ABSTRACT
In 2017, Pennsylvania amended school-entry vaccination requirements including reduction of the provisional period from eight months to the first five days of school and requirement of meningococcal-conjugate vaccine (MCV4) for students entering 12th grade. This cross-sectional study evaluates the impact of these new requirements on clinical practice and vaccination rates among requirement-eligible adolescents within a large pediatric network in metropolitan Philadelphia. We surveyed providers from 24 pediatric primary care facilities across five Southeastern Pennsylvania counties to assess strategies for timely vaccination of children, facilitators and barriers to implementation of these strategies, and attitudes toward the new school vaccine requirements. Vaccination rates post-five-day grace period among eligible 12–18-year-old adolescents were calculated using aggregate electronic health record data and compared pre- and post-policy implementation (2016 vs. 2017) using two-sample tests of proportion. Overall, providers were supportive of the new vaccination requirements and reported that their facilities were equipped to accommodate the increased demand for vaccination visits prior to the beginning of the school year. There were modest increases in Tdap and MCV4 vaccination rates among 12–13-year-old adolescents by mid-September and a significant increase for MCV4 among 17–18-year-old adolescents (p > .001) in all regions. There were also statistically significant increases (p > .001) in MenB and HPV vaccination rates in this older age group. Our results suggest that these amended school-entry vaccination requirements may help improve timely vaccination rates for both required and non-required vaccines, increasing protection among students at the beginning of the school year.
Introduction
The Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) recommends evidence-based childhood vaccinations and vaccination schedules.Citation1 However, recommendations are not enforceable, and the federal government has little leverage to require specific vaccination uptake and adherence as there is no federal enforcement mechanism for noncompliance.Citation2,Citation3 Instead, each state is individually responsible for establishing childhood vaccination regulations such as through school-entry requirements.Citation2,Citation3
Generally, states apply ACIP recommendations as requirements for school-entry, and as of 2018, all 50 states and the District of Columbia required that children be vaccinated before entering kindergarten for the following: diphtheria, tetanus, and pertussis (DTaP); polio; measles and rubella; and varicella (chickenpox).Citation4,Citation5 Almost all states also require vaccination against mumps and hepatitis B.Citation4,Citation5 School-entry vaccination mandates have historically been successful at increasing vaccine uptake and vaccination coverage rates.Citation3,Citation6,Citation7 However, under certain circumstances, children may be allowed to attend school without the required vaccinations, and as such, all states grant exemptions from required vaccinations for medical reasons.Citation4 Almost all (46) states also grant non-medical exemptions for religious or philosophical/personal belief reasons.Citation4,Citation8–Citation10 States may also permit provisional enrollment of non-compliant students while completing the vaccination schedule.Citation11 Prior to 2017, Pennsylvania permitted provisional enrollment – school attendance without complete vaccination or in process of completing a catch-up vaccine schedule – of students for up to 8 months after the start of school.Citation11,Citation12
Children with exemptions from required vaccinations or provisional enrollment without full vaccination compliance are under-vaccinated and may contribute to population vaccination rates below Healthy People 2020 goals to achieve herd immunity.Citation13,Citation14 To address this issue, the state of Pennsylvania enacted new school-entry vaccination requirements for school entry for the 2017–2018 school year.Citation15,Citation16 The most significant changes include:
Replacement of the 8-month provisional period with a 5-day provisional period during which all required doses must be received and documented (unless the student has an exemption or it is medically inappropriate)
New requirement for the second dose of meningococcal conjugate vaccine (MCV4) for entering 12th graders (received at/after 16)
Children who do not comply with the new requirements risk exclusion from school.Citation15
The new school-entry vaccination requirements are intended to ensure that a higher proportion of students are up-to-date with required vaccines and fully protected at the start of school. However, the impact of these changes on vaccination and exemption rates as well as on primary care service delivery have not been systematically evaluated. For example, due to potential barriers to getting their child vaccinated in time to start school, some parents may be inclined to request an exemption rather than risk exclusion, especially since non-medical exemptions are permitted and relatively easy to obtain in Pennsylvania.Citation17 Primary care providers may also be faced with increased demand for vaccination services prior to the beginning of the school year and may also need to implement more reminder-recall activities to identify patients at risk for noncompliance.
The primary objectives of this study were to (1) evaluate implementation of the new Pennsylvania school-entry vaccination requirements from the perspective of clinicians at primary care locations and (2) to evaluate the impact of the new school-entry vaccination requirements on timeliness of up-to-date vaccination rates for school-age adolescents in metropolitan Philadelphia, a region comprised of several different school districts.
