ABSTRACT
Background
Short-term dynamic changes in neutralizing antibodies against EV71 and EV71-IgM after inactivated EV71 vaccine injection are unknown.
Methods
This study was designed as a randomized, open-label study and was registered at ClinicalTrials.gov (NCT03278132). In total, 120 healthy infants aged 6–35 months were randomized 1:1:1 to provide a second blood sample on day 10, day 20, or day 30 after the first vaccine dose, respectively.
Results
According to the per-protocol set, a rapid immune response against EV71 was observed 10 days after the first EV71 vaccine dose, with antibody titers ≥1:8 in 89.19% of participants (95% CI: 74.58–96.97%) on day 10, in 80.65% (95% CI: 62.53–92.55%) on day 20, in 66.67% (95% CI: 49.03–81.44%) on day 30, and in 100% (95% CI: 96.52%-.) on day 60. Based on an ELISA, the percentages of participants positive for EV71-IgM on day 0 and day 60 were 1.71% (2 out of 117) and 82.86% (87 out of 105), respectively.
Conclusions
The EV71 vaccine could be used for contingency vaccination to further control EV71-associated disease outbreaks. Caution should be taken in using the EV71-IgM test for rapid EV71 infection diagnosis after EV71 vaccine administration.
Clinical Trial Registration
ClinicalTrials.gov NCT03278132
KEYWORDS:
Introduction
Hand, foot and mouth disease (HFMD) is commonly reported in children under 5 years across East and Southeast Asia during the hot and humid season.Citation1 Based on the Chinese National Enhanced Surveillance System, annual HFMD outbreaks have occurred since 2008, and 18.2 million HFMD cases and 3.6 thousand deaths associated with HFMD have been reported in mainland China.Citation2 Enterovirus 71 (EV71) and coxsackievirus A16 (CA16) are the most common pathogens causing HFMD, while EV71 is more neurotropic than CA16.Citation3-Citation7 According to reports from large-scale prospective studies performed in Sarawak,Citation8,Citation9 10–30% of hospitalized EV71-associated HFMD patients develop neurological system complications. The results based on national HFMD surveillance during 2008–2015 revealed that 44% of the laboratory-confirmed HFMD cases in mainland China were caused by EV71; moreover, 74% of severe cases and 93% of fatal HFMD cases were caused by EV71.Citation10 EV71 vaccination is the most effective approach for preventing EV71-associated HFMD outbreaks.
Mainland China is the only country worldwide that has licensed vaccines against EV71 since 2015. Since then, a series of studies have been conducted on immunization strategies involving the licensed inactivated EV71 whole-virus vaccine. Sustained high protection against EV71-associated HFMD and persistent immunity were reported based on extended follow-up studies.Citation11-Citation13 Moreover, some reports have focused on the correlates of protection for the inactivated EV71 vaccine,Citation14,Citation15 EV71 disease burden,Citation16 booster immunization strategy,Citation17 and simultaneous administration of the EV71 vaccine with other vaccines.Citation18 However, to date, no data on the short-term dynamic changes in neutralizing antibodies (Nabs) against EV71 after vaccination have been reported.
To further verify the immunogenicity and safety of a licensed EV71 vaccine (Sinovac) after marketing and to preliminarily explore the short-term dynamic changes in Nabs against EV71 after vaccination, we conducted this randomized, open-label study.
Materials and methods
Study design and participants
We conducted a randomized, open-label study in Shangyu district, Shaoxing city, Zhejiang Province from July to September 2017. The study was approved by the ethics committee of the Zhejiang Provincial Center for Disease Control and Prevention and performed by the investigators in accordance with the Declaration of Helsinki of the World Medical Association and Good Clinical Practice. The study was registered at ClinicalTrials.gov (NCT03278132).
Healthy infants and children aged 6–35 months were recruited. Those with a history of HFMD, acute febrile disease, current immunosuppressive therapy, immunodeficiency or history of an allergy to any vaccination or drugs on the day of enrollment were excluded.
Procedures
Written informed consent was obtained from the legal guardians of each participant before beginning any procedure. Eligible participants were assessed through medical history inquiry and physical examinations. After obtaining first blood samples from all the participants before injection, the EV71 vaccine was administered according to a 2-dose schedule (on a 0- and 30-day schedule). In addition, all of the enrolled participants were randomly assigned into 3 groups in a ratio of 1:1:1 to provide the second blood sample on day 10 (group 1), day 20 (group 2), or day 30 (group 3) after first dose, respectively, and the randomization was carried out according to a randomization list (block size = 6) prepared by an independent statistician using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The random allocation of the second blood sample timepoint for each participant was sealed in an envelope with a unique number on the cover that corresponded to the participant code according to the random assignment table. The envelope was not be opened unless the corresponding unique number was assigned to a participant. Persons involved in the randomization procedure did not take part in any other study process.
