ABSTRACT
Protective levels of antibodies induced by the MMR vaccine have been shown to decline over time, but actually there is not a formal recommendation about the opportunity of testing immunized HCWs to investigate the persistence of anti-Mumps IgG. This study aims to evaluate the long-time immunogenicity of MMR vaccination in a sample of medical students and residents of the University of Bari who attended the Hygiene Department for the biological risk assessment (April 2014-June 2018). A strategy for the management of non-responder subjects has been experimented and described. Two thousand students and residents, with documented immunization status (two doses of MMR vaccine), have been tested. 120/2,000 (6%; 95%CI = 5.0–7.1%) subjects did not show anti-Mumps IgG. This percentage was similar among males and females. After a third MMR dose, we noted a seroconversion of 90% of seronegative participants. No serious adverse events were recorded. An important proportion of subjects immunized for MMR do not show an antibodies protective titer. The immunogenicity and the safety of the third dose seem confirmed by our data. Including the screening model described in the routine assessment of the biological risk of medical students and HCWs may be a winning strategy in preventing Mumps nosocomial infection.
Introduction
Mumps is a viral vaccine-preventable disease that can infect infants and adult, in which can occasionally cause severe complications, including orchitis, oophoritis, mastitis, pancreatitis, encephalitis, meningitis and deafness.Citation1 In 2017, WHO reported 552,779 cases of Mumps, worldwide.Citation2 Mumps is endemic throughout the world, and achieving elimination in high-income countries is considered difficult due to the potential Mumps virus importations and the current two-dose vaccination program (“only” 88% effective against the disease). For US, the Healthy People 2020 target was a reduction goal (<500 reported cases annually), rather than an elimination goal.Citation3 The Healthy People 2020 target has not been met since 2013; during this time more than half of the reported Mumps cases were associated with outbreaks.Citation3 To achieve this goal, the universal mass vaccination using combined MMR or MMRV vaccine, both containing live-attenuated virus, is strongly encouraged.Citation4
The Centers for Disease Control and Prevention (CDC) recommends that children should get two doses of MMR vaccine, starting with the first dose at 12 to 15 months of age, and the second dose at 4 through 6 years of age. Teenagers and adults who do not have evidence of immunity against Mumps should get two doses of MMR vaccine with an interval of at least 28 days.Citation4 According to post-licensure data, one dose of MMR vaccine is 78% effective against Mumps, two doses vaccine are 88% effective;Citation4 the seroconversion rate for Mumps is of 94% after single dose.Citation5 Since the introduction of global mass vaccination, the MMR vaccine showed high safety,Citation6 cost-savingCitation7 and efficacy.Citation4
Since 2017, the Advisory Committee on Immunization Practices (ACIP), a committee within CDC, reviewed the available evidence and stated that a third dose of MMR vaccine is safe and effective at preventing Mumps for people exposed to the wild virus. Indeed, ACIP recommended a third dose for persons previously vaccinated with two doses being part of a group or population at increased risk for acquiring Mumps because of an outbreak.Citation8
In Italy, since 2003, the National Immunization Plan recommends two doses of MMR vaccine, following the CDC recommendations.Citation4 In 2017, the Italian Ministry of Health, by Decree-Law n. 73/2017, got compulsory Mumps vaccination.Citation9
Although vaccination strategy was very effective, the vaccine coverage has always been suboptimal,Citation10 far from the minimum coverage (≥95%) planned by Public Health Institutions.Citation11 Indeed, an Italian study settled the prevalence of susceptible for Mumps >20% among 2–14 years-old and >10% among 15–39 years-old subjects.Citation12
The few papers published on this topic suggested that the levels of Mumps antibodies decline over time and it seems that the immunity induced by successful primary immunization may persist for 15–20 years;Citation13 A 2006 study on a US university campus indicated lower levels of mumps neutralizing antibodies among students who had been vaccinated with a second MMR dose >15 years previously than among those who had been vaccinated 1–5 years previously (p > .05).Citation14 In a 2006 study on another US university campus, students with mumps were more likely to have received a second dose of MMR vaccine ≥10 years previously than their roommates without mumps;Citation15 However, another 2006 study from a US college campus identified no such association.Citation16 Furthermore, the impact of immunization strategies on the pattern of the disease could influence the effectiveness and the long-term immunogenicity of the MMR vaccine, as recent studies have shown for pertussis.Citation17 In particular, the reduction of natural booster could be related with a decline of the IgG level in fully vaccinated persons.
