ABSTRACT
Chlamydia trachomatis is the most common bacterial sexually-transmitted pathogen for which there is no vaccine. We previously demonstrated that the degree of phosphate substitution in an aluminum hydroxide adjuvant in a TLR-4-based C. trachomatis serovar E (Ser E) recombinant major outer membrane protein (rMOMP) formulation had an impact on the induced antibody titers and IFN-γ levels. Here, we have extended these observations using outbreed CD-1 mice immunized with C. trachomatis Ser E rMOMP formulations to evaluate the impact on bacterial challenge. The results confirmed that the rMOMP vaccine containing the adjuvant with the highest phosphate substitution induced the highest neutralizing antibody titers while the formulation with the lowest phosphate substitution induced the highest IFN-γ production. The most robust protection was observed in mice vaccinated with the formulation containing the adjuvant with the lowest phosphate substitution, as shown by the number of mice with positive vaginal cultures, number of positive cultures and number of C. trachomatis inclusion forming units recovered. This is the first report showing that vaccination of an outbred strain of mice with rMOMP induces protection against a vaginal challenge with C. trachomatis.
Introduction
Chlamydia trachomatis is the most common sexually-transmitted bacterial infection worldwide.Citation1,Citation2 Genital infections affect mainly sexually-active teenagers.Citation3-5 Newborns can become infected in the birth canal and contract ocular, respiratory and gastrointestinal infections.Citation6-8 Trachoma, a chronic C. trachomatis ocular infection, affecting regions with poor sanitary conditions, is the most common cause of preventable blindness globally.Citation9-11
The clinical sequelae, such as trachoma and pelvic inflammatory disease, are thought not to be directly caused by the pathogen itself, but by induced immune responses, which can clear the infection but can also cause collateral damage.Citation9,Citation12,Citation13 C. trachomatis attracts innate and antigen-specific cells that release immune factors, such as chemokines and cytokines, which are likely to be responsible for the pathology. Results from studies in mice suggest that this pathogenesis is dependent on signaling through toll-like receptor 2 (TLR2), but not TLR4, although both TLR2- and TLR4-deficient mice can clear the infection as well as wild-type mice.Citation14 Studies in humans and mice have shown that TLR2 is the primary pathogen-recognition receptor in the upper genital tract that drives the immune-pathogenic mechanisms associated with C. trachomatis infections.Citation14,Citation15
Many developed countries run C. trachomatis screening programs, although there are gaps in the evidence base for their efficacy.Citation16 The European Center for Disease Prevention and Control (ECDC) recommends national strategies for C. trachomatis control, which include screening programs for at-risk individuals and an evaluation plan for the strategy. Antibiotic therapy is effective against C. trachomatis infections, but due to inappropriate treatment and the large number of asymptomatic patients, long-term sequelae including abdominal pain, infertility, ectopic pregnancy, lymphogranuloma venereum and blindness, can occur.Citation4,Citation5,Citation11,Citation17 The prevalence rate of genital infections has been reported to increase in countries that have established screening programs.Citation18,Citation19 This increase is thought to be due to reduced duration of infection, resulting from antibiotic therapy, which prevents the development of natural immunity.Citation18 Therefore, vaccination could be the best approach to prevent C. trachomatis infections and reduce the burden of disease.Citation20-25 The major outer membrane protein (MOMP), one of the most abundant, immunogenic chlamydial proteins, has been shown to elicit protective immunity in mice, similar to that induced by natural infection.Citation26
Both innate and adaptive immune responses are elicited by natural C. trachomatis infections, with IFN-γ and IL-17 playing important roles as key effectors for both protection and pathological responses.Citation27 At high concentrations, IFN-γ kills the bacteria but at low concentrations, it can enhance pathological responses.Citation12,Citation13 Hence, effective vaccines need to strike the correct balance between inducing a protective response and triggering a pathological reaction.
Phosphate substitution in aluminum-based adjuvants has been reported to modify the immunogenicity of various antigens in vaccine formulations. For example, in an anthrax vaccine containing recombinant protective antigen (rPA) and an aluminum oxyhydroxide AlOOH adjuvant, it was reported that high phosphate substitution was associated with a higher immune response to rPA.Citation28 The authors suggested that this was due to a weaker interaction between rPA and the phosphorylated adjuvant that allowed a more complete water layer between the components and therefore resulted in a rapid release of rPA after injection.
Phosphate substitution in AIOOH-containing adjuvant can affect the adsorption and stability of vaccine components which can have an impact on the bioavailability of antigens, TLR agonists and thus the vaccine’s efficacy.Citation29-31 This phosphate substitution can be achieved by pretreating the AIOOH-containing adjuvant with varying concentrations of phosphate ions in the form of potassium or sodium phosphate.Citation30,Citation31 The phosphate ions displace the surface hydroxyl thereby decreasing its point of zero charge. We recently reported that the cellular and humoral immune responses to a vaccine containing recombinant C. trachomatis serovar E MOMP, and a proprietary adjuvant (SPA08), composed of AIOOH and the TLR4 agonist, E6020, were dependent on the degree of phosphate substitution on the AlOOH component.Citation32
The use of inbred strains of mice has greatly facilitated initial testing of experimental vaccine formulations by providing invaluable data on the assessment of the immune responses of the host which can help improve the protective efficacy of vaccine formulations. Inbred animals only provide information on a limited range of the possible immune responses observed in humans following vaccination. The high incidence and prevalence of chlamydial infections in all parts of the world affecting individuals with very different genetic backgrounds requires vaccine formulations to be tested in outbred individuals.
