RSV-specific serum VNTs in the healthy adult population. For this reason, we assessed RSV-specific serum VNTs in a healthy adult population in the Netherlands outside the RSV-season in two different clinical studies. Here, we present the collective findings that will aid investigators to determine a cutoff value for RSV-specific serum VNT in the future LAV and CHIM studies.
Methods
We determined RSV-specific serum VNTs in two studies: an exploratory study and an FIH vaccine trial investigating an RSV LAV (manuscript in preparation). For the exploratory study, a single blood sample was drawn from 100 healthy male and female adults at the Center for Human Drug Research (CHDR; Leiden, The Netherlands). Blood samples were drawn between 20 and 29 June 2017. Subjects were included if they were 18–45 y of age. Subjects were excluded if they were immunocompromised, had chronic airway diseases, signs of airway infection/common cold within 2 weeks prior to blood sampling or had (active) hay fever or other allergies that involve the airway. Subjects were not allowed to use medication that may affect the immune system within 30 d before blood sample collection. As part of the screening procedure in the FIH vaccine trial, preexisting serum VNTs were determined in 190 volunteers between the end of April and mid-September 2018. Subjects were aged 18–50 y and had to comply with similar in- and exclusion criteria as in the exploratory study. Blood sampling in both studies was performed outside the RSV-season in the Netherlands, which typically occurs annually from November until early April.Citation16,Citation17 The exploratory study was approved by the Medical Review and Ethics Committee Foundation BEBO (Assen, The Netherlands). The FIH vaccine trial (EudraCT number: 2016‐002437‐30) was approved by the Central Committee on Research Involving Human Subjects (CCMO; The Hague, The Netherlands). All subjects provided written informed consent prior to participation in the study. All study procedures were performed in accordance with the Dutch Act regarding Medical Research involving Human Subjects.
Blood sample handling was similar between both studies. Blood was collected in 3.0 mL Clot Activating Tubes. The tubes were centrifuged within 30 min to 2 h of collection at approximately 2000g for 10 min and serum was collected and stored at – 20°C or lower.
All samples were analyzed by Viroclinics B.V. (Rotterdam, The Netherlands). In the VNT assays, a constant amount of RSV-A2 (ATCC® VR-1540™, aimed at 100 plaque forming units/well) was mixed with serial 2 fold dilutions of the subject serum. The serum/virus mixtures were transferred to 96-well plates with Hep-2 cells. Following a 24 h incubation period, cells were fixed and immunostained with a murine monoclonal antibody directed against RSV F protein (Millipore, MAB858), followed by HRP-conjugated goat-anti-mouse antibody (Life technologies, A16072) and TrueBlue (KPL, 50-78-02). The plates were then scanned with an SX UV Analyzer (CTL). Spot counts per well at each serum/antibody concentration were quantified by using the ImmunoSpot/BioSpot software (CTL) and values were used in the inhibitory concentration formula to determine the dilution of serum/antibody that showed the selected 50% reduction point.Citation18 Titers were reported as reciprocal of the dilution.
Analyses were performed using SPSS Statistics for Mac, version 25.0 (IBM Corp) and GraphPad Prism version 6.05 for Windows (GraphPad Software). All titers were log2 transformed prior to analysis. All values above the upper limit of quantification (ULOQ) were set equal to the value of the ULOQ (12.0 log2). Values below the lower limit of quantification (LLOQ) were set to half of the LLOQ (3.9 log2). Normal distribution of log2 serum VNTs in both studies was tested by Shapiro–Wilk test. An independent samples t-test was conducted to compare the means of log2 serum VNT between the exploratory and the FIH vaccine trial. Relative frequency histograms and relative cumulative frequency distribution curves were used to visualize the distribution of VNTs in the two studies. Means, standard deviations (SD) and ranges (minimum, maximum) of log2 VNTs were determined per month for the FIH vaccine trial.
Results
For the exploratory study serum samples were collected from 100 healthy volunteers; the mean age of the subjects was 23 y (range 18–40), 78% were female. For the FIH vaccine trial serum samples from 190 healthy volunteers were collected. The mean age of the subjects was 26 y (range 18–50), the percentage of females in this study was also 78%.
