ABSTRACT
Clinical research is important in establishing the effects of health-care interventions. Vaccine clinical trials are to examine the effectiveness and safety of vaccines for the prevention of diseases. Africa has a high burden of infectious diseases such as malaria, tuberculosis, HIV/AIDS, and Ebola virus disease. Here we report a database surveillance study of vaccine-related clinical trials conducted in Africa. An objective is to address and profile vaccine clinical trials conducted in Africa. Data were extracted from the WHO International Clinical Trials Registry Platform on 22 July 2018 and updated on 05 September 2019. We found that 61% of the 377 clinical trials were registered prospectively and 35% registered retrospectively. About 72% of the trials were single-country studies and within the country, most trials (86%) were single-center studies. The proportion of trials involving multiple African countries was 11% and that of trials involving countries outside of Africa was 16%. The biggest funder of the vaccine trials (34%) was industry, followed by governments (25%) and universities (21%). The most studied diseases were malaria (20%), HIV/AIDS (15%), tuberculosis (7%), and Ebola virus disease (6%). Most of the vaccine trials were conducted in adults (42%). The trials ranged from phase I to phase IV, with most of the trials being in phase I (18%) and phase III (18%). The conduct of vaccine clinical trials in Africa seeks to address the disease epidemics faced by the continent. There is a need for more investments from governmental bodies toward vaccine research in Africa. Further, African country collaborations are needed in efforts to find African solutions to the current infectious disease threats faced by the continent.
Introduction
Vaccines are defined as biological preparations that provide active-acquired immunity to a particular disease. They contain an agent that resembles a disease-causing microorganism and are often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. Vaccines help the immune system fight infections faster and more effectively.Citation1
Over and above, there is overwhelming evidence of the benefits of immunization as one of the most successful and cost-effective health interventions.Citation2,Citation3 The global immunization coverage was 85% by the end of 2017.Citation4 Vaccination averts an estimated 2–3 million deaths from diphtheria, tetanus, pertussis (whooping cough) and measles in children each year.Citation5 Since 2000, the Global Alliance Vaccine Initiative (GAVI) supported vaccinations of over 500 million children in the world’s poorest countries, saving an estimated 7 million lives.Citation6 UNICEF mentioned that if all children were immunized with existing vaccines, nearly 25 million lives could be saved between 2011 and 2020.Citation3
Vaccines continue to save lives reducing the global incidence of polio by 99%. There are many other diseases such as disability and death from mumps, rotaviruses, rubella, yellow fever, pneumococcal diseases, poliomyelitis (polio), diphtheria, tetanus, whooping cough, measles, hepatitis B, human papillomavirus, Haemophilus influenzae type b disease, and epidemic meningococcal A meningitis in which vaccine have contributed in preventing.Citation5,Citation7 While there is evidence that vaccine work, 19.4 million children still don’t receive even the most basic vaccines leaving them vulnerable to vaccine-preventable diseases.Citation5,Citation7 This emphasizes the need for research that will ensure every child gets vaccinated. Diarrhea is also the second leading cause of death in children younger than five in Africa. Vaccine development contributes to the control of infectious diseases. There is a lot of activity in the African continent with regards to the development of new malaria and Ebola vaccines.Citation8–Citation17
Clinical trials are the mainstay in new vaccine development processes, as well as for product license extensions for existing therapies. The conduct of clinical trials in varied populations ensures that the vaccine is safe and effective in varying population groups who would benefit from the vaccines. However, the process of clinical trials is often costly and may have ethical concerns.Citation18
The conduct of clinical trials in Africa is often challenged by the fact that early phase studies are conducted in other parts of the world that do not have the disease epidemics and therefore the context may be different.Citation19 Even though developing countries are usually under-represented in research due to a lack of commercial viability and trained researchers, Africa is emerging as an important destination for researchCitation20–Citation22 and as the conduct of clinical research in Africa increases with the hope to inspire collaboration amongst African researchers to find solutions for Africans. Over the years there has been an increase in clinical research in Africa.Citation21 In the year 2013 alone, 4,060 clinical trials were conducted, and all major pharmaceutical companies have their presence in Africa. Moreover, conducting trials in low- and middle-income countries can be positive for the trial site as it raises research standards, and brings health improvements and seriously needed investment. In Africa, research-led solutions and reductions in disease burden have also brought the greatest impact to high rates of early mortality.Citation22
Sub-Saharan Africa (SSA) is notable in this regard; however, conducting clinical trials in SSA often has its own challenges, such as different regulatory laws and evolving guidelines. Considering scientific factors, stringent regulations, and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)-Good Clinical Practice (GCP) guidelines, it becomes crucial for stakeholders to be aware of the challenges faced when conducting clinical trials in SSA. Besides expanding the investigators’ knowledge, globalization of clinical trials also brings a variety of other benefits such as the ability to produce generalizable results and investment in infrastructure in the host country. There are however risks associated with studies involving countries with social, economic, and health inequalities. There are also other issues to consider, such as whether the rights of those participating in the research are being fully protected.Citation23 Considering the disease burden in SSA and the challenges in the conduct of clinical trials particularly, this paper aims to describe the regional landscape of vaccine clinical trials conducted in the African continent and discuss the addressing of local health needs in the agenda of international vaccine clinical trials.