This study aims to evaluate the long-term immunogenicity of Mumps component of MMR vaccination and the effectiveness of the strategy of a third MMR booster dose in immunized adult subjects who did not show IgG against Mumps (non-responders subjects), outside an outbreak context. Indeed, in the “vaccination era”, the young adults are the age-group at higher risk of lacking anti-Mumps IgG and of disease severe complications.Citation17
Our study has been carried out in Apulia (South Italy, ˜4,000,000 inhabitants), a Region in which previous studies seemed to suggest an important prevalence of people susceptible for Mumps.Citation18,Citation19
Material and methods
The Italian Ministry of Health indicated that the Medical Schools and University Hospital have to apply for students and residents the same procedures provided by Italian Law on the Occupational Health and Safety for HCWs.Citation20 According to these recommendations, in April 2014 the Hygiene Department of the Bari Policlinico University Hospital planned a biological risk prevention program for students and residents of the Medical School of the University of Bari. As part of the program, the susceptibility/immunity against Mumps was assessed.
The study design is retrospective cohort study and the study sample is composed by Apulian students and residents of the Medical School of the University of Bari enrolled from April 2014 to June 2018 in the context of biological risk assessment. The subjects without an available vaccination history, never vaccinated or vaccinated with a single dose or ≥3 doses of MMR vaccine at baseline and with a history of Mumps infection were excluded. We considered in this survey only the subjects who had received, at the time of the enrollment, two doses of MMR vaccine (vaccine basal routine).
To our knowledge, the prevalence of vaccinated but not seroprotected for mumps HCWs is a topic not investigated in literature; for this reason, we were not able to make hypothesis about the prevalence of not seroprotected subjects and then to calculate the sample size. Because our study is a pilot experience, we opted to enroll a very large sample to make our results more consistent.
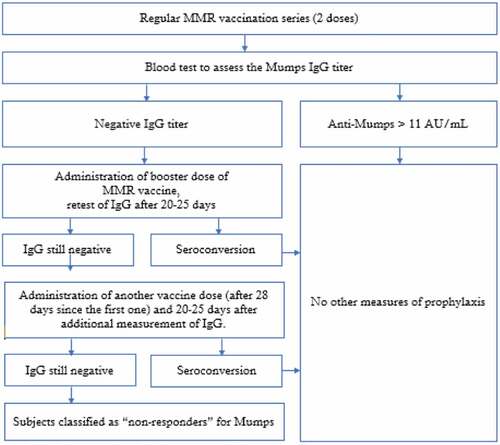
The vaccination status of each enrolled subject was assessed by the Regional Immunization Database (GIAVA).Citation20 For each enrolled participant, a 5 mL serum sample was collected to assess the immunity/susceptibility status for Mumps and tested by chemiluminescence (CLIA), using LIAISON® Measles IgG, a semi-quantitative method, performed with a standardized commercial method (Diasorin). This method defines the subjects with an anti-Mumps IgG titer >11 AU/mL (Arbitrary Unit/mL) as seroprotected.Citation21,Citation22
Tested subjects who showed a non-protective IgG titer received a third booster dose of MMR vaccine (M-M-RVAXPRO, administered subcutaneously in deltoid). Subjects with equivocal tests were retested and, if still equivocal, they were classified as negative. 20–25 days after the vaccination a new blood test was performed to retest IgG titers; if the value found in reevaluation exceeded the cutoff, the subject was classified as seroconverted; if the titer was still negative, another vaccine dose (28 days after the first booster) was administered and after 20–25 days an additional measurement of IgG was performed.
Subjects still seronegative are definitively classified as “non-responders” (Flow-). This management is consistent with protocols applied in some US Medical Schools;Citation23 to our knowledge, no other Italian Medical School applies the protocol we are describing.
Subjects who received the booster doses underwent 1-month follow-up in order to assess the insurgence of adverse effects.
For each enrolled subject a specific form was built, including information on patient id, gender, age at enrollment, dates of the routine MMR vaccine, Mumps IgG titer, date of first booster dose, IgG titers after first booster, date of second booster dose, IgG titers after second booster.
Compiled forms were entered in a database created by Excel spreadsheet and data analysis was performed by STATA MP15 software.
Continuous variables were described as mean±standard deviation and range, categorical variables as proportions, with the 95% confidence interval (95%CI), when appropriate. The Skewness and kurtosis test was used to evaluate the normality of continuous variables, but any of them was normally distributed or normalizable. The Wilcoxon’s rank sum test was used to compare continuous variables between gender, the Wilcoxon’s sign rank test was used to compare continuous variables between time of evaluation. The chi-square and exact Fisher tests were used to compare the proportions.
The multivariate logistic regression was used to assess the determinants of seroconversion after the vaccine basal routine, considering the gender (male vs. female), the age at enrollment (years), the age at the time of the first vaccination at the basal routine (years) and the age at the time of the second vaccination at the basal routine (years) as determinants; the adjusted Odds Ratio (aOR) values were calculated, with the 95%CI.
To assess the determinants of seroconversion after a booster dose, a multivariate logistic regression model was built, considering the seroconversion after booster doses as outcome and analyzing as determinants the gender (male vs. female), the age at enrollment (years), the age at the time of the first vaccination at the basal routine (years), the age at the time of the second vaccination at basal routine (years), the time from the first dose of the vaccine at basal routine to the booster dose (years), the time from the second dose of the vaccine at basal routine to the booster dose (years) and the time from the booster dose to the antibody re-titer evaluation (days); the aOR values were calculated, with the 95%CI.
The Hosmer-Lemeshow test was used to evaluate the goodness-of-fit of the multivariate logistic regression models.
The Protective Antibody Survival (PAS) was defined as the time from the second dose of routine MMR vaccine to the evaluation of the antibody titer.
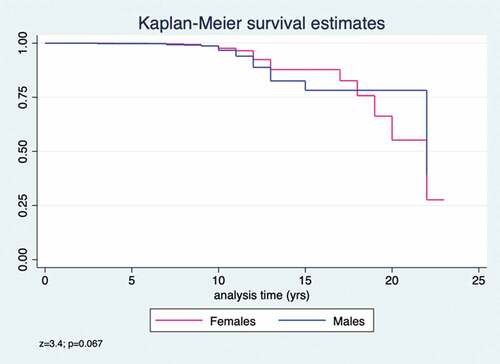
The Kaplan–Meier curves were used to evaluate PAS and the log-rank test was used to evaluate the differences between gender. The median time of PAS was estimated and the incidence rate persons-year of loss of seroprotection; the Incidence Rate Ratio (IRR) was calculated considering the females at denominator and the males at numerator.
To evaluate the determinants of PAS, the multivariate Cox semiparametric regression was used, considering as risk predictors the gender (male vs. female), the age at enrollment (years), the age at the first dose of routine vaccine (years) and the age at the second dose of routine vaccine (years). The Schoenfeld and scaled Schoenfeld residuals test was used to evaluate the proportionality assumption of the multivariate Cox semiparametric regression model; if one of the predictors was not proportional, we opted to stratify the regression model on the non-proportional predictor. The Gronnesby and Borgan test was used to evaluate the goodness-of-fit of the model.
For all tests, a p-value<0.05 was considered statistically significant.
Results
From April 2014 to June 2018 4,563 students and residents have been involved in our biological risk assessment program. The vaccination certificate was available for 4,225/4,563 (92.6%) and 2,000/4,225 (47.3%) received a complete Mumps/MMR vaccination schedule; 1,387 of 2,000 (69.4%) were female. The average age at enrollment was 21.1 ± 2.4 years (range = 18.0–38.0) with a little difference between females (21.1 ± 2.5; range = 18.0–38.0) and males (21.3 ± 2.4; range = 18.0–35.0; z = 2.1; p = .034), that could not affect the results of the study.
All the subjects with a complete baseline vaccination routine (two doses) were tested for anti-Mumps IgG. No one reported a history of Mumps.
1,880/2,000 (94.0%; 95%CI = 92.9–95.0%) subjects showed a protective anti-Mumps IgG titer; this proportion did not show statistically significant difference between females (n = 1,313/1,387; 94.7%; 95%CI = 93.3–95.8%) and males (n = 567/613; 92.5%; 95%CI = 90.1–94.5%; X2 = 3.5; p = .060). The overall geometric mean anti-Mumps IgG titer value was 112.7 (95%CI = 106.8–118.9), without statistically significant difference between females (117.2; 95%CI = 110.0–125.0) and males (103.0; 95%CI = 93.3–113.7; z = 0.9; p = .384). The GMT titer in seroprotected subjects wads equal to 135.3 (95%CI = 129.2–141.6), while in not seroprotected subjects was equal to 6.4 (95%CI = 6.0–6.8; p < .0001).
102/120 (85.0%) seronegative subjects received a booster dose; of these, 81/102 (79.4%) were reevaluated: 73/81 (90.1%; 95%CI = 81.5–95.6%) seroconverted and 8/81 (9.9%; 95%CI = 4.4–18.5%) were still negative. The seroconversion rate after a third booster dose did not show statistically significant difference between females (n = 46/49; 93.9%; 95%CI = 83.1–98.7%) and males (n = 27/32; 84.4%; 95%CI = 67.2–94.7%; X2 = 2.0; p = .253). The anti-Mumps IgG geometric mean titer value after a booster was 56.8 (95%CI = 44.5–72.4), without statistically significant difference between females (66.3; 95%CI = 48.9–89.8) and males (44.8; 95%CI = 29.6–67.6; z = 1.0; p = .332).
All seronegative subjects received one additional dose of MMR vaccine and 7/8 (87.5%) were retested for anti-Mumps IgG: 6/7 subject (85.7%; 95%CI = 42.1–99.6%) seroconverted. The GMT of subjects who received two booster doses were equal to 31.4 (95%CI = 11.6–84.5). Of these 7 subjects re-tested after two booster doses, 6/7 did not declare any chronic disease and 1/7 was affected by hypothyroidism; the subject that did not seroconvert was not affected by any disease.
Overall, of negative subjects at baseline, 99.2% (95%CI = 95.4–99.9%) seroconverted after one or two booster doses. The GMT in seronegative subjects at enrollment after booster dose(s) increased from 6.4 (95%CI = 6.0–6.8) to 66.7 (95%CI = 54.3–81.8; p < .0001).
The multivariate logistic regression shows that seropositivity at enrollment is associated with the age at the time of the second dose of MMR vaccine (aOR = 1.16; 95%CI = 1.08–1.24), while there are no associations with the other determinants (p > .05; ).
Table 1. Analysis of determinants of Mumps IgG seropositivity at the enrollment in a multivariate logistic regression model
The multivariate logistic regression did not show statistically significant association between the outcome of seroconversion after a booster dose and the determinants in analysis (p > .05; Chi-square = 7.1; p = .527).
The average time of PAS was 10.2 ± 3.0 years (range = 0.0–23.0); anti-Mumps IgG loss in 25% of fully vaccinated subjects was estimated to be 19 years (95%CI = 17–22) and the incidence rate year-person of seronegative was 0.006 (95%CI = 0.005–0.007).
We did not find statistically significant difference of estimated PAS per gender (z = 3.4; p = .067; ); the incidence rate persons-year of seronegative is 0.005 (95%CI = 0.004–0.007) in females and 0.007 (95%CI = 0.005–0.010) in males, with an IRR value of 1.4 (95%CI = 0.9–2.0; p = .043).
The multivariate Cox semiparametric regression model showed that being male (HR = 1.5; 95%CI = 1.00–2.15; limits of statistical significance) and a greater age at enrollment (HR = 0.36; 95%CI = 0.28–0.47) were risk factors for PAS ().
Table 2. Analysis of risk predictors of Mumps IgG PAS in a multivariate Cox semiparametric regression model
Regarding the safety of the vaccine, in the 1-month follow-up, we did not find any serious and/or long-term adverse reaction. The most common reactions reported were pain at the injection site, mild fever within 10–15 days after administration and, more rarely, laterocervical lymphadenopathy. All the adverse events regressed in the following days, without sequelae.
Discussion
More than 6% of subjects enrolled, after two doses of MMR vaccine, did not show circulating anti-Mumps IgG; one or more booster doses were needed to elicit an immunity response.
The percentage of seroconversion after a booster was high (90%); overall, the seroconversion rate after one or two doses was of 99%. The GMT increases in seronegative subjects after the booster dose(s) (6 vs. 67), but the value after booster(s) is significantly lower compared to that one of seroprotected subjects at enrollment (67 vs. 115; p < .0001). This difference may be due to an intrinsic factor of the not immunoresponsive subjects or to or the fact that the seroprotected at enrollment may have received natural boosters over time; no differences in GMT were found in the comparison between gender. Regarding the subjects who received two booster doses (n = 8), just 1 was affected by a chronic disease (hypothyroidism) and resulted seroconverted at the re-titer; anyway, the number of subjects who received two booster doses is too small to draw conclusions.
Our model showed that the antibody levels tend to decline after about 10 years from completion of the basal routine; this last value is lower than that reported in the low scientific evidences, which settles it around 15 years.Citation13
No differences in seroprevalence were found between males and females, nor at baseline, nor after eventually booster dose(s). On the other hand, the IRR and the Cox regression suggest that being male is a risk factor for losing circulating antibodies over time; gender differences in the response to vaccines is a topic studied by many authors in literature and the scientific community agrees in recognizing that immunological, hormonal, genetic, microbiota and environmental differences between males and females may also affect the outcomes of vaccination (immunogenicity and probably effectiveness), and in particular, males seem to be less immunoresponsive compared to females.Citation24–26 Further studies are needed to clarify the role of the gender in the response to the Mumps vaccination.
In summary, the time between vaccination and the antibody titer evaluation is a determinant for waning of circulating antibodies and therefore the antibody titer and immunity decrease over the years. Furthermore, the administration of a third booster dose in fully vaccinated but non-seroprotected subjects is effective and safe.
To our knowledge, the introduction of a third (and eventually fourth) booster dose in fully vaccinated but not seroprotected HCWs is a topic very poorly studied in literature. A 2008 studyCitation27 reported the experience in the context of a US university, in which 440 voluntary students and staff, aged 19–30 years and having a documented history of 2 doses of MMR vaccine, were tested for IgG anti-Mumps. Overall, 6% of the participants were seronegative and, after the administration of a third booster MMR dose, 86% of negative subjects seroconverted; these assessments match the results of our study.
Regarding the efficacy of the booster dose, the evidences showed lower attack rates among subjects who received the third booster dose during the outbreak compared with those who had received two doses before the outbreak. Increasing vaccine effectiveness of the third versus the second MMR dose in these studies ranged from 61% to 88%.Citation8,Citation28,Citation29 Many studies evaluated the safety of the third dose of MMR vaccine and no serious adverse events were reported among 14,368 subjects who received the booster dose;Citation8,Citation30,Citation31 these findings are consistent with our data reported in the follow-up of the patients who received the booster dose(s), as no serious and/or long-term reactions were revealed. The main difference between our study and several previous (and above cited) studies is that our study describes a management approach for subjects vaccinated but without circulating antibodies, while the other studies described the management of subjects who came into contact with infected persons during mumps outbreaks.
The strong point of our study is the relevant sample size, it is an argument poorly studied in the literature and the comparison between gender. The major limitation is related to the impossibility to analyze the subjects vaccinated for Mumps immune status in relation to the type of vaccine (MMR vs. MMRV); in addition, it was not possible to evaluate if the subject had ever come into contact with the wild virus. It will be appropriate for the future to repeat the evaluation of the management of the non-responders, on the one hand by expanding the sample in the study, the other part of prolonging the time of follow-up after execution of the vaccination basic routine, in order to describe the trend of immunogenicity over the years.
The results of our study find application above all in some categories of the population, such as the Healthcare workers (HCWs); Including the screening model described in the routine assessment of the biological risk of HCWs may be a winning strategy in preventing Mumps infections. This concept is particularly important for all those HCWs who work in setting with high risk of Mumps circulation (Pediatric Ward, Infectious Diseases Departments), in order to reduce the risk of infection for patients, as it is important to underline that Mumps could be particularly severe in adults, so HCWs should be concerned of to protect themselves.
Emerging evidence seems to suggest that fully MMR vaccine is not an absolute guarantee of immunity against the wild viruses: a 2010 studyCitation32 described a Mumps outbreak investigation in a US hospital that included 7 HCWs (mean age: 34 years), of whom 2 were twice vaccinated with 2 doses of MMR vaccine and 1 HCW with one dose of MMR vaccine. This study showed that to verify the serological state and to implement the appropriate measures of prophylaxis in case of negativity is fundamental even in the vaccinated HCWs.
Abbreviations
| CDC | = | Center for Disease Control and Prevention |
| WHO | = | World Health Organization |
| MMR | = | Measles, Mumps, Rubella |
| HCWs | = | Healthcare Workers |
| GIAVA | = | Regional Immunization Database |
| CLIA | = | Chemiluminescent immunoassay |
Author statement
FPB designed the study, collected results, analyzed data and draft the manuscript. PS and SDN contributed to the collection and analysis of data. AMVL was responsible for the analysis laboratory. ST e CAG revised the protocol of the study and the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Ethical approvals
The research protocol was approved by Apulian Epidemiological Observatory and all human participants gave written informed consent. This research was carried out according to the principles of the Helsinki Declaration.
Additional information
Funding
References
- CDC. Complications of Mumps. [accessed 2019 Mar 22]. https://www.cdc.gov/Mumps/about/complications.html.
- WHO. Mumps reported cases. Last update: 21-Sep-2018 (data received as of 18-Sep-18). [accessed 2019 Feb 24]. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidenceMumps.html.
- CDC. Manual for the surveillance of vaccine-preventable diseases. Chapter 9: Mumps. [accessed 2019 Mar 24]. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt09-Mumps.html.
- CDC. Measles, Mumps, and Rubella (MMR) vaccination: what everyone should know. [accessed 2019 Mar 22]. https://www.cdc.gov/vaccines/vpd/mmr/public/index.html.
- CDC. Epidemiology and prevention vaccine-preventable diseases. Mumps. [accessed 2019 Mar 20]. https://www.cdc.gov/vaccines/pubs/pinkbook/Mumps.html.
- CDC. Measles, Mumps, and Rubella (MMR) vaccine safety. [accessed 2018 Sep 15]. https://www.cdc.gov/vaccinesafety/vaccines/mmr-vaccine.html.
- Zhou F, Reef S, Massoudi M, Papania MJ, Yusuf HR, Bardenheier B, Zimmerman L, McCauley MM. An economic analysis of the current universal 2-dose measles-Mumps-Rubella vaccination program in the United States. J Infect Dis. 2004;189(Suppl 1):S131–45. doi:10.1086/378987.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep. 2018;67(1):33–38. doi:10.15585/mmwr.mm6701a7.
- Italian Ministry of Health. Decree-Law 07 June 2017, n. 73. Urgent provisions on vaccination prevention. G.U. General Serie, n. 130 of 07 June 2017 [accessed 2019 Dec 30]. http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=59548.
- D’Ancona F, D’Amario C, Maraglino F, Rezza G, Ricciardi W, Iannazzo S. Introduction of new and reinforcement of existing compulsory vaccinations in Italy: first evaluation of the impact on vaccination coverage in 2017. Euro Surveill. 2018;23(22). doi:10.2807/1560-7917.ES.2018.23.22.1800238.
- WHO Europe. Eliminating measles and Rubella and preventing congenital Rubella infection. [accessed 2019 Dec 30]. http://www.euro.who.int/__data/assets/pdf_file/0008/79028/E87772.pdf.
- Gabutti G, Guido M, Rota MC, De Donno A, Ciofi Degli Atti ML, Crovari P. Seroepidemiology group. Epidemiol Mumps in Italy Vaccine. 2008;26:2906–11.
- CDC. Immunization of health-care personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and mortality weekly report. Recommendations and Reports/Vol. 60/No. 7. 2011 Nov 25. https://www.cdc.gov/mmwr/pdf/rr/rr6007.pdf.
- Date AA, Kyaw MH, Rue AM, Klahn J, Obrecht L, Krohn T, Rowland J, Rubin S, Safranek T, Bellini W, et al. Long-term persistence of mumps antibody after receipt of 2 measles-mumps-rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J Infect Dis. 2008;197:1662–68. doi:10.1086/590464.
- Cortese MM, Jordan HT, Curns AT, Quinlan P, Ens K, Denning P, Dayan G. Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis. 2008;46:1172–80. doi:10.1086/588450.
- Harling R, White JM, Ramsay ME, Macsween KF, Bosch CVD. van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine. 2005;23:4070–74. doi:10.1016/j.vaccine.2004.10.020.
- Smetana J, Chlibek R, Hanovcova I, Sosovickova R, Smetanova L, Polcarova P, Gal P, Dite P. Serological survey of Mumps antibodies in adults in the Czech Republic and the need for changes to the vaccination strategy. Hum Vaccin Immunother. 2018;14(4):887–93. doi:10.1080/21645515.2017.1412021.
- Tafuri S, Gallone MS, Larocca AM, Germinario C. How will the MMR universal mass vaccination change the epidemiologic pattern of Mumps? A 2012 Italian serosurvey. Am J Infect Control. 2016;44(11):1420–21. doi:10.1016/j.ajic.2016.03.012.
- Tafuri S, Martinelli D, Caputi G, Arbore A, Lopalco PL, Germinario C, Prato R. An audit of vaccination coverage among vaccination service workers in Puglia, Italy. Am J Infect Control. 2009;37(5):414–16. doi:10.1016/j.ajic.2008.10.030.
- Bianchi FP, Gallone MS, Gallone MF, Larocca AMV, Vimercati L, Quarto M, Tafuri S. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: an Italian study among medical students. J Viral Hepat. 2019 Jan;26(1):136–44.
- Tafuri S, Gallone MS, Gallone MF, Pappagallo MT, Larocca AMV, Germinario C. Monitoring the process of Measles elimination by serosurveillance data: the Apulian 2012 study. Vaccine. 2016;34(18):2092–95. doi:10.1016/j.vaccine.2016.03.011.
- DiaSorin. The diagnostic specialist. LIAISON® Mumps IgG. The fully automated solution for antibody detection. [accessed 2019 Dec 30]. https://www.diasorin.com/sites/default/files/allegati_prodotti/scheda_mumps_igg.pdf.
- Cabrillo College. Clinical compliance basics. Health screening. measles, Mumps, Rubella (MMR). [accessed 2018 Nov 04]. https://www.cabrillo.edu/services/health/clinical-compliance/clinical-mumps.html.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–99. doi:10.1146/annurev-cellbio-100616-060718.
- Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016;52(2):198–204. doi:10.4415/ANN_16_02_11.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi:10.1093/trstmh/tru167.
- Date AA, Kyaw MH, Rue AM, Klahn J, Obrecht L, Krohn T, Rowland J, Rubin S, Safranek TJ, Bellini WJ, et al. Long-term persistence of Mumps antibody after receipt of 2 measles-Mumps-Rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J Infect Dis. 2008;197(12):1662–68. doi:10.1086/590464.
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, Marin M, Riley J, Feikin DR, Patel M, et al. Effectiveness of a third dose of MMR vaccine for Mumps outbreak control. N Engl J Med. 2017;377:947–56. doi:10.1056/NEJMoa1703309.
- Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, Lawler J, McLean HQ, Pollock L, Rausch-Phung E, et al. Impact of a third dose of measles-Mumps-rubella vaccine on a Mumps outbreak. Pediatrics. 2012;130:e1567–74. doi:10.1542/peds.2012-0177.
- Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presentation at the Advisory Committee on Immunization Practices meeting; 2017 Oct 25–26; Atlanta (GA). https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/Mumps-03-marlow-508.pdf.
- Albertson JP, Clegg WJ, Reid HD, Arbise BS, Pryde J, Vaid A, Thompson-Brown R, Echols F. Mumps outbreak at a university and recommendation for a third dose of measles-Mumps-rubella vaccine—Illinois, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:731–34. doi:10.15585/mmwr.mm6529a2.
- Bonebrake AL, Silkaitis C, Monga G, Galat A, Anderson J, Trad TJ, Hedley K, Burgess N, Zembower TR. Effects of Mumps outbreak in hospital, Chicago, Illinois, USA, 2006. Emerg Infect Dis. 2010;16(3):426–32. doi:10.3201/eid1603.090198.