ABSTRACT
Mismatch between circulating influenza B viruses and vaccine strains occurs frequently. In a randomized, double-blind, controlled phase III clinical study, healthy children aged 6–35 months were randomized into three groups at a ratio of 2:1:1, received two doses of quadrivalent influenza vaccines (QIVs) or licensed trivalent influenza vaccines (TIVs). The primary objective was to evaluate the non-inferiority immunogenicity of QIV compared with the two TIVs, containing B/Victoria or B/Yamagata strain. Safety information was collected for 28 days after each vaccination. Serious adverse events (SAEs) were monitored for 6 months after the second vaccination. A total of 2146 subjects (QIV: 1069, TIV–Vic: 540, TIV-Yam: 537) were enrolled in this study. QIV was found non-inferior to TIVs for shared strains (A/H1N1 and A/H3N2) and corresponding BY strain based on hemagglutination inhibition (HI) antibodies 28 days after the second dose of vaccination. The resulted geometric mean titer (GMT) ratios (QIV/TIV) were 0.98 (0.89, 1.07) for H1N1, 0.95 (0.85, 1.05) for H3N2 and 0.89 (0.81, 0.98) for BY. And the seroconversion rate differences (QIV-TIV) were −0.46% (−3.24%, 2.31%) for H1N1, −1.95% (−5.54%, 1.65%) for H3N2 and −3.58% (−8.11%, 0.95%) for BY. The BV strain in QIV did not reach the non-inferiority criteria, with GMT of 1:52.25 (vs. 1:61.02 of TIV–Vic) and seroconversion rate of 59.49% (vs. 66.85% of TIV–Vic). No increased safety concerns occurred in QIV group. Candidate QIV can provide good protection for children aged 6 to 35 months, and its immunogenicity and safety were proved.
Clinical Trials Registration
ClinicalTrials.gov number: NCT03859141.
Introduction
Influenza which belongs to acute respiratory infectious diseases is caused by influenza virus. Human influenza viruses mainly include influenza A and B. The primary influenza A subtypes are H1N1 and H3N2, and the primary influenza B viruses are Victoria and Yamagata. Before the 1980s, the main strain spreads in humans was B/Yamagata, but since the 1980s, B/Victoria strain has emerged. Then, both Victoria and Yamagata are mutating and spreading around the world,Citation1 with an annual attack rate of 5%~10% in adults and 20%~30% in children.Citation2 Most persons who contract with the influenza will recover without sequelae. But some people may suffer serious illness, hospitalization, or even death, especially for young children and other people with reduced immunity.Citation3
Vaccination remains the most effective way to prevent influenza and can significantly reduce the risk of attack rate and severe complications. Since 2010, routine annual influenza vaccination for all persons aged ≥ 6 months has been recommended in American.Citation4 In 1952, WHO established Global Influenza Surveillance Network (GISN) and it renamed Global Influenza Surveillance and Response System (GISRS) in 2011. Based on the surveillance data, the WHO recommends the trivalent influenza vaccines (TIV) for the next season, including B/Victoria or B/Yamagata. But mismatch between the two B lineages and vaccines occurs frequently.Citation5–Citation8 For instance, it has been estimated that discrepancies between the circulating B lineage and recommendation vaccine occurred in about one-quarter of seasons from 2000 to 2013.Citation9 Therefore, quadrivalent influenza vaccines (QIVs) which can provide broader protection were expected.
In China, the epidemic of influenza is a serious threat to people’s health. A study based on Severe Acute Respiratory Infection (SARI) surveillance showed that hospitalization rates in 2010 ~ 2011 and 2011 ~ 2012 reached 115/100 thousand and 142/100 thousand, respectively, mainly affecting children under 5 years of age.Citation10 However, the overall coverage in China is less than 2% at present, which is far behind the average of developed countries.Citation11 A study revealed that the vaccination rate is only about 26.4% among children ≤5 years old in 2009-2012.Citation12
Currently, influenza vaccines have not been included in National Immunization Program (NIP) in China. According to the recommendation of China CDC,Citation13 the priority population included children aged 6-59 months, the elderly, people with specific chronic diseases, medical workers, family members or caregivers of infants younger than 6 months, and pregnant or women preparing for pregnant during flu seasons. In China, the licensed influenza vaccines include trivalent and quadrivalent inactivated vaccine. TIVs are used for population ≥6 months of age, 0.25 ml for children aged 6-35 months and 0.5 ml for children and adolescent ≥36 months of age. However, the licensed QIVs are recommended only for population over the age of 3, with one specification of 0.5 ml. Considering the very young children are high-risk group, QIVs for children under 3 years are desperately needed. In this study, we aim to evaluate the immunogenicity and safety of a candidate QIV produced by Sinovac (Beijing) for children aged 6-35 months.
Materials and methods
Study design
This randomized, double-blind, controlled phase Ш clinical trial (ClinicalTrials.gov number: NCT03859141) was conducted in Pizhou and Guanyun County, Xuzhou and Lianyungang City, Jiangsu Province, China. Between February 2018 and March 2018, a total of 2320 subjects aged 6–35 months were enrolled in this study. All subjects were randomized into three groups at a ratio of 2:1:1, received two doses of QIVs or two doses of licensed TIVs, with 1160 subjects in QIV and 580 subjects in each TIV.
Study population
Eligible subjects were children aged 6–35 months, full term delivered, and with a birth weight over 2500 g. The exclusion criteria included the following: (1) seasonal influenza vaccination during the year; (2) seasonal influenza in the past 6 months; (3) axillary temperature > 37°C; (2) acute illness within 3 days prior to vaccination or chronic diseases if any; (4) history of vaccine allergies or severe side effects; (5) any known immunodeficiency; (6) severe malnutrition, congenital malformations, developmental disorders or serious chronic diseases; (7) received blood products, immunosuppressants, hormones and other research drugs within 3 months before vaccination; (8) received live attenuated vaccines within 30 days, or received subunit vaccines or inactivated vaccines within 7 days before vaccination; (9) abnormal physical examination results; (10) any factors or considered inappropriate for clinical trial.
Randomization and double-blind
The randomization and blind setting were entrusted to an independent third party. All vaccines were randomized and subjects were enrolled in sequence. First, SAS software was used to generate two same sets of random numbers. Then, all vaccines were relabeled with a random number and both test and control vaccines packed the same packaging. The random number of the vaccine was corresponding to the group of vaccine. Finally, each subject was assigned a random number in the order they enrolled and given the corresponding vaccine. The internal and external packing between the test and controlled vaccines were both the same.
Vaccine
All vaccines in this study were manufactured by Sinovac (Beijing) Biotech Co., Ltd, in accordance with the Preparation and Verification of Quadrivalent Influenza Vaccine after verified by National Institute for Food and Drug Control in China. QIV contained A/Michigan/45/2015 (H1N1), A/Hong Kong/4801/2014 (H3N2), B/Brisbane/60/2008 (B Victoria lineage) and B/Phuket/3073/2013 (B Yamagata lineage) four viral strains. The comparator TIV–Vic and TIV-Yam contained the unique strain B/Victoria or B/Yamagata, in addition to the shared strains A/H1N1 and A/H3N2. TIVs containing BV or BY strains were both licensed in China. Each subject was administered with either two doses of QIV or comparator TIVs on day 0 and day 28. The approach of the administration was the intramuscular injection of the upper arm lateral deltoid.
The specification of all vaccines was 0.25ml/syringe, contained 7.5 μg hemagglutinin/strain. The lot numbers of QIV and TIV containing BY strain were both 201706002, valid until June 4, 2018. The lot number of TIV containing BV strain was 201706007, valid until June 1, 2018. All vaccines were stored or transported in 2 ~ 8°C and frozen was forbidden.
Immunogenicity assessment
The primary objective was to evaluate the immunogenicity of the QIV non-inferior to that of the two TIV comparators 28 days after the second dose of vaccination. The co-primary endpoints include the following: the lower limits (LL) of the two-sided 95% confidence interval (CI) on the ratio of the GMTs (GMTtest group/GMTcontrol group) should meet or exceed 0.67; meanwhile, the LL of the two-sided 95% CI on the difference between the seroconversion rates (Seroconversiontest group-Seroconversioncontrol group) should meet or exceed −10%. The secondary objective was to evaluate the immunogenicity of the QIV was superior for the unique strains compared with the two TIV comparators. The co-secondary endpoints including the following: the LL of the two-sided 95% CI on the ratio of the GMTs (GMTtest group/GMTcontrol group) should meet or exceed 1.5. Meanwhile, the LL of the two-sided 95% CI on the difference between the seroconversion rates (Seroconversiontest group-Seroconversioncontrol group) should meet or exceed 10%. The positive standard was defined as the hemagglutination inhibition (HI) antibody titers ≥ 1:10. Protective level was defined as the HI antibody titers ≥ 1:40. Seroconversion rates referred that the percentage of subjects with either a prevaccination HI titer <1:10 and a postvaccination HI titer ≥1:40 or a prevaccination HI titer ≥1:10 and a minimum four-fold rise in post-vaccination HI antibody titer.
Safety assessment
Solicited injection-site and systemic reactions were recorded for 7 days after each vaccination. The injection-site reactions include pain, induration, redness, swelling, rash and pruritus; the systematic reactions included fever, diarrhea, activity level, loss of appetite, vomiting and allergic reaction. Unsolicited adverse events were collected for 28 days after each vaccination. Serious adverse events (SAEs) were collected for 6 months after the second dose.
The grading standard of adverse reactions was based on the Guidelines for grading standard of adverse reactions in clinical trials of preventive vaccinesCitation14 produced by Chinese State Food and Drug Administration (SFDA) and Division of microbiology and infectious diseases (DMID) pediatric toxicity tablesCitation15 produced by National Institute of Health (NIH) and National Institute of Allergy and Infectious Diseases (NIAID).
Serological methods
Blood samples were collected pre-vaccination (day 0) and at day 56 (~56 + 7 days) postvaccination. All serum samples were tested by National Institutes for Food and Drug Control. The HI antibody was detected by micro-hemagglutination inhibition test.
Statistical analysis
SAS version 9.4 software was used to conduct statistical analysis. Safety analyses were run on the safety set (SS) and immunogenicity analyses were run on each per-protocol set (PPS). The two TIVs were pooled when comparing the response of H1N1 and H3N2. For BV and BY strains, QIV was compared with the respective TIV containing the same influenza B for inferiority test and with the TIV not containing the same influenza B for superiority test.
Seroconversion rates and GMTs of the four viral strains were the primary indicators. Clopper–Pearson was used to calculate the two-sided 95% CI of the seroconversion rates and CMH-χ2 test was used to compare the rate difference among groups. GMTs after logarithmic conversion were conducted statistical test using group t-test.
Results
Study population
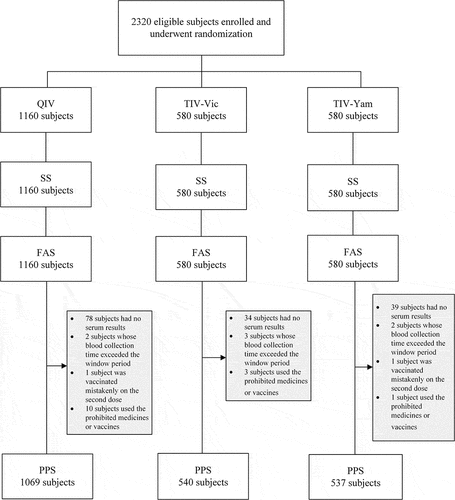
A total of 2320 subjects were enrolled in this study, with 1160 subjects in QIV and 580 subjects in each TIV. All subjects received at least one dose of vaccine and enrolled in a safety set (SS) and full analysis set (FAS). Of the 2320 subjects, 151 subjects had no serum results, of which 78 subjects in QIV, 34 subjects in TIV–Vic and 39 subjects in TIV-Yam. In addition, seven subjects were collected the blood samples after window period with two subjects in QIV, three subjects in TIV–Vic and two subjects in TIV-Yam. One subject in QIV and TIV–Vic was vaccinated wrong with the second dose. Fourteen subjects used prohibited medicines or vaccines during the clinical trial. Finally, 2146 subjects enrolled into the PPS, with 1069 subjects for QIV, 540 subjects for TIV–Vic and 537 subjects for TIV-Yam (). The basic characteristics of subjects are shown in . There were no statistical differences in age, gender, height and weight ().
Table 1. Demographic characteristic of study subjects.
Immunogenicity
The immunogenicity results of H1N1 and H3N2 are shown in . Before vaccination, the antibody levels were balanced and comparable between QIV and Combined-TIV recipients. After two doses of vaccination, the seroconversion rates of H1N1 and H3N2 in QIV were 87.46% (95% CI: 85.33%, 89.39%) and 75.40% (95% CI: 72.70%, 77.95%), respectively. And the difference of seroconversion rates of H1N1 and H3N2 (QIV-Combined-TIV) was −0.46% (95% CI: −3.24%, 2.31%) and −1.95% (95% CI: −5.54%, 1.65%). The corresponding GMTs were 1:329.90 (95% CI: 299.95, 362.85) and 1:136.76 (95% CI: 122.41, 152.80), with the ratio (QIV/TIV) of 0.98 (95% CI: 0.89, 1.07) and 0.95 (95% CI: 0.85, 1.05). In this study, H1N1 and H3N2 strains of QIV both met the primary objective. Meanwhile, seroprotective rates against H1N1 and H3N2 in QIV reached 92.05% and 77.64%, respectively, compared with 39.66% and 23.48% before vaccination.
Table 2. The immunogenicity of H1N1 and H3N2 strains before and after vaccination by groups.
The immunogenicity results of BV and BY strain are shown in . Before immunization, the seroprotective rates of subjects were only about 10% for BV strain and nearly 40% for BY strain. After two doses of immunization, seroconversion rates against BV strain in QIV were 59.49% (95% CI: 56.48%, 62.45%), with the rate differences of −7.36% (95% CI: −12.30%, −2.41%) for QIV-TIV–Vic and 31.56% (95% CI: 26.76%, 36.36%) for QIV-TIV-Yam, respectively. GMT against BV strain in QIV was 1:52.25 (95% CI: 49.01, 55.70), with the ratio of 0.87 (95% CI: 0.79, 0.98) for QIV/TIV–Vic and 1.86 (95%CI:1.70, 2.09) for QIV/TIV-Yam. The LL of 95% CI of difference for seroconversion rates (QIV-TIV–Vic) was <-10%, so the BV strain of QIV did not reach the primary objective. But compared with TIV-Yam, the BV strain of QIV was superior and reached the secondary objective. Seroprotective rate against BV strain of QIV was 69.32% (95% CI: 66.46%, 72.07%), little lower than 78.15% (95% CI: 74.42%, 81.56%) of TIV–Vic. For BY, both the primary and secondary objectives were met: the GMT ratio was 0.89 (95% CI: 0.81, 0.98) and 2.57 (95% CI: 2.29, 2.82) for QIV/TIV-Yam and QIV-TIV–Vic, respectively. Meanwhile, the rate difference of seroconversion rates was −3.58% (95% CI: −8.11%, 0.95%) and 49.07% (95% CI: 44.62%, 53.51%) for QIV-TIV-Yam and QIV-TIV–Vic. The seroprotective rate was 85.03% (95% CI: 82.75%, 87.12%).
Table 3. The immunogenicity of BV and BY strains before and after vaccination by groups.
Safety
The frequencies of solicited adverse reactions are shown in . The incidences were 31.98% for QIV, 32.24% for TIV–Vic and 34.66% for TIV-Yam. There was no significant difference across the three groups (P = .5111). The incidence of grade 3 solicited reactions was 2.67% for QIV, which was higher than those of the TIV comparators (P = .0140). The most common local adverse reaction was redness at the injection site, with the incidence of 1.38%, 1.03% and 1.21% for the QIV, TIV–Vic and TIV-Yam, respectively. While the most frequently systematic reactions were fever followed by diarrhea and loss of appetite, with the incidence of 29.31%, 1.90% and 1.47% for QIV. No significant differences were observed across the three groups for all local and systemic reactions, except for diarrhea. The incidence of diarrhea for QIV was 1.90%, which was lower than those of TIVs (P = .0391).
Table 4. The adverse reactions after vaccination by groups.
A total of 31 SAEs were reported, of which 21 cases in QIV and 5 cases in each TIV. Only two SAEs in QIV were vaccine-related. The incidence was 0.17%. An SAE of upper respiratory infection was observed 3 days after the second vaccination in one 22.6-month-old subject and it resolved after 3 days. The other SAE of infection pneumonia occurred 3 days after the first vaccination in one 18.7-month-old subject, who recovered after 4 days. Both of two SAEs were considered vaccine-related due to the temporal relationship with the immunization.
Discussion
In this study, we evaluate the immunogenicity and safety of a candidate QIV produced by Sinovac (Beijing) based on a randomized, double-blind, controlled phase Ш clinical trial. To our knowledge, there is limited study on the immunogenicity and safety of QIV in young children, especially those younger than 3 years. Our study can provide more data support to the development of QIV for 6-35 months children.
In our study, H1N1, H3N2 and BY strains of QIV all reached the primary objective. The the seroconversion rates were 87.46%, 75.40% and 71.84%, and GMTs were 1:329.90, 1:136.76 and 1:104.30, respectively, which were consistent with the results reported.Citation16–Citation18 Meanwhile, the BY strain of QIV reached the secondary objective. The LL of 95% CI of seroconversion rates for the three strains were all ≥ 40%, and the seroprotective rate points were all ≥ 70%, reaching the criteria of international.Citation19,Citation20
For BV strain of QIV, not the primary but the secondary objective was met. The seroconversion rate of 59.49% and seroprotective rate of 69.32% were little lower than those of reported 66.4%~84.6% and 70.6%~88.0%,Citation16–Citation18 respectively. The LL of 95% CI of the seroconversion rate for BV strain and the seroprotective rate basically reached the international criteria. Through comparison, we found that the immunogenicity of B lineages strains was lower than those of A lineages strains in our study. Several QIV or TIV clinical trials observed the same findings in different age groups and the immune response of BV strain was the lowest among the four influenza strains.21-23 However, non-inferiority of QIVs produced by Sinovac against all strains has been demonstrated in population ≥3 years (unpublished data). Inferiority of BV strain was only found in 6-35 months children. Another study showed the similar results that the GMTs of all strains of QIV were non-inferior to TIV’s except for BV strain in 6-35 months children.24 Meanwhile, a randomized, double-blind, active-controlled phase Ш trial based on children and adolescents 6 months to 18 years of age also found that the seroconversion rate and seroprotective rate against BV strain for QIV were not significantly higher than those of TIV control in subgroup of 6 month to 3 years, but there was a significantly increase in subgroups of 3 to < 9 years and 9 to <19 years.25 Considering the balanced positive rates, GMTs and seroprotective rates before vaccination among the three groups, we excluded the effect of antibody levels pre-vaccination on immune response. The reason why BV strain for QIV not reaching the non-inferiority compared with TIV containing BV strain may be the following: there may exist an interference effect of spectator.Citation26 The immune response resource in lymph nodes is limited in children 6 to 35 months of age because of the immaturity of the immune system, such as the pathway of antigen acquisition, chemotactic factor, activation signal and so on. Therefore, the two B antigens in QIV may compete with each other.
In our study, we also found that the cross-protection was low between the two influenza B. The seroconversion rates of subjects were only 27.93% and 22.78% in TIVs containing certain influenza B strain against the other not containing B strain, with GMTs only 1:27.98 and 1:42.43, respectively. The seroprotective rate against BV strain for TIV-Yam was 42.83% compared with 69.32% for QIV (P<0.0001). And the seroprotective rate against BY strain for TIV-Vic was 61.85% compared with 85.03% for QIV (P<0.0001). A study revealed that eventual cross-protection was estimated to be low in the event of mismatch because of the great antigenic difference between the two B lineages viral strain.Citation27 In addition, this cross-protection effect was not stable. For instance, the BV strain in QIV had a good cross-protection efficacy on BY strain during the 2017–2018 influenza epidemic season, but only 23% in 2015–2016 influenza seasonal. Moreover, the cross-protection was only in a season where the vaccine contained B/Victoria and the circulating strain was B/Yamagata, but not on the opposite.Citation28 Children aged 6 to 35 months were generally susceptible to influenza. However, we found the antibody protective rates of the subjects against BV and BY strains were only 9.12%~10.29% and 37.42%~39.66% before vaccination, respectively. QIVs could prevent influenza A and B in children aged 6-35 months despite high levels of vaccine mismatch.Citation29 Thus, there is a great need for such a population to receive QIV to substitute the corresponding TIV composition. QIV might eliminate the possibility of seasonal mismatched B strains and confer greater protection than traditional TIV.
Regarding safety, 31.98% of QIV, 32.24% of TIV-Vic and 34.66% of TIV-Yam subjects notified solicited reactions. No significant difference was observed across the groups. Most adverse reactions were mild or moderate. Redness at the injection was the most common local adverse reaction; fever constituted the most frequently systematic adverse reactions followed by diarrhea and loss of appetite. While previous studies showed that the local adverse reactions mainly performed pain and redness at the injection site; the most common systematic adverse reactions were irritability/fussiness and drowsiness.Citation17,Citation30 The symptoms of adverse reactions seem to be different. Because of the temporal relationship with the vaccination, the two SAEs in QIV were considered to be vaccine-related, although the etiology was unknown.
There were also some limitations in our study. First, to date, the criteria to evaluate the immunogenicity of QIV non-inferior to the licensed influenza vaccine in pediatric were limited. Guidelines in healthy adults were referred to in our study, which might be higher than those observed in young children. Meanwhile, because immunogenicity is an indirect measure of clinical protection against influenza, an efficacy clinical study might be needed in the future. Second, there was no further analysis on susceptible population. The positive rates were between 35.85% and 74.30% before vaccination, and varied greatly among the four viral strains. The next analysis will be conducted on susceptible subjects to explore the immune response induced by QIV.
Conclusions
In conclusion, the QIV is immunogenic and has aroused no safety concerns, which confer greater protection for children aged 6–35 months.
Disclosure of potential conflicts of interest
Yuansheng Hu, Ningning Jia, Li Xu, and Jing Li are employees of Sinovac Biotech Co., LTD. All other authors: no conflicts.
Ethical statements
This study was conducted in compliance with Guidelines of Good Clinical Practice (GCP)Citation22 and the study protocol was approved by the independent ethics committee of Jiangsu CDC. Written informed consent was obtained from the parent(s) or guardian(s) of each subject before enrollment.
Acknowledgments
We greatly appreciate the volunteers and their parents for supporting this study, and staffs of the Pizhou Center for Disease Control and Prevention and Guanyun Center for Disease Control and Prevention for carrying out this study.
Additional information
Funding
References
- Jennings L, Huang QS, Barr I, Lee PI, Kim W, Buchy P, Sanicas M, Mungall BA, Chen J. Literature review of the epidemiology of influenza B disease in 15 countries in the asia-pacific region. Influenza Other Respir Viruses. 2018;12(3):383–411. doi:10.1111/irv.12522.
- Vaccines against influenza who position paper - november 2012. Wkly Epidemiol Rec. 2012;87(47):461–76. PMID:23210147.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-united states, 2018–19 influenza season. MMWR Recomm Rep. 2018;67(3):1–20. doi:10.15585/mmwr.rr6703a1.
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62.
- Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, Henriques CM, Njouom R, Fasce RA, Yu H, et al. Epidemiological and virological characteristics of influenza B: results of the global influenza B study. Influenza Other Respir Viruses. 2015;9(Suppl 1):3–12. doi:10.1111/irv.12319.
- Orsi A, Colomba GME, Pojero F, Calamusa G, Alicino C, Trucchi C, Canepa P, Ansaldi F, Vitale F, Tramuto F. Trends of influenza b during the 2010–2016 seasons in 2 regions of north and south Italy: the impact of the vaccine mismatch on influenza immunisation strategy. Human Vaccines & Immunotherapeutics. 2018;14(3):523–31. doi:10.1080/21645515.2017.1342907.
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza b lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis. 2014;59(11):1519–24. doi:10.1093/cid/ciu664.
- Trucchi C, Alicino C, Orsi A, Paganino C, Barberis I, Grammatico F, Canepa P, Rappazzo E, Bruzzone B, Sticchi L, et al. Fifteen years of epidemiologic, virologic and syndromic influenza surveillance: A focus on type B virus and the effects of vaccine mismatch in liguria region, Italy. Hum Vaccin Immunother. 2017;13(2):456–63. doi:10.1080/21645515.2017.1264779.
- Montomoli E, Torelli A, Manini I, Gianchecchi E. Immunogenicity and safety of the new inactivated quadrivalent influenza vaccine vaxigrip tetra: preliminary results in children ≥ 6 months and older adults. Vaccines. 2018;6(1):10. doi:10.3390/vaccines6010014.
- Yu H, Huang J, Huai Y, Guan X, Klena J, Liu S, Peng Y, Yang H, Luo J, Zheng J, et al. The substantial hospitalization burden of influenza in central China: surveillance for severe, acute respiratory infection, and influenza viruses, 2010–2012. Influenza Other Respir Viruses. 2014;8(1):53–65. doi:10.1111/irv.12205.
- Yang J, Atkins KE, Feng L, Pang M, Zheng Y, Liu X, Cowling BJ, Yu H. Seasonal influenza vaccination in China: Landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine. 2016;34(47):5724–35. doi:10.1016/j.vaccine.2016.10.013.
- Xu L, Qin Y, Yang J, Han W, Lei Y, Feng H, Zhu X, Li Y, Yu H, Feng L, Shi Y. Coverage and factors associated with influenza vaccination among kindergarten children 2–7 years old in a low-income city of north-western China (2014–2016). PLoS One. 2017;12(7):e0181539. doi: 10.1371/journal.pone.0181539.
- China CDC. Technical Guidelines for Seasonal Influenza Vaccination in China (2018–2019). 2018 edition.
- Note for Guidance on Harmonisation of Requirements for Influenza Vaccines; 1997 [accessed 1997 March 12]. https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-harmonisation-requirements-influenza-vaccines_en.pdf
- US Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for Industry Clinical Data Needed to support the Licensure of Seasonal Inactivated Influenza Vaccines; 2007 [accessed 2007 May 12]. https://www.fda.gov/media/73706/download.
- National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID). Division of Microbiology and Infectious Diseases (DMID). Pediatric Toxicity Tables, November 2007 draft; 2007 [accessed 2007 November]. https://www.niaid.nih.gov/sites/default/files/dmidpedtox.pdf.
- Chinese State Food and Drug Administration (SFDA). Guidelines for grading standard of adverse reactions in clinical trials of preventive vaccines; 2005 [accessed 2005 Oct 14]. http://www.nmpa.gov.cn/WS04/CL2196/322950.html
- Langley JM, Carmona Martinez A, Chatterjee A, Halperin SA, McNeil S, Reisinger KS, Aggarwal N, Huang LM, Peng CT, Garcia-Sicilia J, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: A phase Ш randomized controlled trial in children. J Infect Dis. 2013;208(4):5440–53. doi:10.1093/infdis/jit263.
- Langley JM, Wang L, Aggarwal N, Bueso A, Chandrasekaran V, Cousin L, Halperin SA, Li P, Liu A, McNeil S, et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012–2013: A randomized, double-blind, controlled, multicenter, multicountry, clinical trial. J Pediatric Infect Dis Soc. 2015;4(3):242–51. doi:10.1093/jpids/piu098.
- Wang L, Chandrasekaran V, Domachowske JB, Li P, Innis BL, Jain VK. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine in US children 6-35 months of age during 2013–2014: Results from a phase II randomized trial. J Pediatric Infect Dis Soc. 2016;5(2):170–9. doi:10.1093/jpids/piv041 http://www.nmpa.gov.cn/WS04/CL2196/322950.html
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–6. doi:10.1016/j.vaccine.2012.11.074.
- Greenberg DP, Robertson CA, Landolfi VA, Bhaumik A, Senders SD, Decker MD. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J. 2014; 33(6):630–6. doi:10.1097/inf.0000000000000254.
- Gorse GJ, Falsey AR, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults. Vaccine. 2015; 33(9):1151–9. doi:10.1016/j.vaccine.2015.01.025.
- Pepin S, Dupuy M, Borja-Tabora CFC, Montellano M, Bravo L, Santos J, de Castro JA, Rivera-Medina DM, Cutland C, Ariza M, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6–35months: A multi-season randomised placebo-controlled trial in the northern and southern hemispheres. Vaccine. 2019;37(13):1876–84. doi:10.1016/j.vaccine.2018.11.074.
- Eun BW, Lee TJ, Lee J, Kim KH, Kim DH, Jo DS, Shin SH, Kim H, Kim KH, Kim YK. A randomized, double-blind, active-controlled phase Ш trial of a cell culture-derived quadrivalent inactivated influenza vaccine in healthy south korean children and adolescents 6 months to 18 years of age. Pediatr Infect Dis J. 2019;38(9):e209–e215. doi:10.1097/inf.0000000000002406.
- Chen Q, Shi JC, Ye Q. Immune Interference of Carrier Proteins in Bacterial Glycoconjugate Vaccines. Chin J Biologicals. 2013;26(7):1034–9. doi:10.13200/j.cjb.2013.07.143.chenq.012 (In Chinese).
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28(9):2149–56. doi:10.1016/j.vaccine.2009.11.068.
- Segaloff HE, Leventer-Roberts M, Riesel D, Malosh RE, Feldman BS, Shemer-Avni Y, Key C, Monto AS, Martin ET, Katz MA. Influenza vaccine effectiveness against hospitalization in fully and partially vaccinated children in Israel: 2015–2016, 2016–2017, and 2017-2018. Clin Infect Dis. 2019;69(12):2153–61. doi:10.1093/cid/ciz125.
- Claeys C, Zaman K, Dbaibo G, Li P, Izu A, Kosalaraksa P, Rivera L, Acosta B, Arroba Basanta ML, Aziz A, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: A multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2(5):338–49. doi:10.1016/s2352-4642(18)30062-2.
- Chinese guidelines of Good Clinical Practice (GCP), 2004 edition.