The initial studies assessing protection against trachoma were performed in various models, including humans and non-human primates.Citation11 Most of the work done recently to assess the protective efficacy of vaccine formulations against genital infections utilized inbred mice, in particular C3H/HeN, BALB/c and C57BL/6 mice.Citation33 Currently, one of the most extensive vaccine studies in an outbred species of animals is being performed in koalas (Phascolarctos cinereius).Citation34 These animals that are naturally infected in the eyes and the genitourinary tract by Chlamydia pecorum, suffer long-term sequelae including blindness and infertility, like humans and therefore major efforts are underway to protect them with a vaccine using MOMP as the antigen.Citation35-38
In the context of preclinical development of a human C. trachomatis vaccine, we focused on serovar E rMOMP as a model antigen since serovar E is the most prevalent serovar in human urogenital infections and also the one that induces the highest serological responses.Citation39-42 Here, we report how phosphate substitution affected the ability of the rMOMP vaccine to protect outbreed CD-1 mice against a vaginal challenge with C. trachomatis serovar E. This was achieved using vaccine formulations with different degrees of phosphate substitution on the AlOOH-containing adjuvant, mentioned above, and another formulation containing aluminum phosphate, to represent a high degree of adjuvant phosphate substitution.
Materials and methods
C. trachomatis stocks
Stocks of C. trachomatis serovar E (strain Bour) were prepared in Hela-229 cell culture (American Type Culture Collection, Manassas, VA). Elementary bodies (EBs) were purified using Renografin and frozen at −70°C in SPG (0.02 sucrose, 0.2 M sodium phosphate, pH 7.2, and 5 mM glutamic acid).Citation43 The titer of the EB stocks was determined using HeLa-229 cells.Citation43
Vaccine formulations
The recombinant C. trachomatis serovar E (strain Bour) MOMP (Ser E rMOMP) was expressed in Escherichia coli as inclusion bodies (IBs). Briefly, bacteria were grown in a 20-L fermenter with constant agitation at 37°C, pH 7 and 30% dissolved oxygen. The IBs were extracted and solubilized in 6M guanidine hydrochloride and then exchanged with 8M urea using tangential flow filtration (TFF). The urea solution was applied to an anionic exchange chromatography column (Q Ceramic HyperD® 20 Chromatography Sorbent, Pall Corporation) and Ser E rMOMP was eluted from the column with 50–90 mM sodium chloride. The eluted Ser E rMOMP was refolded by mixing with equal volumes of 10% v/v N-lauroyl sarcosine (NLS), 1 M 1-arginine and dithiothreitol (DTT) to a final concentration of 10 mM. This mixture then underwent buffer exchange with 10 mM Tris-HCl pH 8, 0.06% NLS diafiltration buffer using TFF and a membrane filter with a molecular weight cutoff of 10 kDa. The preparation underwent a second TFF with 50 mM Tris-HCl, pH 8.0 diafiltration buffer. The final purity of the Ser E rMOMP was greater than 90%, as evaluated by SDS-PAGE.
Aluminum hydroxide adjuvant (AlOOH) and aluminum phosphate adjuvant (AlPO4) were acquired from Brenntag (Frederikssund, Denmark). The SPA08 adjuvant was composed of the TLR4 agonist, E6020 (Eisai, USA), adsorbed onto AlOOH. Three SPA08 adjuvanted formulations containing 10 µg of Ser E rMOMP and 40 μg/mL E6020, 2.4 mg/mL elemental Al and no phosphate (SPA08-1), 88 mM (SPA08-3) or 176 mM (SPA08-4) phosphate were prepared as described previously.Citation32 The phosphate to aluminum molar ratio (P:Al) of each adjuvant formulation is reported in . The SPA08-2 formulation, with 8.8 mM phosphate, was not assessed in this study as its immunological characteristics were previously shown to be similar to those of SPA08-1. An aluminum phosphate-based formulation (APF) with a high degree of phosphate substitution (APF/rMOMP) containing 10 µg of Ser E rMOMP was used for comparison.
Table 1. Physicochemical characteristics of Ser E rMOMP-SPA08 formulations
Physicochemical characterization of formulations
The zeta potential, particle size distribution and percentage of MOMP adsorbed for the three SerE MOMP-SPA08 formulations were determined as previously described.Citation32
Mice
Six-week-old outbred female CD-1 mice (Charles River Laboratories) were housed at the University of California, Irvine (UCI) in isolation cubicles at a constant temperature of 24°C with a 12 h light/12 h darkness cycle and fed chow and water ad libitum. The UCI Animal Care and Use Committee approved the animal protocols.
Vaccination protocol
Vaccine formulations containing C. trachomatis Ser E MOMP and SPA08 or APF were extemporaneously prepared in 3 ml glass vials by mixing one volume of Ser E rMOMP stock solution with one volume of the SPA08-1, SPA08-3, SPA08-4 or APF adjuvants. The formulations were mixed manually by inverting the container at least five times to ensure thorough mixing before injecting the mice or conducting physicochemical characterization.
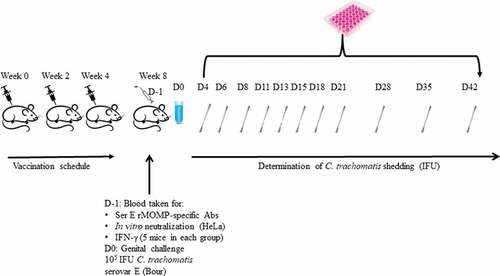
Groups of mice were vaccinated intramuscularly (IM) three times at 3-week intervals with 50 µL of the SPA08-1, SPA08-3, SPA08-4 or APF formulations (). For each formulation, a group of mice was sham-vaccinated at the same time with the same volume of inoculum containing non-phosphate treated SPA08 or APF in phosphate buffered saline (PBS) with no Ser E rMOMP.