Materials and methods
Health-care provider survey
This cross-sectional study used a web-based survey instrument distributed to primary care providers to understand: (1) strategies used by primary care providers to immunize children with required vaccines after the new school-entry vaccination requirements; (2) facilitators and barriers to implementation of these strategies; and (3) attitudes toward the new school-entry requirements. The study population consisted of primary care pediatricians, lead nurses, and practice managers at 24 Children’s Hospital of Philadelphia (CHOP) Pennsylvania primary care sites located in 19 different cities and townships in metropolitan Philadelphia. The survey instrument was administered and distributed via e-mail using REDCap (Research Electronic Data Capture), an encrypted online tool used to distribute surveys and manage databases, to health-care providers and office staff (n = 255). Anonymous completion of the survey indicated consent to participate, and a $5 coffee gift card was offered as appreciation for participation to those who wished to provide their e-mail. The survey instrument was available from June 11 to October 1, 2018 during which time three additional reminders were sent. The survey instrument included the following domains: demographics, practice characteristics, attitudes toward new school-entry requirements, strategies used to implement school-entry requirements within practices, challenges and facilitators to implementation, costs of implementation, and perceived acceptability among parents (Appendix A). For some questions, Likert scales were used to assess agreement with statements.Citation18 All survey responses were collected anonymously.
Descriptive statistics, including frequencies for categorical variables and mean, median, standard deviation, and range for continuous variables, were used to characterize the data.
Vaccination rates
Up-to-date vaccination rates were measured using aggregate electronic health record (EHR) data from participating CHOP primary care sites before and after implementation of the new school-entry vaccination requirements. Aggregate rates were measured as of September 15 in both 2016 and 2017, and individual practices were grouped into the following classifications: New Jersey (Atlantic, Burlington, Camden, and Cape May), Philadelphia county, or Suburbs (including practices in Bucks, Chester, Delaware, and Montgomery counties). New Jersey practices were included as a control since the policy change was only implemented in the state of Pennsylvania. Specifically, up-to-date rates by age were measured for tetanus, diphtheria, and acellular pertussis vaccine (Tdap) and MCV4 vaccine for children ages 12–18 years, stratified by age group (12–13 years, 14–16 years, and 17–18 years old). The 12–13-year-old age group was used as a proxy for 7th-grade school entry, and the 17–18-year-old age group was used as a proxy for 12th-grade school entry. Other vaccines, specifically human papillomavirus vaccine (HPV9) and meningococcal serogroup B vaccine (MenB), were also measured to evaluate the potential impact of the new requirements on recommended vaccines that are not required for school-entry. Differences in vaccination rates were characterized before and after implementation of the school requirement by age group. Specifically, the proportion up-to-date for required vaccines for 1 year before (2016) and the year of (2017) introduction of the new requirements were compared using two-sample tests of proportion. Up-to-date was defined by eligible patients who had received all recommended, valid doses of each vaccine before or during the following time frames: (a) September 2016: January 1 – September 15, 2016 and (b) September 2017: January 1 – September 15, 2017. September 15 was chosen as the start of the school cutoff date to accommodate varying school start dates as well as the five-day grace period. All analyses were performed using StataSE 15.1.
This study was deemed exempt by CHOP’s Internal Review Board.
Results
Provider survey results
Among 255 providers invited to participate, 107 (42% response rate) completed the survey instrument including at least two providers or office staff from each participating clinic (range 2–20). The majority (78%) of survey respondents indicated their role was clinical, either medical doctors or nurse practitioners ().
Table 1. Demographic information of survey respondents
Awareness, communication, and attitudes toward requirements
Most respondents learned about the new school-entry vaccination requirements by a notice from the Pennsylvania Department of Health (31%) or a notice from the Children’s Hospital of Philadelphia (21%). Other sources of information included other clinic staff (16%), notice from the county Department of Health (12%), and other parents (9%). Infrequently (<12%), respondents cited letters from the schools of their own children, school nurses, professional organizations, and the news media. Respondents were able to select multiple options for sources of information.
One third of the responses indicated that their primary care practice did not directly communicate with parents in general about vaccines required for school or send information about new requirements (34%). For those who indicated that they do communicate with parents, the most common methods of communication were phone calls (25%) or in-person during office visits (14%). Only 13% of responses indicated that the respondent did not know about the communication strategy. Other methods of communication included letters (7%) and website updates (5%). For the new 2017–2018 school-entry vaccination requirements, about a third (31%) of survey respondents indicated that they did not know about any additional outreach activities performed by their clinic and 30% indicated that no outreach communication about the new requirements was performed. Among those whose primary care site did perform outreach about the new school-entry vaccination requirements, communication methods included phone calls (20%) as well as posters/fliers in waiting room (11%), letters (4%), and website updates (2%). In-person during office visits was not mentioned as a common communication method for the new requirements.
