ABSTRACT
Enterovirus 71 (EV-A71) and Coxsackievirus A16 (CV-A16) are the two most common pathogens causing hand, foot, and mouth disease (HFMD). Previously, we obtained one candidate live attenuated strain each for EV-A71 and CV-A16; here, we evaluated the safety and immunogenicity of a combinedlive vaccine against EV-A71 and CV-A16 generated from these two candidate strains. Rhesus monkeys were intramuscularly treated with a live combinationvaccine against both EV-A71 and CV-A16 or with either vaccine alone. No fever or atypical clinical signs were observed in any animals. Monkeys vaccinated with the combinationlive vaccine presented no notable pathological changes in the brain, spinal cord, lung, and liver; in contrast, these regions showed inflammatory cell infiltration in monkeys treated with EV-A71 alone or CV-A16 alone. Weak viremia was detected in plasma after inoculation with the combinationvaccine; however, the duration of viral shedding in feces was increased. Biochemical studies revealed a slight increase in aspartate aminotransferase levels in monkeys inoculated with the live combination vaccine; however, histopathological findings did not attribute this change to liver damage. We also found that the live combinationvaccine induced a dual humoral immune response. Cytokine analysis indicated that the combined EV-A71/CV-A16 vaccine significantly down-regulated interleukin-8 production. Here, we have demonstrated that the live attenuated EV-A71/CV-A16 vaccine was safe and could trigger a dual specific immune response. However, its immune protection efficacy requires further investigation.
Introduction
Hand, foot, and mouth disease (HFMD) is an acute and contagious disease that mostly occurs in infants and children younger than 5-years-old. The incidence and mortality of HFMD has been ranked first among the national Class C infectious diseases in China since 2009. Enterovirus 71 (EV-A71) and Coxsackievirus group 16 (CV-A16) are the most common pathogens of HFMD. The monovalent EV-A71 inactivated vaccine has been successfully marketed in China,Citation1-Citation3 but in vitro experiments and clinical studies have confirmed that the EV-A71 monovalent vaccine does not confer cross-protection against CV-A16 or other pathogens associated with HFMD.Citation3,Citation4 Furthermore, clinically, HFMD patients with an EV-A71 and CV-A16 coinfection are more common than those with a single EV-A71 or CV-A16 infection. Thus, the development of a bivalent vaccine against both EV-A71 and CV-A16 may be considered urgent. In our previous study, we developed a CV-A16 candidate vaccine strain that showed attenuated phenotype in a rodent model,Citation5-Citation7 as well as in rhesus monkeys (unpublished data), and an attenuated EV-A71 strain developed from the inactivated vaccine strain FY-23K was also investigated.Citation8 Before a combined vaccine can be used in a clinical setting, it must be ascertained that it is safe and that the immunogenicity of each component is acceptable. In the current study, we combined both candidate attenuated strains into a single vaccine, and the clinical reactions and immune responses induced by the vaccines given separately or combined were compared in rhesus monkeys.
Materials and methods
Viruses and cells
K168-8Ac, a CV-A16 strain (GenBank: KY088084.1) isolated by our group from the feces of HFMD patients in Kunming Children’s Hospital, Yunnan, China, was used to produce the CV-A16 vaccine. The EV-A71 strain FY-23KB (GenBank: EU812515.1) was kindly donated by Professor Qihan Li at the Department of Viral Immunity, the Institute of Medical Biology, Chinese Academic of Medical Sciences (CAMS), which is the parental strain of the inactivated EV-A71 vaccine produced by the Institute of Medical Biology, CAMS.Citation9
The KMB17 human embryonic lung diploid cell line was used as a cell matrix for vaccine production in this study,Citation10 as it is widely used for viral vaccine production in China.Citation9,Citation11,Citation12 Vero cells, which are derived from the kidney of the African green monkey (Chlorocebus sabaeus) and are a commonly used mammalian continuous cell line for vaccine production, were used for virus titration in this study. These two cell line were provide by the Department of Quality Control, the Institute of Medical Biology, CAMS.
Rhesus monkeys
Rhesus monkeys (Macaca mulatta) are recognized as an acceptable nonhuman primate model for studies on CV-A16;Citation13 for this reason, rhesus monkeys were used in this study. All animals were male and aged 5–10 months at immunization. All experimental procedures and animal care protocols were approved by the Yunnan Provincial Experimental Animal Management Association (Approval number SYXK (Dian) K2015-0006) and the Experimental Animal Ethics Committee of the Institute of Medical Biology, CAMS (Approval number [2016] 59). Each monkey was housed alone in individual enclosures. Before beginning the study, all monkeys were confirmed to be negative for CV-A16 and EV-A71 antibodies by a neutralizing antibody assay.