ELISA for C. trachomatis Ser E rMOMP-specific antibodies
Blood was collected for IgG measurements and sero-neutralization assays by periorbital puncture the day before the vaginal challenge. C. trachomatis Ser E MOMP specific IgG, IgG1, IgG2a titers were measured by an enzyme-linked immunosorbent assay (ELISA) as described previously.Citation32,Citation44 Briefly, 96-well plates were coated with EBs in PBS at a concentration of 10 µg per ml (1 μg/well), and blocked with PBS-Tween-milk (0.05% Tween, 1% (w/v) powdered skim milk). The following steps were then carried out in a final volume of 100 µL, followed by three washes with PBS-Tween. Serial 2-fold dilutions of serum samples in PBS-Tween-milk starting from 1/1,000, or 1/10,000, were incubated at 37°C for 1 h. After washing the wells, horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 or IgG2a (BD Pharmingen, San Diego, CA) were added and the plates incubated for 1 h at 37°C. After washing, 2,2ʹ-azinobis (3-ethylbenthiazoline-6-sulfonic acid) (ABTS) was added to the wells and the plates incubated at 37°C for 1 h. The optical density at 405 nm in the wells was measured in an ELISA plate reader (Bio-Rad Laboratories, Richmond, CA).
In vitro sero-neutralization assay
In vitro sero-neutralization assays, performed in 96-well plates as described previously, were repeated three times on different days.Citation32 Briefly, two-fold serial dilutions of mouse sera in Ca2+- and Mg2+-free PBS, pH 7.2, supplemented with 5% guinea pig serum were incubated with C. trachomatis Ser E [1x104 infectious forming units (IFU)] for 45 min at 37°C. The mixtures were added to HeLa-229 cell monolayers, centrifuged for 1 h and incubated at 37°C for 48 h. After washing, the monolayers were fixed with methanol and stained with an in-house monoclonal antibody E4 to MOMP.Citation44 The titer of a sample was the dilution that yielded 50% neutralization relative to the negative control serum from sham-vaccinated mice.
Cell-mediated immune responses: IFN-γ determination
To assess T-cell memory responses, cytokine production was measured in splenic T-cells from five mice per group euthanized the day before the challenge.Citation44,Citation45 In brief, T-cell-enriched splenocytes (105 cells/well) were co-cultured with antigen presenting cells (APCs; 1.25 x 105/well), prepared by irradiating syngeneic, unseparated splenocytes from the harvested spleens (3,000 rads; 137Cs), and then incubated for two hours with either UV-inactivated C. trachomatis Ser E EB at a 1:1 ratio or purified Ser E rMOMP. Concanavalin A was included as a positive control. The supernatants were harvested and stored at −80°C until used for the determination of the IFN-γ concentrations by ELISA (BD Pharmingen, San Diego, CA; Labsystem Multiscan; Helsinki, Finland).
Vaginal challenge
Four weeks after the third vaccine dose the mice were challenged vaginally.Citation46-49 The mice were injected with 2 mg of DepoProvera subcutaneously twice prior to the vaginal challenge (days −10 and −3) to synchronize their estrus cycle in diestrus. The mice were anesthetized and C. trachomatis Ser E (Bour) (105 IFU) were inoculated into their vaginas. The mice were kept in a recumbent position for 10–15 min after the challenge.
Vaginal swabs were collected and cultured twice weekly for the first three weeks, and then once-a-week in weeks 4, 5, and 6 after the vaginal challenge ().Citation47 The swabs were vortexed in 200 μl of sterile SPG and two aliquots from each specimen (100 μl and 10 μl) were inoculated into HeLa-229 cell monolayers in 48-well plates by centrifugation at 1,000xg for 1 h at room temperature. Following incubation at 37°C for 48 h, the chlamydial inclusions were stained with an in-house monoclonal antibody (E4) to MOMP.Citation50
The following criteria were used to determine protection: number of mice with positive vaginal cultures, number of positive vaginal cultures over total number of cultures collected, number of C. trachomatis IFU recovered and length of time of vaginal shedding.
Hydrosalpinx formation
Seven weeks after the vaginal challenge mice were euthanized and their genital tract macroscopically investigated to determine the presence of hydrosalpinx which is an indicator of upper genital tract pathology.Citation51
Statistical analyses
Using a computer model, it was shown that even a vaccine with 50% efficacity would have a significant impact on the clearance of Chlamydia from a defined community.Citation20 Thus, the sample size was based on a criterion of at least 50% efficacity. The number of mice included in the experiments was based on previously published recommendations.Citation52
Student’s t test, Fisher’s exact test and Mann-Whitney U test were used for analyses performed using SigmaStat (version 3.5) software. Differences were considered statistically significant when the p-values was <0.05.
Results
Characterization of vaccine formulations
The SPA08 adjuvant was treated with phosphate ions to increase the phosphate substitution on the AlOOH component to investigate the impact of Ser E rMOMP adsorption on the immunogenicity and protection of the vaccine. The impact of type of aluminum salt and adsorption on immunogenicity and protection was also evaluated using an aluminum phosphate version of the adjuvant (APF), with identical amounts of Ser E rMOMP. The physicochemical characterization of the SPA08 formulations indicated that the zeta potential at neutral pH decreased as the P:Al molar ratio increased, but there was no effect on the particle size or E6020 adsorption (). The percentage of Ser E rMOMP adsorption to AlOOH in the formulation at neutral pH decreased as the phosphate substitution increased primarily due to high electronegativity of the SPA08 adjuvant at high P:Al ratios which decreased the electrostatic interaction between Ser E rMOMP and SPA08 at neutral pH (). In contrast, E6020 remained bound to AlOOH, irrespective of the amount of phosphate substitution.