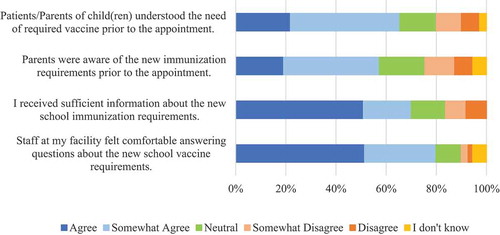
The majority of respondents strongly or somewhat agreed (79%) that “Staff at my facility felt comfortable answering questions about the new school vaccine requirements.” (). The majority of respondents strongly or somewhat agreed (70%) with the statement “I received sufficient information about the new school immunization requirements.” (). Over half of respondents strongly or somewhat agreed (57%) that “Parents were aware of the new immunization requirements prior to the appointment.” (). The majority of respondents strongly or somewhat agreed (66%) with the statement “Patients/Parents of child(ren) understood the need of required vaccines prior to the appointment.” ().
When asked if parents of patients mentioned any of the following communication strategies, 73% of respondents indicated that parents mentioned prompts from their child’s school to get back-to-school vaccinations, compared to 14% of respondents indicating parents mentioned reminder phone calls and 13% who reported that parents mentioned reminder letters.
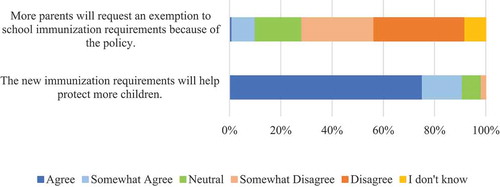
The majority of respondents strongly or somewhat agreed that the new requirements will help protect more children (91%) ().
Practice implementation strategies
A quarter of total respondents (25%) indicated that their practice did not engage in any new activities to meet the new 2017–2018 school-entry vaccination requirements. For respondents (N = 66; 75%) who did indicate practice engagement, the following activities were the most commonly selected: additional staff time for immunization visits (20%), reminder-recall phone calls (16%), extended hours for immunization-only visits (15%), and extended staff time to identify children who were not up-to-date for required vaccines (12%).
When asked which activities their practice would use for the 2018–2019 school year to meet the new requirements, 27% of total participants reported that no new activities would be used. For respondents (73%) who did plan practice engagement, the following activities were the most commonly selected: additional staff time for immunization visits (19%), reminder-recall phone calls (14%), and extended hours for immunization-only visits (14%).
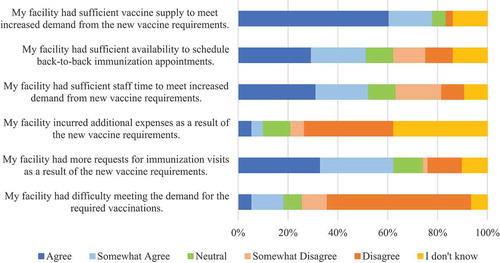
The majority of respondents strongly or somewhat agreed (62%) with the statement “My facility had more requests for immunization visits as a result of the new vaccine requirements” (). However, the majority strongly or somewhat disagreed (68%) that “My facility had difficulty meeting the demand for the required vaccinations” (). The majority of respondents did not know whether (38%) or disagreed (36%) that their facility incurred additional expenses after the new vaccine requirements (). About half of respondents strongly or somewhat agreed (52%) with the statement “My facility had sufficient staff time to meet increased demand from new vaccine requirements” while a similar proportion strongly or somewhat agreed that their facility had sufficient availability to schedule back-to-back immunization appointments” (). Most respondents strongly or somewhat agreed (78%) that their facility had sufficient vaccine supply to meet increased demand from the new vaccine requirements ().
Between publication of the new school-entry vaccination requirements in the spring 2017 and the beginning of the 2017–2018 school year, most respondents answered that the average amount of time between an appointment request and the appointment date for a well visit was more than 2 months (34%) or 1–2 months (25%) while a quarter indicated that they did not know the average timeframe. On the other hand, for that same time period, most respondents answered that the average amount of time between an appointment request and the appointment date for an immunization-only visit was immediately (33%) or 1–2 weeks (42%). When asked about offering recommended, not required, vaccines at the back-to-school immunization visit, the following percentages of respondents indicated that they “Always” offer the vaccine: HPV-9 (88%), MenB (82%), and Hepatitis A (82%) vaccines.
Quotes from survey respondents
Providers and practice managers were also asked to provide additional comments related to the introduction and implementation of Pennsylvania’s new school-entry vaccination requirements. Some respondents mentioned, “For the most part the Pennsylvania school requirements were already met by all our patients” while others mentioned that “it was absolute madness trying to get these kids in” (). Respondents who found that the requirements led to additional workload mentioned scheduling issues and labor costs as the most common challenges their practice faced. One comment noted an unintended consequence of scheduling related to this requirement: “I feel this has caused children who are not up to date with well visits to come in for vaccines only and then some do not return for their annual well check.”
Table 2. Additional comments from survey respondents about implementation
Respondents also noted schools’ limited communication with primary care practices stating “This would have been a great opportunity for collaboration to help keep the patients [up-to-date] and safe … ” and “In many cases, the parent did not know what was due. In some cases, the records of the school were not updated.”