Passaging of CV-A16 and EV-A71 in KMB17 cells and viral titration
KMB17 cells and Vero cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Indianapolis, OH, USA) supplemented with 10% fetal bovine serum. As described previously,Citation14,Citation15 150–200 μl FY-23KB strain or K168-8Ac strain was inoculated into 80% confluent monolayers of KMB17 cells in a 25-cmCitation2 flask (Corning, NY, USA); the cells were cultured until 90% of the cells displayed cytopathic effect (CPE) and then harvested. FY-23KB strain was serially passaged at 35°, while K168-8Ac was propagated at 33°. Virus cultures were treated with three cycles of freeze-thawing before being used as virus seeds for subsequent passages. This was performed consecutively from the first to the fiftieth passage. At each passage, the virus culture was titrated using Vero cells as described previously.Citation16 Briefly, the viruses were serially diluted ten-fold and then inoculated into Vero cells precoated onto 96-well plates (10Citation4 cells/well). Each dilution was added to eight wells. The CPE in each well was observed, and the viral titers were expressed as 50% cell culture infective dose (CCID50) and calculated using the Bethrens-Kärber method.
Preparation of the experimental live combinationvaccine
Viral attenuation by serial passaging was performed using KMB17 cells as described previously.Citation14,Citation15 The fiftieth passages of CV-A16 and EV-A71 in KMB17 cells were mixed at an equal volume at a dose of 10Citation7 CCID50/ml each.
Vaccination
A total of six monkeys were randomly assigned into three groups with two monkeys per group. Group 1 received a live monovalent CV-A16 vaccine at a dose of 10Citation7 CCID50 per monkey, group 2 received a live monovalent EV-A71 vaccine and served as the control group, and group 3 received a live combination vaccine against CV-A16 and EV-A71 at the same dose (107 CCID50 per monkey). The experimental vaccines were intramuscularly administered to the rhesus monkeys on the thigh. The immunization schedule used is shown in .
Table 1. Immunization strategy for the experimental vaccines.
Sample collection
Fecal samples (< 5 g) were collected daily in 15-ml tubes preloaded with PBS (pH 7.4) and weighed. The fecal samples were then frozen at −80°C until required. Chloroform was added to the samples, and the homogenate was allowed to separate into a clear upper aqueous layer for extraction of viral RNA to assess viral shedding by RT-qPCR.
Blood was drawn from the superficial leg vein at 2, 5, 8, 12, 29, and 42 days post inoculation (dpi). Whole blood was kept at room temperature, and serum was separated by centrifugation at 2,100 g for 10 min and stored at −80°C until use for neutralizing antibody detection and biochemical testing for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (γ-GT), uric acid (UA), serum urea, and creatinine (CREA) levels. Plasma samples were prepared from blood collected using EDTA by centrifugation at 13,800 g for 5 min, and stored at −80°C until required for viral load testing and cytokine or chemokine production analysis.
At 14 dpi, one animal per group was anesthetized with an overdose of pentobarbital sodium (> 25 mg/kg) and exsanguinated. The brain, spinal cord, muscle, internal organs, and lymph nodes were removed, fixed with formaldehyde, sectioned, and stained with hematoxylin and eosin (H&E) for pathological examinations.
Viral quantification by RT-qPCR
Supernatants (200 μl) extracted from feces or plasma were used to extract viral RNA for virus quantification using a commercial kit (Axygen, NY, USA); extracted viral RNA was dissolved in 50 μl TE Buffer. One-step RT-qPCR (Takara, Dalian, China) was performed using a fluorescence quantitative PCR instrument (Bio-Rad Laboratories, Hercules, CA, USA). The reverse transcription step involved incubation at 42°C for 5 min. The PCR cycling condition included an initial denaturation of 95°C for 10 sec followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The viral load, expressed as CCID50/ml or Copies/ml, was quantified using a standard curve. The sequences used for the primers and probes are given in .
Table 2. Sequences of primers and probes for RT-qPCR.
Neutralization assays
Serum samples were inactivated at 56°C for 20 min before use. The neutralizing activity of serum was measured in 96-well culture plates using the micro-cytopathic method as described previously.Citation4 Briefly, inactivated serum was diluted two-fold in 96-well plates at dilutions of 1:8–1:4,196 and in two wells for each dilution; thereafter, the solutions were mixed with 50 μl CV-A16 or EV-A71 (32–320 CCID50) at 37°C for 90 min. Next, Vero cells were added to each well at approximately 10Citation4 cells/well and incubated at 37°C for 7 days. After 7 days, the CPE in each well was observed under a microscope, and the highest dilution at which ≥ 50% of cells were protected from CPE was defined as the neutralizing titer for each serum. Three individual experiments were performed for each sample.