Methods
Study design
The International Clinical Trials Registry Platform (ICTRP) is a global initiative that aims to make information about all clinical trials involving human beings publicly available. It was established in 2006 in response to demand from countries through the World Health Assembly for: “a voluntary platform to link clinical trials registers in order to ensure a single point of access and the unambiguous identification of trials with a view to enhancing access to information by patients, families, patient groups and others”. The secretariat of the ICTRP is housed by the World Health Organization (WHO). Additionally, as part of meeting the requirements of the International Committee of Medical Journal Editors (ICMJE), investigators must register their trial with the WHO ICTRP or in ClinicalTrials.gov, which is a data provider to the WHO ICTRP. Here we report a database surveillance study of vaccine-related clinical trials conducted in Africa. One researcher (DN) searched the WHO ICTRP platform using different search terms to find the search combination that yielded more and specific search results (). The researcher (DN) searched the ICTRP with the terms “Immunization OR Vaccine OR Immunisation OR Vaccination” and downloaded the data on the 22 July 2018. The researcher (DN) updated the search on 05 September 2019. The collective data search included all records from the inception of the platform to the latest date searched. A descriptive analysis was conducted on the search outputs.
Table 1. Search strategy combination.
Data management and analysis
Data extraction
Data were extracted from the ICTRP platform and exported into an excel spreadsheet by one researcher (DN). All records were quality checked by a second researcher (KD) to ensure that vaccine clinical trials were included. We defined a vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed. Since the downloaded data showed all the records for vaccine trials conducted worldwide, only trials conducted in one of the 54 countries in Africa were included. The quality checks included assessing each record on whether a vaccine was used as an intervention. Included studies vaccine clinical trials per the definition above and conducted in one of the 54 African countries. The vaccine trials considered were randomized controlled trials in humans evaluating safety, immunogenicity, efficacy or effectiveness; etc. In each record of the included studies, we extracted the following data: date of registration, anticipated last follow-up date, actual last follow-up date, retrospective and prospective registration; disease researched, location of trial, location of principal investigator, intervention type, age range of participants, and funding source. For the trials included in the analysis, published reports were not downloaded. The extracted data were used in Microsoft Excel by performing descriptive analysis on the elements collected to assess the number of studies or percentages per data element extracted.
Results
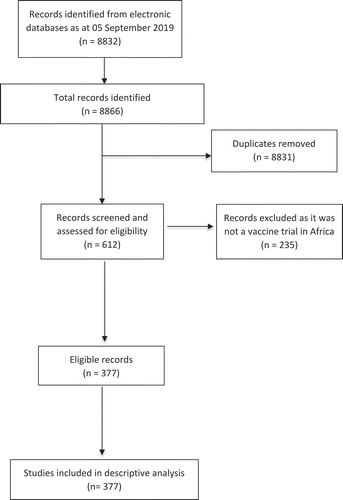
Data obtained from searching the WHO ICTRP database was conducted on 22 July 2018 and updated on 05 September 2019. The search output conducted in July 2018 resulted in 8802 records and the updated search had an additional 34 records. We screened by applying filters on the country, intervention, and title for potentially eligible studies that meet our inclusion criteria. Screening resulted in 612 vaccine-related trials conducted in Africa, however, a second verification of the potentially included records was conducted by screening the individual records to confirm if the study met the inclusion criteria. The second verification resulted in 377 studies which were included in the descriptive analysis ().