While many respondents generally were “glad PA is finally taking a stronger position on making sure kids are properly immunized,” multiple respondents questioned the usefulness and introduction of the new requirements. One comment stated, “I think the letters that came out to families from the school district were too harsh regarding the new vaccine requirements. Most of the families that were affected are families that already believe in vaccines and want to do the right thing. It seems funny to harass that group of parents, when PA still allows other parents to simply write a letter saying they philosophically disagree.”
Vaccination up-to-date rates
Required vaccines for students entering 7th grade: Tdap and MCV4
One dose of Tdap and one dose of MCV4 are required for all students entering 7th grade.Citation19 For all Pennsylvania practices, the proportion of eligible 12–13-year-old children who received one dose of Tdap and MCV4 vaccines by September 15 increased slightly between 2016 and 2017 (89.7% to 90.7% for Tdap and 89.7% to 90.9% for MCV4). Within Philadelphia county practices, the proportion of eligible 12–13-year-old children who received one dose of Tdap and MCV4 vaccines by September 15 of 2016 vs. 2017 increased from 86.9% to 88.5% for Tdap (p = .018) and 86.9% to 88.7% for MCV4 (p = .008). Among suburban practices, there was no significant increase for Tdap (91.0% to 91.7%, p = .079) while MCV4 rates increased from 91.1% to 92.0% (p = .022). However, at the CHOP primary care practices in New Jersey that are outside the purview of these new vaccine requirements, the proportion of eligible 12–13-year-old children who received one dose of Tdap or MCV4 vaccine by September 15 slightly decreased (91.9% to 89.9%, p = .021 and 92.2% to 90.0%, p = .010, respectively).
Required vaccines for students entering 12th grade
An additional booster dose of MCV4 vaccine after 16 years of age is required in Philadelphia for all students entering 12th grade.Citation19 For all Pennsylvania practices, the proportion of eligible 17–18-year-old adolescents who received the second dose of MCV4 vaccine by September 15 increased from 71.4% in 2016 to 78.0% in 2017. Among Philadelphia county practices, the proportion of eligible 17–18-year-old adolescents who received MCV4 in 2016 vs. 2017 increased from 69.8% to 74.2% (p < .001) with a similar significant increase among suburban practices (72.1% in 2016 to 79.8% in 2017, p < .001) (). For New Jersey practices, the proportion of eligible 17–18-year-old adolescents who had received a second dose of MCV4 vaccine by September 15 also significantly increased from 62.2% in 2016 to 69.0% in 2017 (p < .001).
Table 3. Vaccine up-to-date proportions in 2016 vs. 2017 by vaccine, age, county, and dose
Recommended but not required vaccines: MenB and HPV
Two doses of MenB vaccine are recommended using shared clinical decision-making for adolescents 16–23 years of age, with a preference at 16–18 years of age.Citation20 For all Pennsylvania practices, the proportion of eligible 17–18-year-old adolescents who had received the first dose of the MenB vaccine by September 15 significantly increased from 15.5% in 2016 to 46.8% in 2017 (p < .001). Among Philadelphia county practices, the proportion of eligible 17–18-year-old adolescents who received one dose of the MenB vaccine by September 15 increased from 10.2% in 2016 to 40.6% in 2017 (p < .001) (). Within Suburban practices, the proportion of eligible 17–18-year-old adolescents who had received one dose of MenB vaccine by September 15 increased from 18.1% in 2016 to 49.8% in 2017 (p < .001) (). Furthermore, for all New Jersey practices, the proportion of eligible 17–18-year-old adolescents who received one dose of MCV4 vaccine by September 15 also increased from 5.9% in 2016 to 31.7% in 2017 (p < .001) ().
A two-dose series of HPV vaccine is recommended for children ages 9–14 at initial vaccination, preferably at ages 11–12 years, and a three-dose series of HPV vaccine is recommended for children ages 15+ at initial vaccination.Citation20 For all Pennsylvania practices, the proportions of eligible 12–13-year-old children who received the first dose of HPV vaccine by September 15 increased from 50.3% in 2016 to 59.6% in 2017. Among Philadelphia county practices, the proportion of eligible 12–13-year-old children who received a first dose of HPV vaccine by September 15 increased from 71.8% in 2016 to 77.9% in 2017 (p < .001) (), while among suburban practices, the proportion of eligible 12–13-year-old children who received the first dose of HPV vaccine by September 15 increased from 40.3% in 2016 to 51.0% in 2017 (p < .001) (). Similarly, for all New Jersey practices, the proportion of eligible 12–13-year-old children who received the first dose of HPV vaccine by September 15 increased from 21.4% in 2016 to 35.7% in 2017 (p < .001) ().