After obtaining the first blood samples from all the participants in the 3 groups before injection, the EV71 vaccine was administered according to a 2-dose schedule (on a 0- and 30-day schedule). In addition, second blood samples were collected on a 10-day (group 1), 20-day (group 2) and 30-day (group 3) schedule. Finally, third blood samples were collected from all participants 30 days after the second vaccination.
Participants were observed for immediate adverse events for at least 30 minutes after each injection, and the axillary temperature during the following three days and any adverse events occurring within 30 days were recorded by the participants’ guardians on diary cards. Adverse events were graded according to a scale issued by China Food and Drug Administration.Citation11 All serious adverse events (SAEs) during the study period were documented and assessed.
Vaccine and immunogenicity assessment
The Vero cell-based inactivated human EV71 vaccine used in this study was developed by Sinovac Biotech Co., Ltd. (Beijing, China), using the EV71 strain H07 (subgenotype C4) as the seed virus.Citation11 The EV71 vaccine contained 400 U of EV71 antigen with alum adjuvant and was packaged in syringes (0.5 ml per vial).
Measurement of Nabs in sera was performed by Sinovac Biotech Co., Ltd., using a modified cytopathogenic effect assay.Citation19 In addition, immunoglobulin M against EV71 (EV71-IgM) was measured in the pre- and postvaccination serum using enzyme-linked immunosorbent assay (ELISA) with WANTAI EV71-IgM ELISA, a commercial diagnostic kit for IgM antibody to human enterovirus 71.
Statistics
The primary endpoint for immunogenicity assessment was the proportion of participants with an EV71 Nab titer greater than 1:8 that was induced by the two-dose EV71 vaccination. The percentage was established to be greater than 90% based on the premarketing clinical trial results. A sample size calculation by PASS software (version 8.0) with a two-sided α value of 0.05 indicated that 97 participants would be sufficient to provide at least 90% power, ensuring an allowable error no larger than 6% between the proportions of participants with an EV71 Nab titer greater than 1:8 thirty days after two EV71 vaccine doses based on the sample population and overall population.
The primary immunogenicity analysis were run on the per-protocol set (PPS), which included all enrolled subjects who received two-dose vaccinations according to the protocol, provided assessable serum samples both before and 30 days after vaccination, and had no major protocol deviations. EV71 Nab titers were transformed into log10 titers for geometric mean titer (GMT), geometric mean increase (GMI) and 95% confidence interval (CI) calculation. Titers that were lower than 1:8 or higher than 1:16384 were assigned a value of 1:4 or 1:16384, respectively.
For safety assessment, the overall incidences of unsolicited and solicited injection-site and systemic adverse reactions within 30 days after each dose, with 95% CIs, were evaluated in all individuals who received at least 1 vaccine dose.
The chi-square test or Fisher’s exact test was used to analyze categorical data. A paired t test, an ANOVA, or the Kruskal-Wallis test was used for continuous data analysis. Statistical analyses were performed using SAS 9.1. All tests were two-sided, and statistical significance was defined as P < .05.
Results
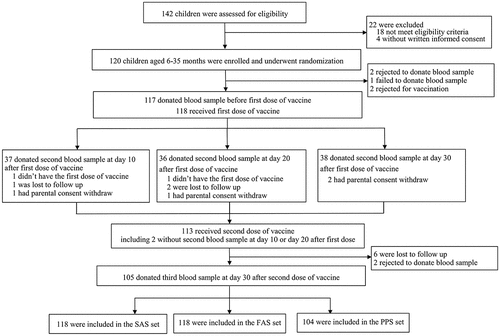
shows the study profile. In total, 142 participants were assessed for eligibility, and of these, 4 children without written informed consent and 18 that did not meet the eligibility criteria were excluded. Ultimately, 120 children aged 6–35 months were enrolled, consisting of 40 children aged 6–12 months, 40 children aged 13–23 months and 40 children aged 24–35 months. In total, 118 participants received the first dose, and 113 participants received the second dose. All participants completed the 30-day safety follow-up after each dose. The serum sample collection rates before the first injection and 30 days after the second injection were 97.5% and 87.5%, respectively. The second serum sample was obtained from 37 (92.5%) participants, 36 (90.0%) participants, and 38 (95.0%) participants on day 10, day 20, and day 30, respectively, after the first injection. The demographic characteristics of the participants are shown in .
Table 1. Demographic characteristics of participants.
Immunogenicity
In total, 104 (86.67%) participants were eligible for the primary immunogenicity analysis in the PPS. Four participants (2 in group 1, 1 in group 2, 1 in group 3) showed a prevaccination antibody titer greater than 1:8, and the prevaccination positive rate for anti-EV71 antibodies was 3.85% (95% CI: 1.06–9.56%), while the prevaccination GMT was 4.97 (95% CI: 4.01–6.16). After two EV71 vaccine doses, the postvaccination proportion of participants with an antibody titer ≥1:8 was 100% (95% CI: 96.52%-.), and the EV71 antibody GMT post-vaccination was 97.13 (95% CI: 75.47–125.00), both of which were statistically higher than the prevaccination values (p < .0001). Seroconversion was observed in 102 participants, with a seroconversion rate of 98.08% (95% CI: 93.23–99.77%).
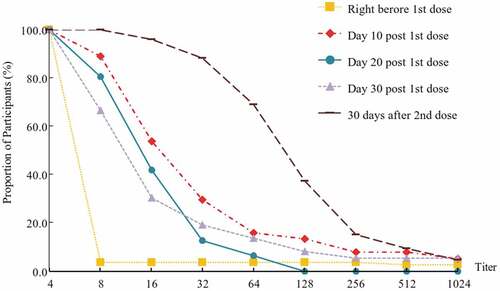
A rapid immune response was observed on day 10 after the first dose, with antibody titers ≥1:8 in 89.19% of participants (95% CI: 74.58–96.97%). The proportions of participants with antibody titers ≥1:8 were 80.65% (95% CI: 62.53–92.55%) and 66.67% (95% CI: 49.03–81.44%) on days 20 and 30, respectively, indicating a decreasing trend in the anti-EV71 antibody titer from day 10 after the first injection (, ). A similar trend was also indicated by the seroconversion rate. GMTs were observed to be 24.59 (95% CI: 14.06–43.02), 12.85 (95% CI: 9.68–17.05) and 13.83 (95% CI: 8.07–23.71) on day 10, day 20 and day 30, respectively. A significant difference in GMT was observed among the three groups (p = .048), and further analysis suggested a significant difference between day 10 and day 30 (p = .042).
Table 2. Immune response to EV71 vaccine after vaccination, in the per-protocol-set.
Safety
No immediate allergic reaction within 30 minutes after injection was observed. During the 60-day follow-up after two EV71 vaccine injections, 70.34% (95% CI: 61.23–78.39%) of participants reported at least one adverse event, and 51.69% (95% CI: 42.31–60.99%) of participants reported at least one solicited adverse reaction. Fifty out of 118 (42.37%, 95% CI: 33.33–51.81%) participants reported at least one solicited adverse reaction within 30 days after the first injection, while 24 out of 113 (21.24%, 95% CI: 14.11–29.93%) participants reported a solicited adverse reaction after the second injection. The incidence of injection-site reactions was 11.86% (95% CI: 6.64–19.10), and the incidence of systemic reactions was 47.46% (95% CI: 38.19–56.85%). Fever was the most commonly reported systemic symptom (34.75%, 95% CI: 26.22–44.06%), while erythema was the most common injection-site symptom (). Most of the reported adverse reactions were mild or moderate, and only 1 participant with a grade 3 allergic reaction was recorded. No SAEs were reported.
Table 3. Frequency of solicited adverse reactions within 30 days after injection of EV71 vaccine.
Results of the EV71-IgM test
The results indicated an interesting phenomenon: a certain proportion of participants (91.89%, 34 out of 37) showed EV71-IgM positivity 10 days after the first dose, while only 2 out of 117 participants (1.71%) were EV71-IgM positive immediately before the first dose. The EV71-IgM that appeared after vaccination may exist for a certain time, with the percentages of participants with EV71-IgM positivity at 100.0% (36 out of 36) and 92.0% (35 out of 38) on day 20 and day 30 after the first vaccine dose, respectively. A total of 87 out of 105 (82.86%) participants showed EV71-IgM positivity 30 days after the second vaccine dose.