Cytokine and chemokine detection
Plasma samples were harvested at the indicated times and stored at −80°C until further experiments. Prior to measurements, plasma was purified by centrifugation at 13, 800 g for 5 min. Interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-6, IL-8, and tumor necrosis factor-α (TNF-α) were quantified as representative cytokines and chemokines using the Luminex-200 System (Luminex, Austin, TX, USA) with a customized Milliplex Kit (Millipore, MA, USA) according to the manufacturer’s instruction. Data analysis was performed with Milliplex Analyst 5.1 software.
Statistical analysis
IBM SPSS 22.0 statistical software was used for all statistical analyses. For raw data or data after transformation with a normal distribution and equal variance, the independent samples t-test-produced t value or one-way analysis of variance (ANOVA)-produced F value was applied for comparisons between two groups or more than two groups, respectively. Otherwise, the nonparametric Mann-Whitney U-test or Kruskal-Wallis test was used. A value of P < .05 was considered statistically significant.
Results
Rhesus monkeys inoculated with all vaccine combinations were clinically asymptomatic
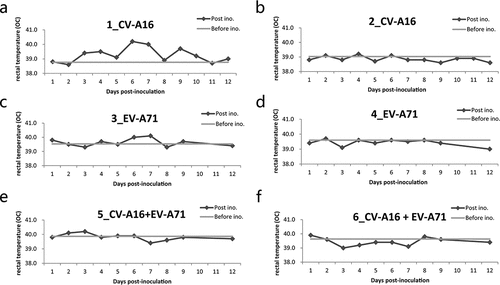
Rhesus monkeys were intramuscularly administered with 7.0 LogCCID50/ml live virus. No monkeys showed clinical signs of HFMD, including mucosa and limb vesicles, compared to the control group. The rectal temperature of the inoculated monkeys with CV-A16 alone ( and ), or EV-A71 alone ( and ) or combination of CV-A16 and EV-A71 ( and ) was measured once daily after infection; no significant increase was observed post inoculation (F = 0.810, P = .639).
Figure 1. Rectal temperature of rhesus monkeys after immunization. Two rhesus monkeys in each group were vaccinated intramuscularly with either CV-A16 alone, EV-A71 alone, or a combination of both at an equal dose of 7 Log CCID50, and rectal temperature was measured daily over the period of twelve days post-immunization. The rectal temperature of the monkeys received CV-A16 alone are represented in (a) and (b); the rectal temperature of the monkeys EV-A71 alone are shown in (c) and (d); the rectal temperatures of the monkeys a live combination of EV-A71 and CV-A16 are shown in (e) and (f). The light gray lines indicate the baseline temperature of each monkey before immunization and the dark gray dotted lines indicate the daily rectal temperature of each monkey over 12 days post-immunization.
The live combinationvaccine attenuated histopathological lesions
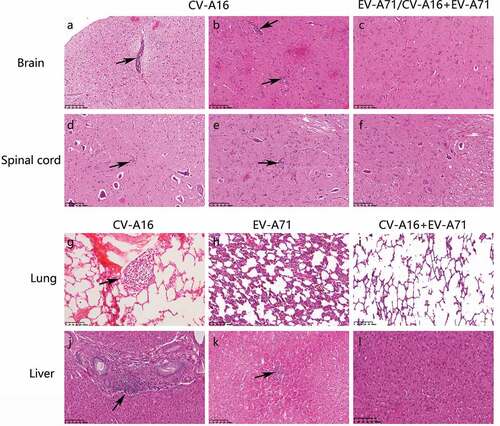
The brain, spinal cord, muscle, lymph nodes, and other internal organs were obtained from the monkeys at 14 dpi, fixed in formaldehyde, and then stained with H&E for histopathological examinations. Glial nodules, a common feature of viral infection, and few lymphocytic cuffs, were observed in the mesencephalon, pons, lumbar, and cervical spinal cord () of the animals vaccinated with CV-A16 alone or EV-A71 alone. Moderate lymphocytic aggregations were observed at hepatic portal areas () and in the liver parenchyma (), and the alveolar walls were thickened along with areas of congestion and edema (). However, animals immunized with the live combinationvaccine presented no notable pathological changes ().
Figure 2. Representative histopathological changes in tissue sections as seen with H&E staining (magnification: 200X). Rhesus monkeys were treated intramuscularly with either CV-A16 alone, EV-A71 alone, or both EV-A71 and CV-A16 at an equal dose of 7 Log CCID50. In each group, one animal was euthanatized, and the brain, spinal cord, visceral organs, and skeletal muscle and lymph nodes were resected for histopathological examination. A few lymphocytic cuffs were observed in the midbrain (a) and pons (b) of the animal treated with CV-A16 alone. Glial nodules representing the aggregates of lymphatic cells in the lumbar (d) and cervical (e) regions were observed in the spinal cord of the animal treated with CV-A16 alone. The enlargement of lymphoid follicles on the alveolar walls of the animal treated with CV-A16 alone is shown in (g). The animal injected with EV-A71 alone presented thickened alveolar walls along with the areas of congestion and edema as shown in (h) Moderate lymphocytic aggregations in portal areas of the liver in the animal treated with CV-A16 alone are shown in (j). A small number of infiltrated inflammatory cells were observed in the liver parenchyma of the animal treated injected with EV-A71 (i). However, no significant pathological changes were observed in the brain (c), spinal cord (f), lung (i), and liver (l).