Descriptive analysis was conducted on the included studies. We found that 55% (205) studies were registered prospectively while 35% were registered retrospectively. There were 10% of the studies with unspecified registration status. Randomized controlled studies were 86% (318) and 9% (33) non-randomized studied with 5% (19) of the vaccine trials not specified. ()
Table 2. Characteristics of participants.
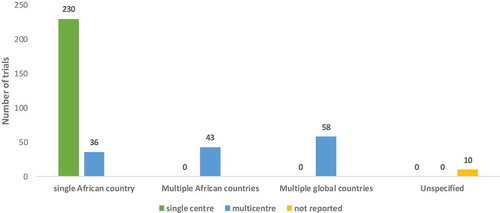
The included studies of the vaccine trials in Africa showed that 70% (266) trials were conducted in single countries and of the single country studies 62% (230) were conducted in a single recruitment center while 9.7% (36) conducted in multiple centers within the one country. Some of the trials (11%) were also conducted in multiple African countries with 15% (58) of trials also having recruitment sites in multiple global countries. We found 3% (10) trials which did not specify their recruitment centers ().
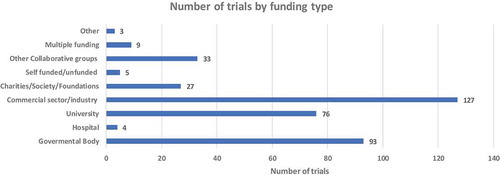
We assessed the funding source of the vaccine trials and found that 33% (127) are largely funded by the Commercial sector/industry. Governmental bodies were the second dominant funder with 24% (93) funded vaccine trials. Universities funded 20% (76) of vaccine-related clinical trials making Universities the third dominant funder. There were notable small number 1% (5) of trials that were self-funded or unfunded ().
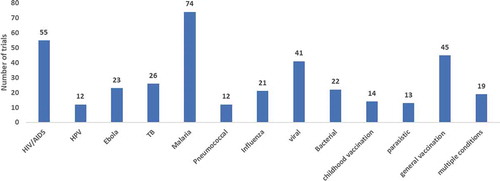
We assessed disease conditions being investigated and found that of the 377 vaccine-related trials, malaria was the most studied condition with 20% (74) trials, the second most studied condition was HIV/AIDS with 14% (55) trials, followed by viral diseases (i.e. conditions caused by a virus that are not listed separately) with 10% (41) trials. There were 7% (26) trials on Tuberculosis infections and 6% (23) trials on Ebola disease. Interestingly, we found the least number of trials investigating parasitic infections such as 4% (13) trials, pneumococcal infections and Human Papilloma Virus (HPV) with 10% (12) trials, respectively. There are 10% (12) trials that listed multiple disease conditions and 15% (59) indicating vaccines as a condition being studied while 12% (45) general vaccination and 3.8% (14) classified as childhood vaccine ().
The majority of vaccine trials were investigating vaccines as a prevention vaccine trials 79% (293) while 8% (28) were treatment trials and, 12% (45) indicated as other (). Of the prevention vaccine trials, two were measuring cytokine levels or an immune response in order to determine the effectiveness of the vaccine used.
Table 3. Characteristics of studies.