For all Pennsylvania practices, the proportions of eligible adolescents 17–18 years of age who initiated (first dose) or completed (second and/or third dose) the series of HPV vaccine by September 15 increased from 76.5% in 2016 to 79.5% in 2017 and 64.0% in 2016 and 69.2% in 2017, respectively. At Philadelphia practices, the proportions of eligible adolescents 17–18 years of age who initiated or completed the series of HPV vaccine by September 15 increased from 82.1% in 2016 to 85.8% in 2017 (p < .001) and 68.8% in 2016 to 73.4% in 2017 (p < .001), respectively (). Significant increases were also noted among eligible 17–18-year-old adolescents in Suburban practices (73.7% in 2016 to 76.5% in 2017, p < .001 for series initiation and 61.8% in 2016 to 67.4% in 2017, p < .001 for series completion) (). Similarly, for all New Jersey practices, the proportions of eligible adolescents 17–18 years of age who initiated or completed the series of HPV vaccine by September 15 also increased from 59.0% in 2016 to 63.8% in 2017 (p = .002) and 47.5% in 2016 and 54.8% in 2017 (p < .001), respectively ().
Discussion
In our web-based survey among pediatric providers from a large pediatric network in Pennsylvania, we found that implementation of new 2017–2018 Pennsylvania school-entry vaccination requirements that significantly shortened the grace period for compliance and added a new recommendation for older adolescents did result in increased demand for vaccination services. However, survey results indicated that practices largely appeared to be well equipped to manage the increased demand. Our results also suggest that the new requirements may have contributed to increases in timely vaccination rates, especially for MCV4 among 17–18 years old adolescents. The findings of increases in vaccination coverage of Tdap and MCV4 vaccines in 7th graders were similarly reflected in other analyses performed by the Pennsylvania Department of Health.Citation21 We observed concurrent increases among New Jersey practices, suggesting that our results may reflect general trends in adolescent vaccination rates, or that the Pennsylvania new school-entry requirements influenced recommendation practices across the entire primary care network.
While our findings suggest that the new school-entry vaccination requirements were not disruptive for practices and may have contributed to increased vaccine uptake, communication and implementation of the new requirements revealed areas for improvement. The majority of respondents agreed that they had been informed about the new requirements and felt comfortable talking to the parents about them. Although over half (57%) of providers indicated that parents knew of the new requirements before the scheduled visit, there remained a sizable proportion of providers who interacted with parents who were not familiar with the requirements (21%). Children’s school was the primary source of information to parents about the new school-entry requirements; however, our results suggest that parents were not always aware of what was due or if their child was already up-to-date on vaccinations from the communication from their child’s schools. Schools also did not communicate with practices. Some providers indicated an increased workload or “stampede” on their practices, which in some cases, could have been prevented as some parents requested appointments for students who were already up to date. If a child’s up-to-date status with new school-entry vaccination requirements could be communicated more efficiently between schools, parents, and clinics, this could improve schools’ ability to have accurate vaccination information and minimize a sense of urgency among parents. Practices would need to accommodate fewer parental vaccination requests around the time of school entry. This may also decrease the overall number of students that were provisionally enrolled or missing school due to vaccine up-to-date status. As such, a partnership between schools and primary care practices in the months leading up to school-entry could be useful in increasing efficiency and decreasing parental confusion. Local and state health departments can also play a role in improving timely access to vaccination information through immunization information systems (IIS). Finally, a sizable proportion of providers (21%) did not feel comfortable answering questions about the new school-entry vaccine requirements, which could be amended in the future by more communication from health departments about policy changes.
As for other communication approaches directly with parents, posters and fliers were more commonly used by practices in waiting rooms after the announcement of the new school-entry vaccination requirements. Reminder-recall e-mails or text messages did not comprise a large proportion of the communication strategy. This is surprising considering the well-documented success of reminder-recall messaging (ex. calls, e-mails, text messages, letters) in the promotion of health behaviors, including vaccination, especially in adolescents.Citation22–Citation24 This could potentially be adopted in the future considering 43% of providers acknowledged that parents may not have been aware of changes.
Vaccination up-to-date rates at the same time point in 2016 and 2017 were compared to assess the success of these new school-entry vaccination requirements in ensuring students are up-to-date with required vaccines at the beginning of the school year. Since we used September 15 as our evaluation date, we assumed this captured all of the catch-up vaccinations performed in the new provisional enrollment period of 5-days post-school start in 2017. While statistically significant, none of the absolute differences in Tdap and MCV4 vaccination coverage for the 12–13-year age (7th-grade school entry) group between 2016 and 2017 were large. This may be due to a ceiling effect as vaccination rates for these vaccines were already quite high. However, a much larger absolute difference was noted between 2016 and 2017 in the second dose for the 17–18-year age, or 12th-grade school entry, group, hinting at the usefulness of the new requirements in the adoption of booster doses of vaccinations in adolescents. This is a particularly critical age group since high school seniors could soon be entering the high-risk environments of college campuses, where they could be susceptible to outbreaks of infectious diseases such as meningococcus due to enhanced person-to-person transmission associated with crowded living conditions and specific social behaviors.Citation25–Citation28
The rise in MenB and HPV initiation and dose-completion rates for all doses and age groups was also a notable result which could be due to multiple reasons. Providers may be recommending that children initiate or complete the dose when patients are coming in for their school-entry vaccinations, hence bundling all the required and recommended vaccines they can give into that visit. According to our survey, 88% of respondents said they “Always” offered HPV9, and 82% said they “Always” offered MenB, indicating buy-in from clinicians to promote vaccination of not just required but also recommended vaccines. The increase in HPV vaccination rates in our study could also be due to the trend of increasing HPV vaccine rates among adolescents across the United States, influenced by growing vaccine acceptance and stronger HPV vaccine recommendations that have been promoted to improve vaccine uptake.Citation29 This is evident through the similarly increased vaccination rates in New Jersey, where this policy change did not take place.