Weak viremia in the experimental vaccine-inoculated rhesus monkeys
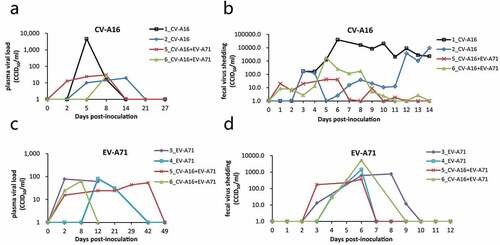
Next, we measured the plasma viral load by RT-qPCR. The CV-A16 viral load was undetectable in the plasma from all experimental groups, except in one monkey inoculated with CV-A16 alone, in which the viral load exceeded 1,000 CCID50/ml at 5 dpi (); the lower limit of detection for CV-A16 was 10 CCID50/ml. The plasma EV-A71 viral load in all animals immunized with EV-A71 alone or combined with CV-A16 was below 100 CCID50/ml (). There was no statistically significant difference in the plasma EV-A71 viral load between the two groups (t = 0.044, P = .965).
Figure 3. Viral load in plasma and feces. Plasma and feces were collected at the indicated time points to detect the viral RNA load after immunization with EV-A71 alone, CV-A16 alone, or the combined live vaccine. The viral shedding of CV-A16 in plasma over 27 days (a) and in feces over 14 days (b) are shown following the CV-A16 alone and combination vaccination, respectively. (c) and (d) indicate the viral shedding of EV-A71 in plasma and feces during the same period in the animals immunized with EV-A71 alone and the combination vaccine, respectively.
Extended virus shedding in experimental vaccine-inoculated rhesus monkeys
Enteroviruses are transmitted through the fecal-oral route. Thus, viral shedding in the feces may reflect the replication rate of the virus in the body and was evaluated by RT-qPCR in this study. In the group treated with CV-A16 only, fecal virus shedding occurred at 5–14 dpi, while that in the combined group occurred only at 5–6 dpi; furthermore, the extent of viral shedding was significantly lower than that in the CV-A16 alone group (P < .001) (). The group treated with EV-A71 only and the combination treatment group both shed the virus starting at 3–4 dpi, and shedding lasted a further 3–4 days (). The extent of viral shedding between the two groups was not statistically significant (P = .610).
Hemato-biochemical changes in response to immunization
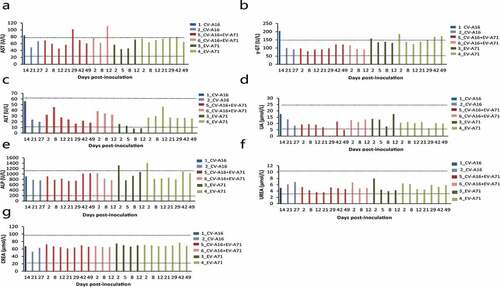
At 2, 5, 8, 12, 21, 29, and 42 dpi, blood serum was extracted to determine the levels of AST, ALT, ALP, γ-GT, UA, urea, and CREA, which are generally accepted to be reliable indicators of liver, kidney, and muscle damage. The results showed an increase in AST levels at 12 and 29 dpi in the combination treatment group (). The animals immunized with EV-A71 alone were found to have slightly elevated ALP levels at 2 dpi (). A mild increase in γ-GT was observed in both animals from the EV-A71 alone group and in one animal from the CV-A16 alone group. The renal function indicators UA and urea, as well as one muscle damage indicator, CREA, were within normal ranges in all experimental animals ().
Figure 4. Measurement of biochemical markers in monkeys inoculated injected with EV-A71 alone, CV-A16 alone, or the combined vaccine. The serum levels of AST (a), γ-GT (b), ALT (c), UA (d), ALP (e), urea (f), and CREA (g) were measured by using an automatic analyzer. The black dotted line represents the normal ranges of these indices. The blue, red and green histograms represent the animals injected with CV-A16 alone, combination vaccine, and the EV-A71 alone, respectively.
Combined immunization produces dual seroconversion
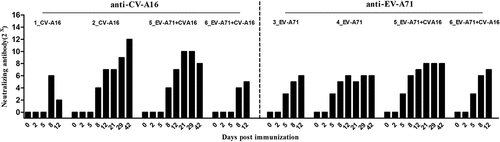
Blood samples were collected at 2, 5, 8, 12, 21, 29, and 42 dpi, and serum was isolated for neutralization antibody detection by the micro-cytopathic method. The animals developed CV-A16 and EV-A71-neutralizing antibody responses almost simultaneously in the single treatment and combination treatment groups, beginning at 8 and 5 dpi, respectively (). There were no significant differences in the levels of EV-A71- and CV-A16-specific neutralizing antibodies between the single groups and the combination group (P = .311 and P = .74, respectively), indicating that a combination of these two live viruses probably had no effect on their individual specific antibody responses.
Plasma IL-8 levels were reduced in combination-treated rhesus monkeys
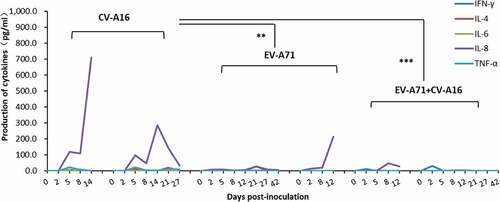
Plasma was isolated from whole blood samples collected at 2, 5, 8, 12, 21, 29, and 42 dpi for the quantitative analysis of IFN-γ, IL-4, IL-6, IL-8, and TNF-α using a high-sensitivity bead-based multiplex assay. In all groups, the levels of IFN-γ, IL-4, IL-6, and TNF-α were less than 50 pg/ml (), below the confidence interval of the standard curves. However, IL-8 production was increased in the plasma of both animals immunized with CV-A16 alone, and in the plasma of one animal in the combination group (), while that in the EV-A71 alone group showed no obvious changes compared to baseline levels (). Plasma IL-8 levels in the CV-A16 alone group were significantly higher than those in all other groups (P < .001 and P = .002, respectively).
Discussion
FY-23 is prototype strain of the EV-A71 vaccine marketed in China. We found that this strain can be continuously passaged on KMB17 cells at 35° with a stable propagation. When it was serially passaged by 28 times (P28), no pathogenicity or death was observed in neonatal mice, and no obvious pathological damage was detected in the central nervous system.Citation14 However, when we immunized rhesus monkeys with P28, we found that rhesus monkeys still had obvious herpes and developed significant viremia, pulmonary edema and hemorrhage in the lungs. We inferred that FY-23 strain could meet the safety requirements if it was passaged by more than P28, thus P50 was selected for testing in this study. K168-8AC strain was isolated and identified by our group, which was defined as a candidate strain of CV-A16 vaccine. In vivo experiments showed that the survival rate of suckled mice was infected with the early passage of this strain only about 20%, and their central nervous systems and muscle tissues showed serious pathological injuries.Citation17We found that the K168-8Ac strain could be continuously passaged at 33°C When the strain was passaged by 17 times (P17), the survival rate of the inoculated suckling mice was greatly improved, and no obvious changes were found in histopathological examination or pathological damage was significantly reduced.Citation6Therefore, we inferred that P50 viruses may have attenuated phenotypes, which was tested in this study.
It is reported that rhesus monkeys aged 3 to 3.5 years were used to evaluate inactivated vaccine efficacy of EV-A71,Citation18while neonatal rhesus monkeys, 2 to 3 month of age, were confirmed to be sensitive to EV-A71.Citation19,Citation20For CV-A16, the infectious process was verified by using rhesus monkeys aged 6–8 months,Citation13and the same research fellows also found that rhesus monkeys aged 1–2 month or aged 5–10 month developed similar clinical symptoms with in humans.Citation21
In previous studies, typical viremia symptoms were observed in the peripheral blood at 3–9 dpi in rhesus monkeys inoculated with the CV-A16 live virus,Citation13,Citation22 and the blood CD14+ cell population was found to be one of the primary sites for CV-A16 proliferation.Citation22 However, in our study, no obvious viremia was observed, even at high doses (7 LogCCID50/ml); however, it must be noted that a viral load of over 1,000 CCID50/ml was observed in the plasma of one animal in the CV-A16 alone group at 5 dpi. We therefore concluded that plasma may not be ideal for evaluating CV-A16 infection, because CV-A16 may be latent in peripheral blood mononuclear cells without a lytic model. In contrast, the viral strains used in this study – K168-8Ac and FY-23-KB – were attenuated through continuous passaging, and our previous study showed the attenuated virulence of these two strains through tissue pathology (unpublished data). Thus, we speculated that these weak viremia symptoms may have been another attenuated phenotype of these two candidate stains. It was noticed that the trend of viral loads in the feces and in plasma of monkeys inoculated with CV-A16 alone, were different from that of in the combination group. We speculated the interferences would be exsited between EV-A71 and CV-A16, as they share the same one of functional receptors, scavenger receptor class B member 2 (SCARB2),Citation23 thereby this combination strategy might decrease the replication activity of EV-A71 or CV-A16 live viruses.
In this study, liver function tests revealed a significant increase in AST in animals immunized with the combination vaccine, while ALP and γ-GT were elevated in the EV-A71 alone group and CV-A16 alone group, possibly because a live vaccine was used. A phase I clinical trial of an EV-A71 inactivated vaccine indicated that it stimulated an immediate rise in ALT levels in the test subjects, but did not cause liver or kidney damage.Citation24 Previous Chinese clinical studies found that AST and ALP levels were increased in some HFMD patients,Citation25 but no significant difference was observed between severe and mild HFMD patients,Citation26 nor between patients with HFMD caused by EV-A71 or CV-A16 infection.Citation27
In this study, a combined live attenuated EV-A71 and CV-A16 vaccine was administered to rhesus monkeys by intramuscular injection. After one immunization dose, the neutralizing antibody titer of CV-A16 and EV-A71 reached a maximum of 1:4,096 and 1:256, respectively. In clinical trials of the inactivated EV-A71 vaccine, 1:8 was defined as the threshold for vaccine efficacy evaluation.Citation3 However, one dose of the inactivated CV-A16 experimental vaccine was found to induce neutralizing antibodies at a maximum of 1:64 in rhesus monkeys, while a titer of 1:256 was reached after three doses; however, this did not protect the rhesus monkeys against CV-A16 infection.Citation13 It was previously reported that the Genomic Mean Titers (GMTs) of neutralizing antibodies against EV-A71 and CV-A16 in rhesus monkeys were 38 and 40, respectively, after the first immunization with a combination inactivated EV-A71 and CV-A16 vaccine; the GMTs then increased to 740 and 800, respectively, after the second dose of this combinationvaccine.Citation28 In the current study, the live attenuated EV-A71 and CV-A16 combination vaccine produced effective neutralizing antibodies against both EV-A71 and CV-A16. For EV-A71, it was established that how 1:8 was determined as a threshold for EV-A71 neutralizing antibody level for evaluating vaccine efficacy.Citation3However, for CV-A16, it is difficult to estimate the level of specific antibody is enough to protect from infection, as the existing neutralizing antibody seemed not be sufficient to protect against natural infection.Citation13 Thus, whether our live attenuated combined vaccine confers effective protection requires further in vivo investigation.
Cellular immunity is closely related to the severity of diseases caused by viral infections; cytokines and chemokines, which are major immune regulatory factors, play an indispensable role in this response. In this study, it was found that plasma IL-8 levels in the CV-A16 alone group were significantly increased at 8–14 dpi, and that one animal in the combined group showed a similar increase, while this effect was not observed in the animals in the EV-A71 alone group. IL-8, also known as CXCL8, belongs to the CXC protein family and was the first chemokine found in the human brain; it plays an important role in the recruitment and activation of neutrophils. IL-8 can also promote the recruitment of neutrophils, B cells, and T cells.Citation29 Clinically, it was reported that IL-8 expression was significantly increased in patients with encephalitis or pulmonary edema caused by EV-A71 infection,Citation30 and a significant increase in IL-8 in the plasma of patients with severe EV-A71 infections was also observed,Citation31 suggesting that the severity of EV-A71 infection may be related to IL-8 production. In our study, a marked increase in plasma IL-8 was observed in rhesus monkeys immunized with CV-16 alone. Whether this increase was related to histopathological damage to further influence the safety of this single component vaccine remains unknown. In conclusion, the safety and immunogenicity of a live combination vaccine against both EV-A71 and CV-A16 using a rhesus model were evaluated, showing that the vaccine is safe and effective for inducing specific immune responses. However, given the limited number of rhesus monkeys used in this study, we still need to further confirm its effectiveness and protective efficacy in a larger population of non-human primate animals, and the mechanisms by which the combination of EV-A71 and CV-A16 induce weakened histopathological signs as compared to EV-A71 alone or CV-A16 alone are worthy of further studies.
Genetic recombination is a safety concern about live attenuated vaccines. Widespread use of live attenuate vaccines might increase the potential risk of the recombination of the vaccine strains and circulating viruses.Citation32It was demonstrated that genetic recombination is frequently occurring in enteroviruses. In this case, the recombination of EV-A71 and CV-A16 attenuated vaccine strains would be worried. Epidemiological data showed that inter-typicrecombination may exist in the EV-A71 and CV-A16,were possibly located in the genome regions of 5ʹUTR, P1 and P2 of EV-A71 and in P3 and 3ʹUTR regions of CV-A16.Citation33 However, these combination events are not conversed to a particular gene or a genome region, but across the entire genome.Citation34 To confirm whether the FY-23KB attenuated strain and K168-8AC attenuated strain used in this study recombined in vivo, we need to further isolate the replicated viruses from the inoculated monkeys for sequencing, alignment and similarity analysis of the whole genome. Meanwhile, virulence of these isolated viruses from feces or other samples should be investigated, as horizontal transmission is another important potential safety issue for live attenuated vaccines.
Limitations
Dose is one of vital parameters in administering vaccines; however, it was not investigated in this study due to the limited number of monkeys. It is known that specific humoral immunity plays important roles in the resistance to enteroviruses infection, so the current study focused on the humoral immune response (IgG) induced by the combined live attenuated vaccine, and the muscosal immunity (IgA), that is a different characteristic of live attenuated vaccines from other forms of vaccine, was not concerned, thus more attention should be paid to that in the following study. Furthermore, a challenge experiment was not carried out in the current study as more than three monkeys per group would be needed for evaluating protective effect, and it would be a top priority in our future study.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We are grateful to Kaili Ma, Yun Li, and Lipeng Zhou in the Good Laboratory Practice of Drug (GLP) Center of the Institute of Medical Biology, CMAS for their professional help with animal manipulation. We are also grateful to Qingling Wang, Xi Wang, and Yunguang Hu in the Department of Quality Control in our institute for their extensive assistance with histopathological examinations. We would like to thank Wenhai Yu at the Medical Primate Center of our institute for generously providing the normal reference range of biochemical indicators in the blood of healthy rhesus monkeys.
Additional information
Funding
References
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, Wen SQ, Liu Y, Yin WD, Li RC, Wang JZ. Safety and immunogenicity of a novel human enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, phase I clinical trial. Vaccine. 2012;30:3295–303. doi:10.1016/j.vaccine.2012.03.010.
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–32. doi:10.1016/S0140-6736(13)61049-1.
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–37. doi:10.1056/NEJMoa1303224.
- Yang T, Li H, Yue L, Song X, Xie T, Ma S, Meng H, Zhang Y, He X, Long R, et al. A comparative study of multiple clinical enterovirus 71 isolates and evaluation of cross protection of inactivated vaccine strain FY-23 K-B in vitro. Virol J. 2017;14:206. doi:10.1186/s12985-017-0872-8.
- Zhang Y Study on the key technology of iyophilized experimental attenuated live attenuated vaccine of coxsackievirus A16[dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College; 2018. doi:10.3168/jds.2017-14085
- Yang SZ Attenuated characteristics and bioprocess assessment of CA16 vaccine candidates [dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College; 2015.
- Jiang GJ The selection of candidate strain of attenuated live coxsackievirus A16 vaccine [dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College; 2014. doi:10.1094/PDIS-06-14-0565-PDN
- Zhu F Development of EV71 live attenuated vaccines and the attenuation mechanism research [dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College; 2016.
- Zhang Y, Wang L, Liao Y, Liu L, Ma K, Yang E, Wang J, Che Y, Jiang L, Pu J, et al. Similar protective immunity induced by an inactivated enterovirus 71 (EV71) vaccine in neonatal rhesus macaques and children. Vaccine. 2015;33:6290–97. doi:10.1016/j.vaccine.2015.09.047.
- Guo R, Cao YY, Dai ZZ, Qu SR, Zhuang JY. Characteristics of a human diploid cell line, KMB-17. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1981;3:226–30.
- Mao JS, Dong DX, Zhang HY, Chen NL, Zhang XY, Huang HY, Xie RY, Zhou TJ, Wan ZJ, Wang YZ, et al. Primary study of attenuated live hepatitis A vaccine (H2 strain) in humans. J Infect Dis. 1989;159:621–24. doi:10.1093/infdis/159.4.621.
- Liang Y, Che Y, Yang B, Zhan F, Li H, Guan X, Zhang Y, Yin Q, Li C, Li J, et al. Immunogenicity and safety of an F-genotype attenuated mumps vaccine in healthy 8- to 24-month-old children. J Infect Dis. 2019;219:50–58. doi:10.1093/infdis/jiy469.
- Wang J, Zhang Y, Zhang X, Hu Y, Dong C, Liu L, Yang E, Che Y, Pu J, Wang X, et al. Pathologic and immunologic characteristics of coxsackievirus A16 infection in rhesus macaques. Virology. 2017;500:198–208. doi:10.1016/j.virol.2016.10.031.
- Zhu FL, Li H, Xie TH, Yang T, Song X, Wang X, Shen D, Liu ZL, Yue L, Xie ZP. Dynamic changes of biological characteristics of EV-A71 strains continuously passaged in vitro at different temperatures. Chin J Viral Dis. 2016;6:113–18.
- Yang SZ, Xie TH, Li H, Jiang GJ, Long RX, Yang T, Yue L, Luo FY, Zhu FL, Xie ZP. Characteristics of live attenuated coxsackeivirus A16 candidate vaccine strain. Chin J Biol. 2015;28:441–48.