Discussion
This report is the first study to focus on the short-term dynamic changes in Nab and IgM against EV71 after EV71 vaccine injections. The immunogenicity results showed a rapid immune response induced by the first inactivated EV71 vaccine dose, which was similar to the results for the inactivated hepatitis A vaccine observed in other studies,Citation20,Citation21 indicating that inactivated vaccines may induce a fast immune response after vaccination. According to the previous phase 3 clinical trials of the EV71 vaccine, a titer of 1:16 could be correlated with the vaccine’s protection against EV71-associated diseases.Citation13 In this study, approximately half of the recipients were observed to rapidly acquire protection against EV71-associated diseases only 10 days after the first injection. The robust and rapid immune response induced 10 days after the first dose indicated the possibility of using the EV71 vaccine for contingency vaccination to further control EV71-associated disease outbreaks. A decreasing trend in the EV71 antibody titer was observed based on the serum testing data on day 10, day 20 and day 30 after the first dose, while the GMT on day 30 was significantly lower than that on day 10. This result was similar to the result derived from a previous phase 2 clinical trial,Citation22 indicating the need for complying with the two-dose regimen.
The EV71-IgM test performed on serum is usually used for rapid diagnosis of recent infection with EV71.Citation1,Citation23 Recently, queries have arisen from guardians of HFMD patients who received the EV71 vaccine and had positive EV71-IgM results in mainland China. However, according to the extrafollicular antibody response theory,Citation24 IgM may persist for a certain time after primary vaccine injection, which was also observed in this study. The data in this study indicate that using the EV71-IgM test for rapid EV71 infection diagnosis after EV71 vaccine administration would be inappropriate or at least that caution should be taken.
The safety and immunogenicity results in this study were consistent with a previous large-scale phase 3 clinical trial,Citation13 which may be considered validation of the adequate safety and immunogenicity of the Sinovac inactivated EV71 vaccine. Furthermore, as the same safety data collection method was used in this study and the previous large-scale phase 3 clinical trial, the safety results were highly consistent between the two studies, with 51.69% participants in this study reporting at least one solicited adverse reaction, while the proportion was 51.4% in the previous phase 3 clinical trial. The most commonly reported systemic symptom observed in both studies was fever (34.8% in this study vs 34.7% in the previous phase 3 study), while the most common injection-site symptom in both studies was erythema (8.47% in this study vs 6.7% in the previous phase 3 study), indicating the good practice of this study and the reliability of the results.
The main study limitation was that the sample size was relatively small; in fact, only 120 participants were enrolled, with 40 participants in each group, according to the protocol based on a statistical calculation. However, considering the high proportion of participants with antibody titers ≥1:8 and a relatively narrow confidence interval on day 10 after the first injection, as well as the good consistency of the data in this study with data reported in previous phase 2 and 3 clinical trials,Citation13,Citation22 the result revealed in this study are reliable based on such a small sample size. As no control group was set, which could be another study limitation, providing a reasonable explanation for the emergence and maintenance of EV71-IgM after vaccination with the EV71 vaccine is difficult, and further studies are needed in the future.
Disclosure of potential conflicts of interest
Zeng Gang and Zeng Ji are employees of Sinovac Biotech Co., Ltd., which was a sponsor of this study. The other authors do not have any commercial or other association that might pose a conflict of interest.
Author Contributions
Conceptualization: Wang SY, Zeng G, Lv HK
Data curation: Wang SY, Zeng G, Zhang XP, Gan ZK, Fan JQ, Zeng J, Chen YP, Liang ZZ, Hu XS
Formal analysis: Wang SY, Zeng J
Investigation: Wang SY, Zhang XP, Gan ZK, Fan JQ, Chen YP, Liang ZZ, Hu XS, Lv HK
Methodology: Wang SY, Zeng G, Lv HK
Supervision: Lv HK, Zeng G
Writing - original draft: Wang SY
Writing - review & editing: Wang SY, Zeng J, Zhang XP, Gan ZK, Fan JQ, Chen YP, Liang ZZ, Hu XS, Zeng G, Lv HK
Disclaimer
The opinions expressed in this manuscript are those of the authors and may not necessarily reflect those of Sinovac Biotech Co., Ltd. All coauthors approved the final version of the manuscript.
Acknowledgments
We thank all the investigators from the Zhejiang Provincial Center for Disease Control and Prevention and Shangyu District Center for Disease Control and Prevention.
Additional information
Funding
References
- World Health Organization Western Pacific Region. A guide to clinical management and public health response for hand, foot and mouth disease (HFMD). Manila: World Health Organization Western Pacific Region; [accessed 2019 Apr 10]. https://iris.wpro.who.int/bitstream/handle/10665.1/5521/9789290615255_eng.pdf.
- Chinese center for disease control and prevention. National notifiable disease reported system (HFMD) from 2008 to 2017. http://www.chinacdc.cn/jkzt/crb/bl/szkb/cbw_2274/. Accessed 10 April 2019.
- Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics. 2007;120:e244–52. doi:10.1542/peds.2006-3331.
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang J-R, Shih S-R. An epidemic of enterovirus 71 infection in Taiwan. Taiwan enterovirus epidemic working group. N Engl J Med. 1999;341:929–35. doi:10.1056/NEJM199909233411301.
- Lin TY, Chang LY, Hsia SH, Huang YC, Chiu CH, Hsueh C, Shih S-R, Liu -C-C, Wu M-H. The 1998 enterovirus 71 outbreak in Taiwan: pathogenesis and management. Clin Infect Dis. 2002;34(Suppl 2):S52–7. doi:10.1086/338819.
- Lin TY, Twu SJ, Ho MS, Chang LY, Lee CY. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg Infect Dis. 2003;9:291–93. doi:10.3201/eid0903.020285.
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang Y-T, Yao X, Chu K, Chen Q-H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–32. doi:10.1016/S0140-6736(13)61049-1.
- Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, Clear D, Del Sel S, Chieng CH, Tio PH, Cardosa MJ, et al. Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infect Dis. 2009;9:3. doi:10.1186/1471-2334-9-3.
- Ooi MH, Wong SC, Podin Y, Akin W, Del Sel S, Mohan A, Chieng C, Perera D, Clear D, Wong D, et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis. 2007;44:646–56. doi:10.1086/522508.
- An ZJ, Liu Y, Liao QH, et al. Guidelines for use of inactivated enterovirus type 71 vaccine. Chin J Vacc Immun. 2016;6(4):458–64.
- China Food and Drug Administration. The standard guidelines for adverse reactions grading of vaccine clinical trials. [accessed 2019 Apr 10]. http://wwwsdagovcn/WS01/CL0844/9350_5html.
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–37. doi:10.1056/NEJMoa1303224.
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–28. doi:10.1056/NEJMoa1304923.
- Hu Y, Zeng G, Chu K, Zhang J, Han W, Zhang Y, Li J, Zhu F. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: A further observation. Hum Vaccin Immunother. 2018;14:1517–23. doi:10.1080/21645515.2018.1442997.
- Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, Xia JL, Li J, Zhu FC. Two-year efficacy and immunogenicity of Sinovac enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15:129–37. doi:10.1586/14760584.2016.1096782.
- Wei M, Meng F, Wang S, Li J, Zhang Y, Mao Q, Hu Y, Liu P, Shi N, Tao H, et al. 2-year efficacy, immunogenicity, and safety of vigoo enterovirus 71 vaccine in healthy chinese children: a randomized open-label study. J Infect Dis. 2017;215:56–63. doi:10.1093/infdis/jiw502.
- Jin P, Li J, Zhang X, Meng F, Zhou Y, Yao X, Gan Z, Zhu F. Validation and evaluation of serological correlates of protection for inactivated enterovirus 71 vaccine in children aged 6–35 months. Hum Vaccin Immunother. 2016;12:916–21. doi:10.1080/21645515.2015.1118595.
- Zhang Z, Liang Z, Zeng J, Zhang J, He P, Su J, Zeng Y, Fan R, Zhao D, Ma W, et al. Immunogenicity and safety of an inactivated enterovirus 71 vaccine administered simultaneously with hepatitis B vaccine and group A meningococcal polysaccharide vaccine: a phase 4, open-label, single-center, randomized, noninferiority trial. J Infect Dis. 2019;220:392–99. doi:10.1093/infdis/jiz129.
- Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, Gao F, Yang L, Yao X, Shao J, et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy Chinese infants and children. PLoS One. 2013;8:e79599. doi:10.1371/journal.pone.0079599.
- Ren YH, Chen JT, Wu WT. Evaluation on different immunization schedules of hepatitis A vaccine healive. Chin J Vaccines Immun. 2003;9:219–21.
- Wang DM, Shi ZH, Zhu HM. The observation of antibody dynamic in children who inactivated hepatitis A vaccine Chinese. J Vaccines Immunization. 2010;16(1):4–4.
- Li YP, Liang ZL, Xia JL, Wu JY, Wang L, Song LF, Mao Q-Y, Wen S-Q, Huang R-G, Hu Y-S, et al. Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: a phase II, randomized, double-blind, placebo-controlled trial. J Infect Dis. 2014;209:46–55. doi:10.1093/infdis/jit429.
- National Health Commission of People’s Republic of China. The guidelines for the diagnosis and treatment of hand foot and mouth disease; 2018. edition.
- MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi:10.1034/j.1600-065X.2003.00058.x.