The physicochemical characteristics of the formulation containing APF adjuvant were similar to those of SPA08-4, with a strongly negative zeta potential and comparable Ser E rMOMP percentage adsorption (). However, the particles in the APF formulation were significantly smaller than those in the SPA08-adjuvanted formulations.
Association of P:Al molar ratio with total C. trachomatis Ser E rMOMP-specific IgG levels and their neutralizing capacity
Ser E rMOMP-specific antibody response
High IgG titers in serum were observed in the four groups of mice immunized with C. trachomatis Ser E rMOMP independently of the adjuvant formulation used (). Although mice immunized with SPA08-1 had lower IgG geometric mean titers (GMTs) (877,997; range 398,099–1,936,000) than those immunized with SPA08-3 (1,882,000; range 1,375,000–2,575,000), SPA08-4 (1,529,000; range 1,153,000–2,027,000) or APF (2,940,668; range 1,280,000–5,120,000) the differences were not statistically significant.
Figure 2. C trachomatis rMOMP serovar E-specific antibody titers determined by ELISA. Serum samples were drawn four weeks after the third vaccine dose from 10 mice. Each dot represents the results for one mouse and the horizontal lines represent the geometric mean titer (GMT) for the group of mice
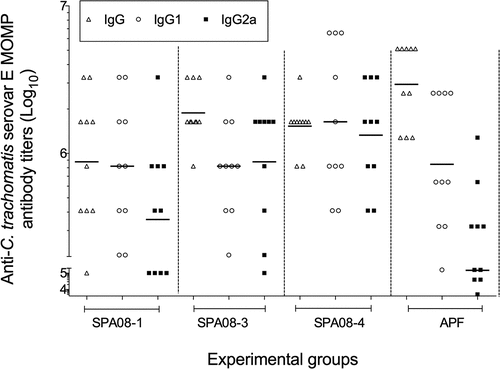
The IgG2a GMTs increased as the P/Al molar ratios increased (). Similar IgG1 GMTs were elicited by SPA08-1 and SPA08-3, while the IgG1 GMTs elicited by SPA08-4 and APF were higher. The IgG1/IgG2a ratio for APF was 5.7 compared with a range from 0.93 to 2.3for the SPA08 formulations.
In vitro sero-neutralization assay
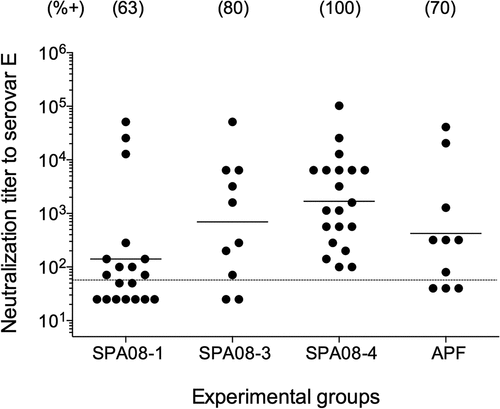
Serum antibodies were evaluated for their ability to neutralize C. trachomatis Ser E infectivity in vitro (). There was a trend for more mice to have neutralizing antibodies as the P:Al molar ratio increased: 12/19 (63%) in the SPA08-1 group; 8/10 (80%) in the SPA08-3 group and 20/20 (100%) in the SPA08-4 group. Positive neutralizing titers were observed in 7/10 (70%) of the mice in the APF group. The neutralizing GMTs were 141 (range: 25–51,200), 397 (range: 25–51,200), 1,685 (range: 100–102,400), and 422 (range: 40–40,960) for the SPA08-1, SPA08-3, SPA08-4, and APF groups, respectively.
Association of P:AL molar ratio with ser E rMOMP-specific IFN-γ production
Ser E MOMP-specific cellular responses were tested by ex vivo stimulation of primed spleen T-cell with either Ser E EBs (Ct-E EB) or purified Ser E rMOMP. T-cell stimulation with purified Ser E rMOMP led to more IFN-γ production than stimulation with EBs for all three SPA08 groups (). The IFN-γ levels were higher for the SPA08-1 and SPA08-3 groups than those in the SPA08-4 group for both type of stimuli. The primed spleen cells from APF-immunized mice secreted the lowest quantities of IFN-γ when stimulated with either Ser E EB or rMOMP ().
Table 2. IFN-γ levels from stimulated spleen T-cells taken four weeks after the third vaccination from five mice in each group
Culture results following vaginal challenge
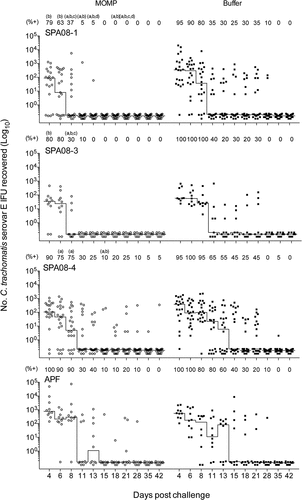
Vaginal swabs were collected from the CD-1 mice following the vaginal challenge twice weekly for the first three weeks and weekly thereafter. There were fewer mice with at least one positive vaginal culture in the rMOMP/SPA08-1 and rMOMP/SPA08-3 groups compared with their respective sham-vaccinated control groups, but not in the rMOMP/SPA08-4 group, however, the differences were not statistically significant (; ). The median number of days to negative culture was significantly shorter in the vaccinated groups compared with the sham-vaccinated control groups, except for the rMOMP/APF group. Infection was cleared faster in the rMOMP/SPA08-1 and rMOMP/SPA08-3 groups than in the rMOMP/SPA08-4 and rMOMP/APF groups. Moreover, there was no difference in the range of number of days to negative culture between rMOMP/APF and its sham-vaccinated control group. Significant differences in the median number of IFUs recovered/mouse were observed for the SPA08-1 and SPA08-4 groups and their respective sham-vaccinated control groups, but not for the SPA08-3 and APF and their sham-vaccinated control group. The mice in the APF immunized group were not protected compared with the mice in the sham-immunized group.