- Yang E, Cheng C, Zhang Y, Wang J, Che Y, Pu J, Dong C, Liu L, He Z, Lu S, et al. Comparative study of the immunogenicity in mice and monkeys of an inactivated CA16 vaccine made from a human diploid cell line. Hum Vaccin Immunother. 2014;10:1266–73. doi:10.4161/hv.28083.
- Jiang GJ, Li H, Yang T, Liu ZL, Xie TH, Long RX, Yue L, Luo FY, Xie ZP. Biological characteristics of clinical isolates of coxsackievirus group A type 16. Chin J Biol. 2014;27:607–11.
- Dong C, Wang J, Liu L, Zhao H, Shi H, Zhang Y, Jiang L, Li Q. Optimized development of a candidate strain of inactivated EV71 vaccine and analysis of its immunogenicity in rhesus monkeys. Hum Vaccin. 2010;6:1028–37. doi:10.4161/hv.6.12.12982.
- Zhongping X, Hua L, Ting Y, Zhengling L, Min F, Tianhong X, Runxiang L, Dong S, Guangju J, Lei Y, et al. Biological characteristics of different epidemic enterovirus 71 strains and their pathogeneses in neonatal mice and rhesus monkeys. Virus Res. 2016;213:82–89. doi:10.1016/j.virusres.2015.11.007.
- Zhao T, Zhang Z, Zhang Y, Feng M, Fan S, Wang L, Liu L, Wang X, Wang Q, Zhang X, et al. Dynamic interaction of enterovirus 71 and dendritic cells in infected neonatal rhesus macaques. Front Cell Infect Microbiol. 2017;7:171. doi:10.3389/fcimb.2017.00171.
- Wang J Study of Coxsackievirus A16-infected rhesus monkeys macaques model and immunologic characteristics [dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College, 2017.
- Qi SD, Wang JJ, Zhang XL, Shen F, Li QH, Li YY, Liu LD, He ZL. Study on proliferation of coxsackievirus A16-infected peripheral blood mononuclear cells in rhesus monkeys. J Microbes Infections(Chinese). 2015;10:140–46.
- Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med. 2009;15:798–801. doi:10.1038/nm.1992.
- Meng FY, Li JX, Li XL, Chu K, Zhang YT, Ji H, Li L, Liang ZL, Zhu FC. Tolerability and immunogenicity of an inactivated enterovirus 71 vaccine in Chinese healthy adults and children: an open label, phase 1 clinical trial. Hum Vaccin Immunother. 2012;8:668–74. doi:10.4161/hv.19521.
- Liu D, Wu JH, Zhu QJ. The statistical analysis of laboratory examination of hand, foot and mouth diseases in wuhan city in 2009. J Mathe Med. 2011;24:565–67.
- Gao LZ, Li SJ, Zuo J, Tang L. Study on changes of electrocardiogram and biochemical indexes of 128 children with EV71 severe hand-foot-and mouth-disease. China Med Pharm. 2017;7:69–72.
- HZ Y, Chu C, Ji W, XD L, XH Z, BQ L, Xu J, WF Z. Analysis of 139 cases of hand, foot and mouth disease. J Pract Med. 2011;27:4469–70.
- Li M, Duan Y, Yang X, Yang Q, Pang B, Wang Y, Ren T, Wang X, Zhao Z, Liu S. Intradermal injection of a fractional dose of an inactivated HFMD vaccine elicits similar protective immunity to intramuscular inoculation of a full dose of an Al(OH)3-adjuvanted vaccine. Vaccine. 2017;35:3709–17. doi:10.1016/j.vaccine.2017.05.060.
- Mamik MK, Ghorpade A. CXCL8 as a potential therapeutic target for HIV-associated neurocognitive disorders. Curr Drug Targets. 2016;17:111–21. doi:10.2174/1389450116666150626124544.
- Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, Su IJ, Liu CC. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–70. doi:10.1086/376998.
- Ye N, Gong X, Pang LL, Gao WJ, Zhang YT, Li XL, Liu N, Li DD, Jin Y, Duan ZJ. Cytokine responses and correlations thereof with clinical profiles in children with enterovirus 71 infections. BMC Infect Dis. 2015;15:225. doi:10.1186/s12879-015-0965-1.
- Kew OM, Wright PF, Agol VI, Delpeyroux F, Shimizu H, Nathanson N, Pallansch MA. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ. 2004;82:16–23.
- Yip CCY, Lau SKP, Woo PCY, Yuen K-Y. Human enterovirus 71 epidemics: what’s next? Emerg Health Threats J. 2013;6:19780–19780. doi:10.3402/ehtj.v6i0.19780.
- Mandary MB, Poh CL. Changes in the EV-A71 genome through recombination and spontaneous mutations: impact on virulence. Viruses. 2018;10:320. doi:10.3390/v10060320.