Limitations
Our study does have important limitations. The provider survey was administered several months after the 2017 school-entry requirements were introduced so there may be recall bias with the events regarding school-entry vaccination visits. This might have led some respondents to underestimate the workload increase. However, due to our sample size, reasonable response rate, and geographic distribution of practices across different community types, we believe that we captured a representative sample of themes regarding the introduction of the new 2017–2018 school-entry vaccine requirements.
For vaccination rates, we used age groups as a proxy for grade in school; therefore, we are likely not capturing all children entering either 7th or 12th grade. Vaccination rates are also only measured among patients from one hospital system in the greater Philadelphia area. These patients only represent a small proportion of students and, therefore, have limited generalizability. Lastly, because we wanted to measure implementation shortly after the introduction of the new school-entry vaccination requirements, we measured vaccination rates only 1 year after implementation and did not measure trends over time. New Jersey showed similar increases in vaccination rates without the new requirements, suggesting that the changes we observed could be influenced by other factors. Similar trends among New Jersey patients may also reflect a spillover effect as strategies used by Pennsylvania practices to identify and vaccinate patients may have been adopted across the entire network. Continuing measurement of vaccination rates over time will provide more observation points to better evaluate trends and school-entry vaccination requirements’ impact.
Conclusion
While the adoption of the new 2017 school-entry vaccination requirements led to increased demand for vaccinations, the shorter grace period and the new requirement for adolescents appeared to be readily accommodated with minimal disruption among practices. Most importantly, the new requirements may have positively impacted timely vaccination up-to-date rates, ensuring that students are protected at the beginning of the school year. Moving forward, partnerships between the health department, schools, clinics, and parents can help improve efficient and effective implementation. As other states consider changes to the architecture of their school-entry vaccination requirements, our results contribute to current evidence demonstrating that more stringent school-entry vaccination requirements can increase timely vaccination up-to-date rates of school-age children, decreasing overall population susceptibility to preventable diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to acknowledge the Vaccine Education Center at the Children’s Hospital of Philadelphia for support of this project. We would also like to acknowledge Peter Camacho and Daniel Singleton from CHOP’s Department of Biomedical and Health Informatics for their invaluable support with electronic health record data extraction.
References
- Advisory Committee for Immunization Practices. Birth-18 years and catchup immunization schedules for providers | CDC 2018 [Internet]. Centers Dis. Control Prev. 2018 [accessed 2018 Apr 20]. https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html
- Ducharme J. FDA head says the federal government may have to set vaccine policies if state laws continue to allow outbreaks. Time [Internet]. [accessed 2019 Jun 6]. 2019. https://time.com/5534592/fda-commissioner-federal-government-vaccine-policies/
- Malone KM, Hinman AR. Vaccination mandates: the public health imperative and individual rights [Internet]. In: Law in public health practice. 2003. p. 262–84. [accessed 2019 Jun 6]. https://www.cdc.gov/vaccines/imz-managers/guides-pubs/downloads/vacc_mandates_chptr13.pdf
- State School Immunization Requirements and Vaccine Exemption Laws [Internet]. [accessed 2019 Jun 3]. https://www.cdc.gov/phlp/docs/school-vaccinations.pdf
- ProCon.org. State-by-state: vaccinations required for public school kindergarten - Vaccines - ProCon.org [Internet]. ProCon.org. 2018 [accessed 2019 Jun 4]. https://vaccines.procon.org/view.resource.php?resourceID=005979