Table 3. Vaginal culture results from mice challenged vaginally with C. trachomatis Ser E
Upper genital tract pathology: hydrosalpinx formation
To determine protection against long-term sequelae, at seven weeks following the vaginal challenge mice were euthanized and their genital tract macroscopically inspected. None of the mice had findings consistent with hydrosalpinx formation, suggesting limited upper genital tract pathology.
Discussion
We have previously shown that phosphate substitution in the adjuvant used in a C. trachomatis Ser E rMOMP/SPA08 vaccine increased MOMP-specific IgG and decreased IFN-γ secretion in immunized mice.Citation32 In the current study, we confirmed that as the P:Al molar ratio in the Ser E rMOMP/SPA08 vaccine formulations increased, the ELISA and neutralizing antibody titers also increased while the IFN-γ cellular response decreased. Hence, the formulation with the highest P:Al ratio, SPA08-4, compared with the other formulations, induced the highest neutralizing antibody GMT and the lowest IFN-γ cellular response. This formulation was associated with the lowest protection in the C. trachomatis vaginal challenge mouse model, thus, it would seem that high IFN-γ (Th1 response), rather than neutralizing antibodies, correlated better with protection in this model. The component within the vaccine that polarizes the rMOMP immunity toward an IFN-γ immune response profile and critically contributes to protection is the TLR4 agonist, E6020. This serves as a cautionary tale the fact that small changes within the formulation, unrelated to the concentration of antigen or TLR4 agonist, can turn a protective vaccine with a type I immune profile into a non-protective vaccine with a predominantly type II character, with far-reaching implications. The mechanisms by which phosphate substitution, protein adsorption and TLR-agonist adsorption influence the profile of the immune response against rMOMP are currently unknown. It is, however, plausible that these critical formulation parameters impact the residence time of the antigen and TLR-agonist at the site of injection, the uptake of the adjuvant – antigen complex by APCs and antigen presentation.Citation32,Citation53
The role of IFN-γ in clearance and protection against chlamydia infection is supported by evidence from both human and animal studies.Citation54-57 Despite the vigorous antibody response to chlamydia infection or in vaccine models across different mammalian species, the role of antibodies is still poorly understood with some studies reporting protection and others a disease-enhancing effect.Citation58-60 This is not surprising due to the huge heterogeneity of the antibody responses and our incomplete understanding of this critical component of the immune system. The results from in vitro experiments performed decades ago demonstrated that following a C. trachomatis infection, mice mounted humoral responses that resulted in the production of antibodies some of which were protective and others which had neutral or disease enhancing effects.Citation50,Citation59,Citation61 The protective effect of monoclonal and polyclonal antibodies against MOMP has also been shown in passive transfer experiments.Citation58,Citation62-64 The only C. trachomatis vaccine undergoing evaluation in clinical trials is based on a polyvalent recombinant MOMP formulation that elicits high levels of neutralizing antibodies.Citation58,Citation65,Citation66
The Ser E rMOMP/APF formulation induced a neutralizing antibody response characterized by high levels of total IgG, predominantly IgG1 subclass, and low levels of IFN-γ, suggestive of a type II immune response, primarily. However, it did not provide protection. This demonstrates that, in the present model, Ser E rMOMP antibodies with in vitro neutralizing capacity failed to protect against C. trachomatis Ser E challenge, therefore, the protection would appear to be dependent on an IFN-γ-mediated mechanism. The apparent discordance between the in vivo and in vitro results could be explained by the fact that the rMOMP antibodies exert their function via Fc receptors (FcRs) in vivo but that the FcRs are not necessary for in vitro neutralization. Also, the antibody isotype and the host cell type used for the in vitro neutralization assay can affect the results.Citation50,Citation59,Citation61 Thus, this apparent negative effect is likely to be due to this particular vaccine preparation since protective results have been observed with rMOMP and native MOMP in other formulations.Citation21,Citation26,Citation65,Citation67-69 As seen with other recombinant vaccines, such as those for HBV and HPV, many of the highly protective B-cell epitopes are located in conformation-dependent domains. Thus, it may be that in this model the humoral immune protective responses are not fully achieved and hence play a secondary role to the cell-mediated immune responses.Citation70-73 Future studies such as passive immunity transfer with different subclasses of rMOMP antibodies, could address this question to improve our understanding about which antibody-dependent mechanisms are protective and to de-risk further attempts to develop chlamydia vaccines.
The search for an effective C. trachomatis vaccine started more than a century ago, and although whole-organism vaccines were found to induce protective responses, their development stopped when enhanced disease was observed in some immunized individuals, a phenomenon known as vaccine-related immunopathology.Citation9,Citation11,Citation21,Citation23,Citation74,Citation75 Although this phenomenon is rare, it has been reported for other infectious diseases, such as RSV and measles.Citation76,Citation77 The rMOMP antigen, produced by recombinant technology in E. coli, is free from chlamydial contaminants that could be responsible for unwanted immune responses.
The efficacy of experimental vaccine formulations is tested in different animal models.Citation78,Citation79 To facilitate the interpretation of immunological responses the majority of vaccination experiments in animal models are performed in inbreed strains. This approach is extremely helpful for characterizing immune responses to vaccination and determining which correlate with protection. A limitation of using inbred animals is that they do not have the highly heterogeneous genetic diversity that humans have. The heterogeneous human genetic diversity means their response to the same immunological stimuli can vary. To mimic the heterogeneity better, we used outbred female CD-1 mice in this study. To our knowledge this is the first report of vaccine-induced protection in an outbred mouse strain against a vaginal challenge with C. trachomatis. This represents a significant step forward in chlamydia vaccine development. Previous reports have shown protection using homologous rMOMP in inbred mice challenged in the genital tract with C. trachomatis or C. muridarum.Citation65,Citation67,Citation80
One limitation of the study is the lack of overt pathogenicity of the C. trachomatis serovar E in mice. The serovar E is a human pathogen with little affinity for murine cells and tissues which could explain its lack of pathogenicity. Moreover, C. trachomatis is generally not very virulent, even in humans. Infections are frequently asymptomatic, with overt pathogenicity in female upper genital tract typically detected only during severe acute infections or after years of chronic infection. Hence, reproducing C. trachomatis pathology in a time-contracted animal model is a challenge. The absence of hydrosalpinx formation, an indicator of upper genital tract pathology, confirms that none of the vaccinated or sham-vaccinated control mice developed long-term sequelae during the study. These results support the limited pathogenic nature of C. trachomatis in mice when delivered by the vaginal route.Citation81-83
In conclusion, our results showed that the SPA08 vaccine formulation with no phosphate substitution provides better protection than formulations with phosphate substitution against a vaginal C. trachomatis challenge in mice, probably via a Th1 mechanism. Furthermore, it establishes the ability of a vaccine formulated with rMOMP to elicit protection in individuals with a broad diversity of genetic backgrounds.
Disclosure of potential conflicts of interest
SFA, SG and VS are employees of Sanofi Pasteur. LV was employed by Sanofi Pasteur at the time this work was performed. SP, DFT, CC, LMdM declare no conflicts of interest.
Acknowledgments
The authors thank Ausra Mancevski for her supportive role and helpful discussion on the concept and design of this study. The authors acknowledge medical writing and editorial assistance from Margaret Haugh, PhD (MediCom Consult, Villeurbanne, France), funded by Sanofi Pasteur, and editorial assistance and manuscript coordination from Sandrine Buisson (Sanofi Pasteur, Lyon, France).
Additional information
Funding
References
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi:10.1371/journal.pone.0143304.
- CDC. Sexually transmitted disease surveillance 2017. prevention DoS, ed. Atlanta, GA: Department of Health and Human Services; 2018. p. 1–168
- Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–36. doi:10.1001/jama.291.18.2229.
- Ness RB, Smith KJ, Chang CC, Schisterman EF, Bass DC. Prediction of pelvic inflammatory disease among young, single, sexually active women. Sex Transm Dis. 2006;33:137–42. doi:10.1097/01.olq.0000187205.67390.d1.
- Stamm W. Chlamydia trachomatis infections of the adult. In: Holmes KK, WE Stamm PS, Piot P, Wasserheit JW, Corey L, Cohen MS, Watts DH, editors. Sex transm dis. New York, NY: McGrawHill Book Co.; 2008. p. 575–93.
- Arth C, Von Schmidt B, Grossman M, Schachter J. Chlamydial pneumonitis. J Pediatr. 1978;93:447–49. doi:10.1016/S0022-3476(78)81155-X.
- Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16:235–44. doi:10.1053/j.spid.2005.06.004.
- Stutman HR, Rettig PJ, Reyes S. Chlamydia trachomatis as a cause of pneumonitis and pleural effusion. J Pediatr. 1984;104:588–91. doi:10.1016/S0022-3476(84)80554-5.
- Schachter J, Dawson CR. Human chlamydial infections. Littleton (Mass): PSG Pub. Co.; 1978.
- Wang SP, Grayston JT. A potency test for trachoma vaccine utilizing the mouse toxicity prevention test. Am J Ophthalmol. 1967;63:Suppl:1443–54. doi:10.1016/0002-9394(67)94130-X.
- Taylor HR. Trachoma: a blinding scourge from the Bronze Age to the twenty-first century. Victoria (Australia): Haddington Press Pry Ltd; 2008.
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi:10.1038/nri1551.
- Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi:10.1128/IAI.70.6.2741-2751.2002.
- Darville T, O’Neill JM, Andrews CW Jr., Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–97. doi:10.4049/jimmunol.171.11.6187.
- Karimi O, Ouburg S, de Vries HJ, Pena AS, Pleijster J, Land JA, Morre SA. TLR2 haplotypes in the susceptibility to and severity of Chlamydia trachomatis infections in Dutch women. Drugs Today (Barc). 2009;45(Suppl):B:67–74.
- European Centre for Disease Prevention and Control. Guidance on chlamydia control in Europe - 2015. Stockholm, Sweden: ECDC; 2016.
- Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–92. doi:10.1097/00007435-199207000-00001.
- Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. doi:10.1086/jid.2005.192.issue-10.
- Gotz H, Lindback J, Ripa T, Arneborn M, Ramsted K, Ekdahl K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis. 2002;34:28–34. doi:10.1080/00365540110077001.
- de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–27. doi:10.1016/0264-410X(95)80022-6.
- Farris CM, Morrison RP. Vaccination against chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79:986–96. doi:10.1128/IAI.00881-10.
- Rockey DD, Wang J, Lei L, Zhong G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines. 2009;8:1365–77. doi:10.1586/erv.09.98.
- de la Maza LM, Zhong G, Brunham RC. Update on Chlamydia trachomatis vaccinology. Clin Vaccine Immunol. 2017;24:e00543–16.
- Zhong G, Brunham RC, de la Maza LM, Darville T, Deal C. National Institute of Allergy and Infectious Diseases workshop report: “Chlamydia vaccines: the way forward”. Vaccine. 2017;37:7346–54.
- Phillips S, Quigley BL, Timms P. Seventy years of chlamydia vaccine research - limitations of the past and directions for the future. Front Microbiol. 2019;10:70. doi:10.3389/fmicb.2019.00070.
- Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–60. doi:10.1128/IAI.73.12.8153-8160.2005.
- Vasilevsky S, Greub G, Nardelli-Haefliger D, Baud D. Genital Chlamydia trachomatis: understanding the roles of innate and adaptive immunity in vaccine research. Clin Microbiol Rev. 2014;27:346–70. doi:10.1128/CMR.00105-13.
- Watkinson A, Soliakov A, Ganesan A, Hirst K, Lebutt C, Fleetwood K, Fusco PC, Fuerst TR, Lakey JH. Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions. Clin Vaccine Immunol. 2013;20:1659–68. doi:10.1128/CVI.00320-13.
- Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, HogenEsch H, Mancinelli R, Hem SL. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine. 2009;27:888–92. doi:10.1016/j.vaccine.2008.11.078.
- Iyer S, HogenEsch H, Hem SL. Effect of the degree of phosphate substitution in aluminum hydroxide adjuvant on the adsorption of phosphorylated proteins. Pharm Dev Technol. 2003;8:81–86. doi:10.1081/PDT-120017526.
- Ljutic B, Ochs M, Messham B, Ming M, Dookie A, Harper K, Ausar SF. Formulation, stability and immunogenicity of a trivalent pneumococcal protein vaccine formulated with aluminum salt adjuvants. Vaccine. 2012;30:2981–88. doi:10.1016/j.vaccine.2012.02.038.
- Visan L, Sanchez V, Kania M, de Montfort A, de la Maza LM, Ausar SF. Phosphate substitution in an AlOOH - TLR4 adjuvant system (SPA08) modulates the immunogenicity of Serovar E MOMP from Chlamydia trachomatis. Hum Vaccin Immunother. 2016;12:2341–50. doi:10.1080/21645515.2016.1168958.
- Pal S, Peterson EM, de la Maza LM. Induction of protective immunity against a Chlamydia trachomatis genital infection in three genetically distinct strains of mice. Immunology. 2003;110:368–75. doi:10.1046/j.1365-2567.2003.01748.x.
- Polkinghorne A, Hanger J, Timms P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet Microbiol. 2013;165:214–23. doi:10.1016/j.vetmic.2013.02.026.
- Kollipara A, Polkinghorne A, Wan C, Kanyoka P, Hanger J, Loader J, Callaghan J, Bell A, Ellis W, Fitzgibbon S, et al. Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Vet Microbiol. 2013;167:513–22. doi:10.1016/j.vetmic.2013.08.009.
- Kollipara A, Wan C, Rawlinson G, Brumm J, Nilsson K, Polkinghorne A, Beagley K, Timms P. Antigenic specificity of a monovalent versus polyvalent MOMP based Chlamydia pecorum vaccine in koalas (Phascolarctos cinereus). Vaccine. 2013;31:1217–23. doi:10.1016/j.vaccine.2012.12.057.
- Kollipara A, George C, Hanger J, Loader J, Polkinghorne A, Beagley K, Timms P. Vaccination of healthy and diseased koalas (Phascolarctos cinereus) with a Chlamydia pecorum multi-subunit vaccine: evaluation of immunity and pathology. Vaccine. 2012;30:1875–85. doi:10.1016/j.vaccine.2011.12.125.
- Nyari S, Booth R, Quigley BL, Waugh CA, Timms P. Therapeutic effect of a Chlamydia pecorum recombinant major outer membrane protein vaccine on ocular disease in koalas (Phascolarctos cinereus). PLoS One. 2019;14:e0210245. doi:10.1371/journal.pone.0210245.
- Marangoni A, Foschi C, Nardini P, D’Antuono A, Banzola N, Di Francesco A, Ostanello F, Russo I, Donati M, Cevenini R, et al. Chlamydia trachomatis serovar distribution and other sexually transmitted coinfections in subjects attending an STD outpatients clinic in Italy. New Microbiol. 2012;35:215–19.
- Morre SA, Rozendaal L, van Valkengoed IG, Boeke AJ, van Voorst Vader PC, Schirm J, de Blok S, van Den Hoek JA, van Doornum GJ, Meijer CJ, et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: an association with clinical manifestations? J Clin Microbiol. 2000;38:2292–96.
- van Duynhoven YT, Ossewaarde JM, Derksen-Nawrocki RP, van der Meijden WI. van de Laar MJ. Chlamydia trachomatis genotypes: correlation with clinical manifestations of infection and patients’ characteristics. Clin Infect Dis. 1998;26:314–22. doi:10.1086/516291.
- Verweij SP, Lanjouw E, Bax CJ, Quint KD, Oostvogel PM, Dorr PJ, Pleijster J, de Vries HJ, Peters RP, Ouburg S, et al. Serovar D and E of serogroup B induce highest serological responses in urogenital Chlamydia trachomatis infections. BMC Infect Dis. 2014;14:3. doi:10.1186/1471-2334-14-3.
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–76. doi:10.1128/IAI.31.3.1161-1176.1981.
- Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–62. doi:10.1128/IAI.62.8.3354-3362.1994.
- Cheng C, Bettahi I, Cruz-Fisher MI, Pal S, Jain P, Jia Z, Holmgren J, Harandi AM, de la Maza LM. Induction of protective immunity by vaccination against Chlamydia trachomatis using the major outer membrane protein adjuvanted with CpG oligodeoxynucleotide coupled to the nontoxic B subunit of cholera toxin. Vaccine. 2009;27:6239–46. doi:10.1016/j.vaccine.2009.07.108.
- de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–97. doi:10.1128/IAI.62.5.2094-2097.1994.
- Pal S, Peterson EM, de la Maza LM. Susceptibility of mice to vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect Immun. 2001;69:5203–06. doi:10.1128/IAI.69.8.5203-5206.2001.
- Buwitt-Beckmann U, Heine H, Wiesmuller K-H, Jung G, Brock R, Akira S, Ulmer A. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–89. doi:10.1002/eji.200424955.
- Swenson CE, Schachter J. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex Transm Dis. 1984;11:64–67. doi:10.1097/00007435-198404000-00002.
- Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–91. doi:10.1128/IAI.56.4.885-891.1988.
- de la Maza LM, Peterson EM, Goebel JM, Fennie CW, Czarniecki CW. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun. 1985;47:719–22. doi:10.1128/IAI.47.3.719-722.1985.
- Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. Ilar J. 2002;43:244–58. doi:10.1093/ilar.43.4.244.
- HogenEsch H, O’Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018;3:51. doi:10.1038/s41541-018-0089-x.
- Al-Zeer MA, Al-Younes HM, Lauster D, Abu Lubad M, Meyer TF. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy. 2013;9:50–62. doi:10.4161/auto.22482.
- Batteiger BE, Xu F, Johnson RE, Rekart ML. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis. 2010;201(Suppl 2):S178–89. doi:10.1086/652400.
- Rank RG, Whittum-Hudson JA. Protective immunity to chlamydial genital infection: evidence from animal studies. J Infect Dis. 2010;201(Suppl 2):S168–77. doi:10.1086/652399.
- Zhong GM, Peterson EM, Czarniecki CW, Schreiber RD, de la Maza LM. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–57. doi:10.1128/IAI.57.1.152-157.1989.
- Olsen AW, Lorenzen EK, Rosenkrands I, Follmann F, Andersen P. Protective effect of vaccine promoted neutralizing antibodies against the intracellular pathogen Chlamydia trachomatis. Front Immunol. 2017;8:1652. doi:10.3389/fimmu.2017.01652.
- Peterson EM, Cheng X, Motin VL, de la Maza LM. Effect of immunoglobulin G isotype on the infectivity of Chlamydia trachomatis in a mouse model of intravaginal infection. Infect Immun. 1997;65:2693–99. doi:10.1128/IAI.65.7.2693-2699.1997.
- Poston TB, Darville T. Chlamydia trachomatis: protective adaptive responses and prospects for a vaccine. Curr Top Microbiol Immunol. 2018;412:217–37. doi:10.1007/82_2016_6.
- Peterson EM, Cheng X, Pal S, de la Maza LM. Effects of antibody isotype and host cell type on in vitro neutralization of Chlamydia trachomatis. Infect Immun. 1993;61:498–503. doi:10.1128/IAI.61.2.498-503.1993.
- Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–82. doi:10.1016/S0264-410X(97)00206-5.
- Pal S, Bravo J, Peterson EM, de la Maza LM. Protection of wild-type and severe combined immunodeficiency mice against an intranasal challenge by passive immunization with monoclonal antibodies to the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Infect Immun. 2008;76:5581–87. doi:10.1128/IAI.00574-08.
- Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–83. doi:10.1128/IAI.00622-10.
- Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the major outer membrane protein. J Infect Dis. 2015;212:978–89. doi:10.1093/infdis/jiv137.
- Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections. Curr Opin Infect Dis. 2017;30:77–86. doi:10.1097/QCO.0000000000000343.
- Carmichael JR, Pal S, Tifrea D, de la Maza LM. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine. 2011;29:5276–83. doi:10.1016/j.vaccine.2011.05.013.
- Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, Luis M, et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009;182:8063–70. doi:10.4049/jimmunol.0804375.
- Tifrea DF, Pal S, Popot JL, Cocco MJ, de la Maza LM. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J Immunol. 2014;192:5201–13. doi:10.4049/jimmunol.1303392.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci. 1992;89:12180–84. doi:10.1073/pnas.89.24.12180.
- Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116:1167–73. doi:10.1172/JCI28607.
- Qiu S, Zhang J, Tian Y, Yang Y, Huang H, Yang D, Lu M, Xu Y. Reduced antigenicity of naturally occurring hepatitis B surface antigen variants with substitutions at the amino acid residue 126. Intervirology. 2008;51:400–06. doi:10.1159/000205265.
- Ionescu-Matiu I, Kennedy RC, Sparrow JT, Culwell AR, Sanchez Y, Melnick JL, Dreesman GR. Epitopes associated with a synthetic hepatitis B surface antigen peptide. J Immunol. 1983;130:1947–52.
- Nicolle C, Cuenod A, Baizot L. Etude experimentale du trachome. Arch Instit Pasteur de Tunis. 1913;4:157–82.
- Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31:1892–97. doi:10.1016/j.vaccine.2013.01.024.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. A typical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–80. doi:10.1001/jama.1967.03130250057008.
- van Drunen Littel-van den Hurk S, Watkiss E. Pathogenesis of respiratory syncytial virus. Current Opinion in Virology. 2012;2:300–05. doi:10.1016/j.coviro.2012.01.008.
- Levine MM. New generation vaccines. New York, NY: Informa Healthcare USA, Inc.; 2010.
- Plotkin SA, Orenstein WA, Offit PA. Plotkin’s vaccines. Philadelphia (PA): Elsevier; 2018.
- Olsen AW, Theisen M, Christensen D, Follmann F, Andersen P. Protection against Chlamydia promoted by a subunit vaccine (CTH1) compared with a primary intranasal infection in a mouse genital challenge model. PLoS One. 2010;5:e10768. doi:10.1371/journal.pone.0010768.
- Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Lett. 1981;12:111–15. doi:10.1111/j.1574-6968.1981.tb07622.x.
- Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–16.
- Darville T, Andrews CW Jr., Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–73. doi:10.1128/IAI.65.8.3065-3073.1997.