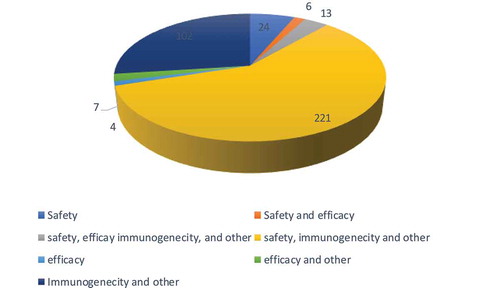
Based on the analysis, vaccine clinical trials were for different study purposes i.e. prevention and treatment. We further assessed the outcome measured in these studies and found that the outcomes ranged from safety, immunogenicity which included other secondary outcomes at 60% (221) of the trials to the least studied outcome i.e. efficacy at less than 1% (4) trials, 26% (95) studied immunogenicity and other outcomes, 6% (24) studied safety only as an outcome, 3% (13) trials studied safety, efficacy, immunogenicity and other outcomes, 2% (7) trials studied efficacy and other outcomes and less than 2% (6) trials studied safety and efficacy as outcomes ().
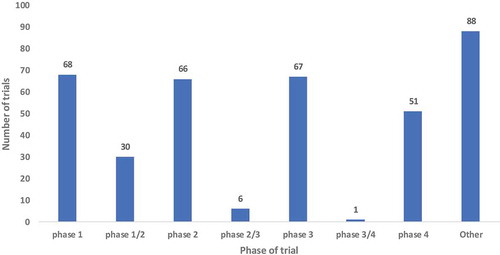
Interestingly, there were almost the same number of vaccine trials in Phases I, II and III at 18% in each phase with a slight drop in Phase IV vaccine trials 13% (51) (). Most of the African vaccine trials were conducted in adults’ population 41% (156) while 28% (107) conducted on multiple ages. There was however a small representation of vaccine trials conducted in infants and toddlers, 22% and 6%, respectively. A small number of trials conducted research in adolescents 2% (6) ().
There were different study designs of the vaccine clinical trials 71% (262) parallel-group trial design, 10% (36) single group trial design, 3% (11) with other study design, 1% (4) factorial trial design and less than 1% (3) of the trials indicating crossover trial design, respectively ().
Discussion
In Africa, the successful implementation of vaccination programs resulted in a drastic reduction of the burden of vaccine-preventable diseases (VPD). Most vaccinations in Africa are delivered through the Expanded Program on Immunization (EPI); a combination of infrastructural, programmatic and financial processes aiming at systematically delivering the much-needed vaccines to all eligible target populations in a country. Here we present findings of vaccine clinical trials conducted in Africa and show that most trials are in a single African country with multiple recruitment sites within one country. South Africa, Egypt, and Nigeria are among the top countries in Africa conducting vaccine clinical research.Citation24,Citation25 This is indicative of the African continent advancing in finding solutions for themselves. Strong collaboration in the African continent is observed in 42 vaccine trials within African countries and extend beyond the borders of the continent with recruitment sites in multiple global countries. Such observation is encouraging especially in the process of vaccine development which can be tailor-made for the African context.
While there is so much activity in vaccine research in Africa, the question is how the vaccine in developmental stages would be scaled up and marketed in Africa. We show here that the commercial sector or industry is funding most of the trials followed by governmental bodies. This suggests that both commercial and governmental sectors understand the benefits of vaccination. Although the funding is generally from international sponsors, this then begs the question of why local governmental bodies or commercial institution not fund vaccine research in Africa.
It is reasonable to point out that the commercial sector would be funding many vaccine trials because they have resources to conduct vaccine clinical research which is often very costly. However, industries are focused on long-term return on investments when the vaccine becomes licensed. There would be far more financial gains compared to the investment made in the developmental process. Governmental bodies are also seeing the need for research and innovation; however, they are limited in terms of budgetary allocations to other needs of their countries such as education, infrastructure, water supply, housing, etc. While budget allocations are often an issue in prioritizing research, African governmental bodies are making strides to invest in research, particularly vaccine research as this, in the long run, will yield a return on their investments by achieving the sustainable millennium developmental goals (SDGs). Our findings suggest that governmental funding is received from international governmental agencies, particularly the National Institute of Health mainly funding HIV and TB vaccine trials in Africa. SDG3 seeks to improve the general health and wellbeing of all people and support the research and development of vaccines and medicines for communicable and non-communicable diseases that primarily affect developing countries.Citation26
The data presented indicate that vaccine research is conducted to ensure the goals of SDG3 are met. Meeting the SDG3 goals by 2030 may be ambitious however considerable efforts are being made with vaccine clinical research in Africa focusing on malaria, HIV/AIDS, other viral diseases, tuberculosis, Ebola, pneumococcal infections and Human Papilloma Virus (HPV). These diseases are in the top list of diseases in the SSA and hence the interest in conducting vaccine research in the African continent in order to assess the effectiveness of candidate vaccines in high burden countries.Citation27
Vaccine clinical research is often focused on adult populations who are considered not as vulnerable as children and adolescents due to ethical reasons,Citation28 however, a recent study by Wajja et al. has shown that the so-called vulnerable populations are an important target population for a new TB vaccine and for other vaccines which are relevant at school-age. However, in most endemic settings there is limited experience of clinical trials research among adolescents while they also fall outside the routine vaccination programs.Citation29 The findings of Wajja et al. showed that infection with Schistosoma mansoni has no effect on vaccine-inducted T cell response among Ugandan adolescents in primary school. As tuberculosis is one of the top infectious disease in SSA, an effective vaccine is urgently needed and therefore such findings strongly suggest that vaccine clinical research is feasible in resource-limited settings with high burden of disease providing hope for new TB vaccine in the near future which is highly needed.Citation30
The conduct of early phase studies in children has been difficult due to reasons related to ethics, acceptability, rarity, standardization, endpoints, safety, dosing, and feasibility. Over the past decade, there have been several developments including novel clinical trial design, in silico pharmacology and micro-dosing that have significantly enhanced the ability of investigators to conduct early phase studies in children.Citation31 While the evolution of research is creating a series of new challenges, African vaccine trials only include 22% of infants and just about 2% of adolescents. This suggests that Africa is increasingly adapting to changing regulatory environment to allow clinical research in vulnerable populations like children although, regulatory authorities may still need to deliberate on issues of participants in vaccine trials to ensure that there are no unreasonable or serious risks of illness or injury associated with those trial designs. The advances in clinical research thus challenge the orthodox way of thinking about clinical trials however measures should be in place to ensure that all participants receive the full benefits of scientific innovation.Citation19,Citation32
Malaria is also affecting most parts of the African continent such as Malawi, Kenya, and Ghana and through the vaccine research of pilot studies in these countries which has enabled the approval of the world’s first malaria vaccine, RTS, S/AS01, for use in the context of the pilot implementations.Citation33–Citation35 It is encouraging to see that the majority of vaccine clinical research conducted in these aimed at addressing this devasting pandemic. Research conducted in the continent is largely focusing on seeks to address the disease burden in Africa, in turn making progress toward reaching the SDG3 goals. Though there are some highlights with regards to vaccine clinical research in Africa there are however general inequalities which may need to be looked at in combination with the advances in vaccine research. For example, South Africa has a generalized HIV epidemic, which has stabilized over the last four years at a national antenatal prevalence of around 30%. In 2009, an estimated 17.9% of the adult population was living with HIV. This is estimated to be 5.63 million people, including 3.3 million women and 334 000 children. HIV infection fuels the tuberculosis epidemic, with more than 70% of patients co-infected with both infections. The highest prevalence of TB infection is among people aged 30–39 years who are living in townships and informal settlements. The TB infection rate places South Africa as the third country with the highest level of TB in the world after India and China. There is a wide variation in HIV and TB prevalence across age, race, gender, socio-economic status, and geographical location.Citation36
Eradication of infectious disease in Africa is rooted in solid implementation strategies of the health-care services aimed at reducing socio-economical inequalities. The data here suggest an equal distribution of trials in different phases, although, there are slightly fewer trials in Phase IV which is meant to unpack the implementation process and challenges post-licensure. Vaccine trials at early stages are greatly encouraged however the whole pipeline of clinical research needs to be considered taking into account that African countries may have challenges with the implementation of such interventions. Not only does the consideration for implementation required, having the whole process in mind allows for countries to collect data on the incidence of diseases. Such an example is the recently chimeric dengue vaccine.Citation37 There is a pressing need for guidelines focused on the clinical evaluation of dengue vaccines in exposed populations, to collect accurate dengue incidence data across multiple transmission seasons, and to conduct Phase 2 or 3 bridging studies, post-Phase 3 follow-up safety studies and Phase 4 post-licensure trials to better elucidate vaccine immunogenicity, protective efficacy, or safety in endemic areas where multiple dengue types and other flaviviruses circulate where flavivirus vaccines are widely used.Citation38
Recent trials of the vaccine candidate MVA85A, designed to boost BCG efficacy, showed high levels of immunogenicity in UK adults but poor levels in South African infants, highlighting a frustrating but common inconsistency of responses across populations.Citation39,Citation40 This inconsistency of response is not limited to TB vaccines but is also observed in malaria and HIV vaccine development, where vaccine candidates often appear highly effective in early phase trials and yet perform below expectation in efficacy trials.Citation41 Contributing factors such as socioeconomic status could also have a role to play in the inconsistencies where effective vaccines in high-income countries perform poor in SSA. Social factors include poverty-reduction strategies in both low and middle-income countries and encompass both social security and social assistance interventions. These typically consist of the provision of regular, noncontributory, monetary benefits to households living in poverty and extreme poverty.Citation42 Such interventions could be looked at holistically to assist implementation programs for these new vaccines.
Conclusion
Here we described the landscape of vaccine trials conducted in Africa and found that the African continent is conducting research aiming to address the local burden of disease in infectious diseases and maternal-child health through vaccination as a prevention method. Africa remains the preferred choice of clinical research, particularly with the large number of clinical trials which include local and international research sites highlighting the interest taken by African researchers to collaborate and find solutions specific for the African context. With the complexity of diseases and the need for preventative interventions for vulnerable populations in the continent, regulatory authorities are becoming more flexible in accepting different types of study designs conducted in children. More local governmental funding is an issue that needs consideration as governmental agencies have to split the costs for other societal needs. The value of investing in research has far-reaching benefits in the long term where state health facilities will not be overloaded with patients seeking various treatments. These findings may be useful to stakeholders for decision-making regarding the conduct of vaccine clinical trials in Africa and provides potential mechanisms to advance these trials by providing infrastructures to enable developing these vaccines within the continent. To a broader context, implementation issues are also a key factor to be considered to ensure that health inequalities are reduced particularly in the low and middle-income countries.
Limitation of the study
Our search strategy could have underestimated the number of studies as the search term used did not include inoculation/s. Even though this search strategy may have covered most clinical trials in Africa, the term “inoculation” or “inoculations” did also pick a comparable number of studies that we may have missed. Additionally, since this study is a descriptive study, the findings presented here only provide an overview of the conduct of vaccine research in Africa. A more systematic literature review that fulfills the PRISMA protocol may be an option in the future to unpack the findings contained here.
Authors contribution
DN, KD, and CSW conceived the study. DN extracted the data and KD and LM screened and analyzed the data. DN wrote the first manuscript. DN, KD, LM, CSW contributed to interpreting and shaping of the argument of the manuscript and participated in writing the final draft of the manuscript. All the authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
This study was based on an analysis of existing ICTRP data records and ethics approval was not required as the records in ICTRP are publicly available.
Acknowledgments
The authors are grateful to the South Africa Medical Research Council, Cochrane South Africa for providing funding. The views expressed in this publication are those of the author(s) and not necessarily those of the SAMRC.
Additional information
Funding
References
- Plotkin SA. Vaccines, vaccination, and vaccinology. J Infect Dis. 2003;187(9):1349–1359. doi:10.1086/jid.2003.187.issue-9.
- Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff (Millwood). 2016;35(2):199–207. doi:10.1377/hlthaff.2015.1086.
- Ozawa S, Clark S, Portnoy A, Grewal S, Stack ML, Sinha A, Mirelman A, Franklin H, Friberg IK, Tam Y, et al. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001–2020. Bull World Health Organ. 2017;95(9):629–38. doi:10.2471/BLT.16.178475.
- World Health, O. 9 in 10 infants worldwide received vaccinations in 2017. [accessed Sept 05]. https://www.who.int/immunization/newsroom/2018_infants_worldwide_vaccinations/en/.
- World Health, O. Immunization. [accessed 2019 Sept 05]. https://www.who.int/news-room/facts-in-pictures/detail/immunization.
- GAVI TVA. Annual progress report 2018. GAVI The Vaccine Alliance; 2018.
- World Health, O. Immunization coverage. [accessed Sept 05]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, et al. Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315(15):1610–23. doi:10.1001/jama.2016.4218.
- World Health Organisation. Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;91(4):33–51.
- Agnandji ST, Fernandes JF, Bache EB, Obiang Mba RM, Brosnahan JS, Kabwende L, Pitzinger P, Staarink P, Massinga-Loembe M, Krähling V, et al. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. PLoS Med. 2017;14(10). doi:10.1371/journal.pmed.1002402.
- Coller BAG, Blue J, Das R, Dubey S, Finelli L, Gupta S, Helmond F, Grant-Klein RJ, Liu K, Simon J, et al. Clinical development of a recombinant Ebola vaccine in the midst of an unprecedented epidemic. Vaccine. 2017;35(35):4465–69. doi:10.1016/j.vaccine.2017.05.097.
- Gsell PS, Camacho A, Kucharski AJ, Watson CH, Bagayoko A, Nadlaou SD, Dean NE, Diallo A, Honora DA, Doumbia M, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis. 2017;17(12):1276–84. doi:10.1016/S1473-3099(17)30541-8.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389(10068):505–18. doi:10.1016/S0140-6736(16)32621-6.
- Heppner DG Jr., Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, Link CJ, Das R, Xu ZJ, Sheldon EA, et al. Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis. 2017;17(8):854–66. doi:10.1016/S1473-3099(17)30313-4.
- Malvy D, McElroy AK, de Clerck H, Gunther S, van Griensven J. Ebola virus disease. Lancet. 2019;393(10174):936–48. doi:10.1016/S0140-6736(18)33132-5.
- Carter RJ, RGBS, Dawson P 2, Gassama I 3, Kargbo SAS 2, Petrie 4CR, Mohamed Hashim Rogers MS 2, Luman ET. STRIVE (Sierra Leone Trial to Introduce a Vaccine Against Ebola); 2015.
- World Health, O. Malaria vaccine: WHO position paper, January 2016 - Recommendations. Vaccine. 2018;36(25):3576–77. doi:10.1016/j.vaccine.2016.10.047.
- Puppalwar G, Mourya M, Kadhe G, Mane A. Conducting clinical trials in emerging markets of sub-Saharan Africa: review of guidelines and resources for foreign sponsors. Open Access J Clin T. 2015;7:23–34. doi:10.2147/OAJCT.S77316.
- Ndebele P, Blanchard-Horan C, Shahkolahi A, Sanne I. Regulatory challenges associated with conducting multicountry clinical trials in resource-limited settings. J Acquir Immune Defic Syndr. 2014;65(Suppl 1):S29–31. doi:10.1097/QAI.0000000000000037.
- Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health. 2018;17(1):37. doi:10.1186/s12939-018-0748-6.
- Okpechi IG, Swanepoel CR, Venter F. Access to medications and conducting clinical trials in LMICs. Nat Rev Nephrol. 2015;11(3):189–94. doi:10.1038/nrneph.2015.6.
- Marchetti E, Mazarin-Diop V, Chaumont J, Martellet L, Makadi MF, Viviani S, Kulkarni PS, Preziosi MP. Conducting vaccine clinical trials in sub-Saharan Africa: operational challenges and lessons learned from the meningitis vaccine project. Vaccine. 2012;30(48):6859–63. doi:10.1016/j.vaccine.2012.09.008.
- da Silva RE, Amato AA, Guilhem DB, de Carvalho MR, Novaes M. International clinical trials in Latin American and Caribbean Countries: research and development to meet local health needs. Front Pharmacol. 2017;8:961. doi:10.3389/fphar.2017.00961.