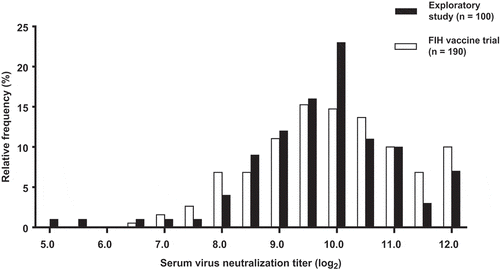
Shapiro–Wilk test was used to examine the normality of log2 serum VNTs in both studies and was not significant (p> .05) for both studies, indicating normal distribution. The normal distribution of log2 serum VNT is also visually apparent in , illustrating the prevalence and distribution of log2 serum VNT. Virus neutralizing titers were most frequently observed in the range of 9.0 to 10.5 log2. Values above the ULOQ were observed in a subset of samples (n = 5 [5.0%] in the exploratory study and n = 11 [5.8%] in the subsequent FIH vaccine trial), this contributed to the small peak in relative frequency observed at 12.0 log2 ().
Figure 1. Relative frequency histogram of log2 serum VNTs of the exploratory study (black bars) and the FIH vaccine trial (open bars).
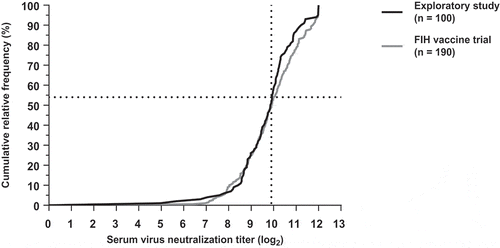
The relative cumulative distribution curves of the exploratory and FIH vaccine trial overlap until a VNT of approximately 9.9 log2 (). also shows that approximately 54% of the healthy adult volunteers in both studies had a VNT below 9.9 log2 (dotted line). There is a slight difference between the cumulative distribution curves for titers above 9.9 log2 due to relatively more values above 9.9 log2 in the FIH vaccine trial compared to the exploratory study ( and ). There was no statistical difference in mean log2 VNT for the exploratory study (mean = 9.7, standard deviation [SD] = 1.3) and the FIH vaccine trial (mean = 9.9, SD = 1.3); p= .3.
Figure 2. Cumulative relative frequency distribution of log2 serum VNTs of the exploratory study (black curve) and the FIH vaccine trial (gray curve). Dotted line marks the 54% of subjects in both studies with a VNT ≤ 9.9 log2.
The mean (SD) log2 serum VNT of the six-month screening period of the FIH vaccine trials was summarized per month (). The lowest mean log2 VNT values were observed in August and were the highest in May and June. The total range of serum VNTs observed in this period ranged from 6.4 to 12.0 log2.
Table 1. Serum virus neutralization titer per month.
Discussion
To our knowledge, this is the first publication that reports in this detail on the levels and distribution of RSV neutralizing serum antibodies in healthy adults outside the RSV-season. We found that the exploratory study and FIH vaccine trial yielded overall comparable results. The relative cumulative distributions of serum VNTs were similar in both studies, especially up to a VNT of 9.9 log2. There was no significant difference in mean serum VNT between the two studies, indicating the consistency of VNTs in two separate cohorts. In addition, we found that mean log2 serum VNT were quite similar for most month screening period of the FIH vaccine trial. Interestingly, a considerable lower mean log2 serum VNT was observed in August 2018 compared to the other months. This difference could be due to cross-sectional sampling and small sample sizes when months were compared. We did not observe an apparent trend of increasing or decreasing log2 VNT during these months.