- Simpson JE, Hills RA, Allwes D, Rasmussen L. Uptake of meningococcal vaccine in Arizona schoolchildren after implementation of school-entry immunization requirements. Public Health Rep [Internet]. 2013; 128:37–45. [accessed 2017 Aug 30]. http://www.ncbi.nlm.nih.gov/pubmed/23277658
- Perkins RB, Lin M, Wallington SF, Hanchate AD. Impact of school-entry and education mandates by states on HPV vaccination coverage: analysis of the 2009–2013 National Immunization Survey-Teen. Hum Vaccin Immunother [Internet]. 2016;12:1615–22. [accessed 2017 Aug 29]. http://www.tandfonline.com/action/journalInformation?journalCode=khvi20
- Center for Disease Control and Prevention. State school immunization requirements and vaccine exemption laws. Cal Heal Saf Code NH Code Admin R He-P 301 [Internet]. 1203; 13:1–19. [accessed 2018 Apr 23]. https://www.cdc.gov/phlp/docs/school-vaccinations.pdf
- Constable C, Blank NR, Caplan AL. Rising rates of vaccine exemptions: problems with current policy and more promising remedies. Vaccine [Internet]. 2014;32:1793–97. [accessed 2017 Aug 30]. http://linkinghub.elsevier.com/retrieve/pii/S0264410X14001480
- Omer SB, Richards JL, Ward M, Bednarczyk RA. Vaccination policies and rates of exemption from immunization, 2005–2011. N Engl J Med [Internet]. 2012;367:1170–71. [accessed 2017 Aug 24]. http://www.nejm.org/doi/abs/10.1056/NEJMc1209037
- Seither R, Calhoun K, Street EJ, Mellerson J, Knighton CL, Tippins A, Underwood JM. Vaccination coverage for selected vaccines, exemption rates, and provisional enrollment among children in kindergarten — United States, 2016–17 school year. MMWR Morb Mortal Wkly Rep [Internet]. 2017;66:1073–80. [accessed 2017 Dec 19]. http://www.cdc.gov/mmwr/volumes/66/wr/mm6640a3.htm
- Mellerson JL, Maxwell CB, Knighton CL, Kriss JL, Seither R, Black CL. Vaccination coverage for selected vaccines and exemption rates among children in kindergarten — United States, 2017–18 school year. MMWR Morb Mortal Wkly Rep [Internet]. 2018;67:1115–22. [accessed 2019 Jun 4]. http://www.cdc.gov/mmwr/volumes/67/wr/mm6740a3.htm?s_cid=mm6740a3_w
- Office of Disease Prevention and Health Promotion. Immunization and infectious diseases | Healthy people 2020 [Internet]. Heal. People. 2020 [accessed 2019 Jun 3]. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases
- Whittington MD, Kempe A, Dempsey A, Herlihy R, Campbell JD. Impact of nonmedical vaccine exemption policies on the health and economic burden of measles. Acad Pediatr [Internet]. 2017;17:571–76. [accessed 2017 Aug 17]. http://linkinghub.elsevier.com/retrieve/pii/S1876285917300682
- Pennsylvania School Boards Association. School immunization changes now final | PSBA [Internet]. 2017 [accessed 2017 Sep 25]. https://www.psba.org/2016/10/school-immunization-approved/
- Pennsylvania S of. 28 Pennsylvania Code: Chapter 23. Subchapter C. Immunization [Internet]. [accessed 2017 Sep 25]. http://www.pacode.com/secure/data/028/chapter23/subchapCtoc.html
- Pennsylvania S of. 028 Pa. Code § 23.84. Exemption from immunization. [Internet]. [accessed 2019 Jul 25]. https://www.pacode.com/secure/data/028/chapter23/s23.84.html
- Losby J. CDC coffee break: using Likert scales in evaluation survey work [Internet]. Atlanta. 2012 [accessed 2019 Jun 7]. https://www.cdc.gov/dhdsp/pubs/docs/CB_February_14_2012.pdf
- City of Philadelphia Department of Public Health Division of Disease Control Immunization Program. Philadelphia immunization requirements for school entry (2017/2018) [Internet]. Philadelphia. 2017 [acessed 2017 Sep 27]. http://kids.phila.gov/wp-content/uploads/2017/08/2017-18-school-immunization-requirement.pdf
- Centers for Disease Control and Prevention. Birth-18 Years Immunization Schedule | CDC 2019 [Internet]. Centers Dis. Control Prev. 2019 [accessed 2019 Jul 9]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
- Mohanty S, Delamater P, Feemster K, Buttenheim AM. 8 months to 5 days: what happened when Pennsylvania changed the vaccination regulations for provisional enrollment? Hum Vaccin Immunother. 2019;1–5. doi:10.1080/21645515.2019.1673120.