- research, C. P. Why these are the top countries for conducting clinical trials. [accessed 2019 June 06]. http://www.confidenceresearch.com/2016/06/why-these-are-the-top-countries-for-conducting-clinical-trials/.
- Simpkin V, Namubiru-Mwaura E, Clarke L, Mossialos E. Investing in health R&D: where we are, what limits us, and how to make progress in Africa. BMJ Glob Health. 2019;4(2):e001047. doi:10.1136/bmjgh-2018-001047.
- World Health Organisation. SDG3 ensure healthy lives and promote wellbeing for all at all ages; 2017.
- DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1859–922.
- Elliott AM, Roestenberg M, Wajja A, Opio C, Angumya F, Adriko M, Egesa M, Gitome S, Mfutso-Bengo J, Bejon P, et al. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res. 2018;1:2. doi:10.12688/aasopenres.
- Wajja A, Namutebi M, Apule B, Oduru G, Kiwanuka S, Akello M, Nassanga B, Kabagenyi J, Mpiima J, Vermaak S, et al. Lessons from the first clinical trial of a non-licensed vaccine among Ugandan adolescents: a phase II field trial of the tuberculosis candidate vaccine, MVA85A. Wellcome Open Res. 2018;3:121. doi:10.12688/wellcomeopenres.
- Vekemans J, Gebreselassie N, Zignol M, Friede M, Kasaeva T, Swaminathan S. A new tuberculosis vaccine: breakthrough, challenges, and a call for collaboration. Lancet Infect Dis. 2019;19(2):123–25. doi:10.1016/S1473-3099(19)30003-9.
- Van Hoof W, Meesters K, Dossche L, Christiaens D, De Bruyne P, Vande Walle J. Ethical considerations of researchers conducting pediatric clinical drug trials: a qualitative survey in two Belgian university children’s hospitals. Eur J Pediatr. 2018;177(7):1003–08. doi:10.1007/s00431-018-3151-9.
- Rieder M, Hawcutt D. Design and conduct of early phase drug studies in children: challenges and opportunities. Br J Clin Pharmacol. 2016;82(5):1308–14. doi:10.1111/bcp.v82.5.
- Guerra Mendoza Y, Garric E, Leach A, Lievens M, Ofori-Anyinam O, Pircon JY, Stegmann JU, Vandoolaeghe P, Otieno L, Otieno W, et al. Safety profile of the RTS,S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother. 2019;391(10200):1–13.
- Schuerman L. RTS,S malaria vaccine could provide major public health benefits. Lancet. 2019;394(10200):735–36. doi:10.1016/S0140-6736(19)31567-3.
- Tinto H, Otieno W, Gesase S, Sorgho H, Otieno L, Liheluka E, Valea I, Sing’oei V, Malabeja A, Valia D, et al. Long-term incidence of severe malaria following RTS,S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect Dis. 2019;19(8):821–32. doi:10.1016/S1473-3099(19)30300-7.
- SANAC, 2013.
- Back AT, Lundkvist A. Dengue viruses - an overview. Infect Ecol Epidemiol. 2013;3:1–21.
- Edelman R, Hombach J. “Guidelines for the clinical evaluation of dengue vaccines in endemic areas”: summary of a World Health Organization technical consultation. Vaccine. 2008;26(33):4113–19. doi:10.1016/j.vaccine.2008.05.058.
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–28. doi:10.1016/S0140-6736(13)60177-4.
- Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, Ngwira B, Sichali L, Nazareth B, Blackwell JM, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359(9315):1393–401. doi:10.1016/S0140-6736(02)08353-8.
- Li S, Plebanski M, Smooker P, Gowans EJ. Editorial: why vaccines to HIV, HCV, and malaria have so far failed-challenges to developing vaccines against immunoregulating pathogens. Front Microbiol. 2015;6:1318. doi:10.3389/fmicb.2015.01318.
- Dowd JB, Fletcher HA, Boccia D. Social determinants and BCG efficacy: a call for a socio-biological approach to TB prevention. F1000Res. 2018;7:224. doi:10.12688/f1000research.