Previous studies have shown that subjects with relatively low VNTs are more susceptible to RSV-infection and high serum VNTs have a protective effect against RSV infection in healthy adults.Citation13,Citation14,Citation15 Similarly, high serum VNTs might also prevent shedding and the subsequent immune response of RSV LAV in healthy adults. However, there is no established absolute threshold for protection against RSV infection for serum VNT in healthy adults. In fact, a study by Hall et al.Citation14 showed that even healthy adults with relatively high antibody levels could be (re)infected when challenged with a wild-type RSV. Because there is no absolute threshold of protection, we suggest that the distribution of RSV-specific serum VNT in the healthy adult population should be taken into account when determining a cutoff value for VNT to be used as an eligibility criterion. For example, if only the lower third of the population is to be included, then a cutoff serum VNT of 9.3 log2 or lower should be used (). In addition, can be used to estimate the amount of subjects in the healthy adult population with a suitable serum VNT. For the previous example (cutoff: VNT≤9.3 log2) 54% of the population will have a suitable serum VNT. These estimations will help investigators to anticipate recruitment rates accordingly.
DeVincenzo et al.Citation11 determined serum microneutralization titers against an RSV (A) Memphis 37 strain as part of a screening procedure for a challenge study. Similar to our study, they showed a normal distribution of serum neutralizing antibodies. Interestingly, lower titers were observed in comparison to our studies. The timing of blood sampling in relation to the RSV-season was not mentioned in this study.Citation11 Assay variability, such as the differences in readout and the use of the Memphis 37 strain compared to the RSV-A2 in this study could be a possible explanation for the observed difference. International standardization of RSV neutralization assays and subsequent availability of International Standard reference sera is recommended to improve the comparison between studies.Citation19 Recently, a World Health Organization (WHO) International Standard antiserum has become available for RSV-A.Citation20 The neutralization assay used in this study was included in the collaborative study to establish this WHO International Standard.Citation20 Unfortunately, this standard was not yet available during the execution of this study.
We performed both studies outside of the Dutch RSV-season because this would reduce the risk of concurrent wild-type RSV infections. Concurrent wild-type RSV infections can potentially interfere with the assessment of immunogenic endpoints in LAV trials, as natural infection can cause a significant increase in serum neutralizing antibodies.Citation14,Citation21 We therefore hypothesize that lower serum VNTs can be observed outside the RSV-season, due to the decreased incidence of RSV infection.Citation22 However, there is insufficient data on RSV-specific serum VNTs of the healthy adult population throughout the year to test this hypothesis. Nonetheless, the timing of sampling – and study conduct in general – outside the RSV-season could be beneficial, especially to prevent concurrent wild-type RSV infection during trials.
Some limitations should be noted. The ULOQ of the virus neutralization assay was set to the highest observed titer that was initially observed during the validation of the assay. However, in a later stage, serum VNTs above the ULOQ were observed in a small percentage of subjects. These titers were set equal to the ULOQ (approximately 12.0 log2) of the validated range of the neutralization assay. The mean and range of VNTs are therefore likely to be slightly underestimated. However, this should not interfere with the interpretation of the presented results, since screening should be based on the inclusion of the lower percentiles of the presented VNT distribution. We expect that the values above the ULOQ would have followed the downslope of the normal distribution (). There were relatively more female subjects included in both studies; however, we are not aware of any male-female differences regarding the prevalence of neutralizing antibodies or their protective effects. Due to variable screenings rates during the FIH vaccine trial, there were considerable differences in the amount of subjects screened per month. Therefore, no formal statistical tests were performed to compare mean log2 serum VNTs between months for the FIH vaccine trial.
In conclusion, this article describes the prevalence, distribution, and relevance of predetermining serum VNTs in healthy adults outside the RSV-season in the Netherlands. The presented results will help future RSV LAV and CHIM studies to determine cutoff values for VNT to be used as eligibility criteria. This, in turn, could improve the chance to detect a meaningful immune response in healthy adults after vaccination with an RSV LAV or increase the rate of successful inoculation after inoculation with a wild-type virus in RSV CHIM studies. Furthermore, the presented results will facilitate investigators to more accurately estimate recruitment rates when VNT is used as an eligibility criterion. Further research is needed to optimize the assessment of immunogenic endpoints in early clinical research with healthy adult volunteers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the nurses of CHDR for their support in the blood sample collection and the bio-technicians of Viroclinics for performing the neutralization assay. In particular Chaveli Els, Sakinie Misiedjan, Asma Azmani, and Penelope Koraka. Furthermore, we would like to thank Marieke L. de Kam for her input into the preparation of this paper.
Additional information
Funding
References
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35(12):519–30. doi:10.1542/pir.35-12-519.
- Kwon YS, Park SH, Kim MA, Kim HJ, Park JS, Lee MY, Lee CW, Dauti S, Choi WI. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect Dis. 2017;17(1):785. doi:10.1186/s12879-017-2897-4.
- Chatzis O, Darbre S, Pasquier J, Meylan P, Manuel O, Aubert JD, Beck-Popovic M, Masouridi-Levrat S, Ansari M, Kaiser L, et al. Burden of severe rsv disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect Dis. 2018;18(1):111. doi:10.1186/s12879-018-3002-3.
- Stein RT, Bont LJ, Zar H, Polack FP, Park C, Claxton A, Borok G, Butylkova Y, Wegzyn C. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol. 2017;52(4):556–69. doi:10.1002/ppul.23570.
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390(10098):946–58. doi:10.1016/S0140-6736(17)30938-8.
- Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018. doi:10.1016/S1473-3099(18)30292-5.
- PATH. Vaccine Resource Library - RSV Vaccine and mAb Snapshot. 2019 Apr 5 [Accessed 2019 July 6]. http://vaccineresources.org/details.php?i=1562.
- Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O’Shea AF, Gruber WC, Murphy BR. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25(42):7372–78. doi:10.1016/j.vaccine.2007.08.014.
- Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–84. doi:10.1007/978-3-64238919-1_13.
- DeVincenzo JP, McClure MW, Symons JA, Fathi H, Westland C, Chanda S, Lambkin-Williams R, Smith P, Zhang Q, Beigelman L, et al. Activity of oral als-008176 in a respiratory syncytial virus challenge study. N Engl J Med. 2015;373(21):2048–58. doi:10.1056/NEJMoa1413275.
- DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–14. doi:10.1164/rccm.201002-0221OC.
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. A randomized, double-blind, placebo-controlled study of an rnai-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107(19):8800–05. doi:10.1073/pnas.0912186107.
- Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with a2 respiratory syncytial virus. Antiviral Res. 2004;63(3):191–96. doi:10.1016/j.antiviral.2004.04.005.
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–98.
- Watt PJ, Robinson BS, Pringle CR, Tyrrell DA. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine. 1990;8:231–36.
- De Gier B, Mooij SH, Hahne SJM State of infectious diseases in the Netherlands, 2017. Bilthoven, the Netherlands: rijksinstituut voor volksgezondheid en milieu (RIVM); 2018. Report No.: RIVM Rapport 2018-0032.
- Broberg EK, Waris M, Johansen K, Snacken R, Penttinen P. European influenza surveillance N. Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 european countries, 2010 to 2016. Euro Surveill. 2018;23:5. doi:10.2807/1560-7917.ES.2018.23.5.17-00284.
- Zielinska E, Liu D, Wu HY, Quiroz J, Rappaport R, Yang DP. Development of an improved microneutralization assay for respiratory syncytial virus by automated plaque counting using imaging analysis. Virol J. 2005;2:84. doi:10.1186/1743-422X-2-84.
- Hosken N, Plikaytis B, Trujillo C, Mahmood K, Higgins D. Participating laboratories working G. A multi-laboratory study of diverse rsv neutralization assays indicates feasibility for harmonization with an international standard. Vaccine. 2017;35(23):3082–88. doi:10.1016/j.vaccine.2017.04.053.
- McDonald JU, Rigsby P, Dougall T, Engelhardt OG, Study P. Establishment of the first who international standard for antiserum to respiratory syncytial virus: report of an international collaborative study. Vaccine. 2018;36(50):7641–49. doi:10.1016/j.vaccine.2018.10.087.
- Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78(11):1493–97. doi:10.1002/jmv.20724.
- Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodriguez-Tenreiro C, Sly P, Ramilo O, Mejias A, Baraldi E, Papadopoulos NG, Nair H, et al. Respiratory syncytial virus seasonality: A global overview. J Infect Dis. 2018;217(9):1356–64. doi:10.1093/infdis/jiy056.