- Rand CM, Vincelli P, Goldstein NPN, Blumkin A, Szilagyi PG. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J Adolesc Heal [Internet]. 2017;60:113–19. [accessed 2019 Jul 25]. https://www-sciencedirect-com.proxy.library.upenn.edu/science/article/pii/S1054139X1630355X
- Schwebel FJ, Larimer ME. Using text message reminders in health care services: A narrative literature review. Internet Interv [Internet]. 2018;13:82–104. [accessed 2019 Jul 10]. http://www.ncbi.nlm.nih.gov/pubmed/30206523
- Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev [Internet]. 2018;1:CD003941. [accessed 2019 Jul 10]. http://www.ncbi.nlm.nih.gov/pubmed/29342498
- Imrey PB, Jackson LA, Ludwinski PH, England AC, Fella GA, Fox BC, Isdale LB, Reeves MW, Wenger JD. Meningococcal carriage, alcohol consumption, and campus bar patronage in a serogroup C meningococcal disease outbreak. J Clin Microbiol [Internet]. 1995;33:3133–37. [accessed 2017 Aug 14]. http://www.ncbi.nlm.nih.gov/pubmed/8586688
- Kumar A, Murray DL, Havlichek DH. Immunizations for the college student: a campus perspective of an outbreak and national and international considerations. Pediatr Clin North Am [Internet]. 2005;52:229–41. [accessed 2017 Aug 14]. http://linkinghub.elsevier.com/retrieve/pii/S0031395504001373
- MacDougall DM, Langley JM, Li L, Ye L, MacKinnon-Cameron D, Top KA, McNeil SA, Halperin BA, Swain A, Bettinger JA, et al. Knowledge, attitudes, beliefs, and behaviors of university students, faculty, and staff during a meningococcal serogroup B outbreak vaccination program. Vaccine [Internet]. 2017;35:2520–30. [accessed 2017 Aug 14]. http://linkinghub.elsevier.com/retrieve/pii/S0264410X17301767
- Centers for Disease Control and Prevention. Meningococcal | community Settings Risk Factors | CDC [Internet]. 2017 [accessed 2017 Aug 14]. https://www.cdc.gov/meningococcal/about/risk-community.html
- Centers for Disease Control and Prevention. HPV | understanding HPV Coverage | CDC [Internet]. Centers Dis. Control Prev. 2018 [accessed 2019 Jun 21]. https://www.cdc.gov/hpv/partners/outreach-hcp/hpv-coverage.html
Appendix A.
CHOP primary care practice survey questions
At which CHOP primary care site do you work?
Broomall/Drexel Hill/Media
Central Bucks
Chadds Ford
Chestnut Hill
CHOP campus
Coatesville
Cobbs Creek
Flourtown
Haverford
Highpoint
Indian Valley
Karabots
Kennett Square
Newtown
Norristown
North Hills
Paoli
Pottstown
Roxborough
South Philadelphia
Springfield
West Chester
West Grove
Physician Floats
What is your professional role?
Medical Director/Nurse Practitioner
Registered Nurse
Office Manager
Other (please specify):
How many years have you been practicing medicine/nursing?
<5 years
5–9 years
10–15 years
16–20 years
21–30 years
>30 years
Not applicable (practice manager, non-clinician, etc.)
How did your find out about the new school immunization requirements? Please check all that apply:
Notice from the Pennsylvania Department of Health
Notice from your county Department of Health
Notice from the Children’s Hospital of Philadelphia
Other clinic staff
Received a letter from school for my own children
Other parents
Other (please specify):
How does your practice communicate with families about vaccines required for school? Please check all that apply:
Phone call
Website update
Letter
We do not send information/directly communicate about new requirements to families
I don’t know
Other (please specify):
Did your practice perform any outreach communications related specifically to the new 2017–18 school requirements? Please check all that apply:
Phone call
Website update
Letters
Posters/fliers in waiting room
None
I don’t know
Other (please specify):
Which of the following activities were used by your practice to meet the new vaccination requirements? Please check all that apply:
Extended staff time to identify children who were not up-to-date for required vaccines
Reminder-recall letters
Reminder-recall phone calls
Extended hours for well visits
Extended hours for immunization only visits
Additional staff time for immunization visits
None
Other (please specify):
Which of the following activities will your practice use for the 2018–19 school year to meet the new vaccination requirements? Please check all that apply:
Extended staff time to identify children who were not up-to-date for required vaccines
Reminder-recall letters
Reminder-recall phone calls
Extended hours for well visits
Extended hours for immunization only visits
Additional staff time for immunization visits
None
Other (please specify):
Please indicate your agreement with the following statements:
(10) Between the publication of the new requirements and the beginning of the school year (prior to September 1, 2017), what was the average amount of time between an appointment request and the appointment date for a well visit?
Immediately
1–2 weeks
3–4 weeks
1–2 months
More than two months
I don’t know
(11) Between the publication of the new requirements and the beginning of the school year (prior to September 1, 2017), what was the average amount between an appointment request and the appointment date for an immunization-only visit?
Immediately
1–2 weeks
3–4 weeks
1–2 months
More than two months
I don’t know
(12) Please indicate your agreement with the following statements:
(13) Did parents of patients mention any of the following? Please check all that apply:
Reminder letters
Reminder phone calls
Prompts from their child’s school to get back-to-school immunizations
(14) How often do you or your practice offer the following recommended, but not required vaccines at the time of a back-to-school immunization visit?
(15) Are there any other comments you would like to provide related to the introduction and implementation of Pennsylvania’s new school immunization requirements? Please include here: