ABSTRACT
This phase Ⅱ, randomized, controlled trial aimed to evaluate the safety and immunogenicity of a various Sabin IPV preparations. Six hundred infants aged 60 ~ 90 days received one of five different vaccines: low- (group A), medium- (group B) or high-D antigen content (group C) of an experimental Sabin IPV, control Sabin IPV (group D) or control Salk IPV (group E), on a 0-1-2 month schedule. Participants were observed and followed up within 30 days of each dose to assess safety. Serum samples were collected before the first dose and 30 days after the third dose to assess immunogenicity. After three doses, type-1 seroconversion rates of groups A–E were 99.1%, 100.0%, 99.1%, 99.0%, and 93.4%, respectively; type-2 seroconversion rates were 93.5%, 97.1%, 98.1%, 95.1%, and 91.5%, respectively; and type-3 seroconversion rates were 95.4%, 98.1%, 98.1%, 95.1%, and 100.0%, respectively. Only type-1 seroconversion rates differed significantly for group E. The incidences of injection-site redness (A: 21.9%, B: 23.7%, C: 29.4%, D: 16.2%, E: 12.7%), swelling (A: 6.7%, B: 6.8%, C: 5.0%, D: 0.0%, E: 1.7%) and pain (A: 5.0%, B: 6.8%, C: 7.6%, D: 0.0%, E: 0.9%) all were significantly higher for experimental vaccines relative to control groups. No SAEs were detected related to vaccination, and most adverse reactions were mild or moderate in severity. In conclusion, the experimental Sabin IPVs with low-, medium-, and high-D antigen content all revealed good safety and immunogenicity profiles although being more reactogenic than the control vaccines.
Introduction
Poliomyelitis (or polio) is a severe infectious disease caused by any of three serotypes of poliovirus (types 1, 2, and 3). It can strike anybody (mainly children <5 years old) and invade the central nervous system to cause the irreversible flaccid paralysis in one of every 200 infected persons.Citation1 There is no cure, but there are safe and effective vaccines to control the prevalence of polio since the 1950s.Citation2 In 1988, the World Health Assembly established the Global Polio Eradication Initiative (GPEI) and resolved to eradicate polio globally by the year 2000.Citation3 Since then, the strategies to achieve polio eradication in all polio-endemic countries were maintaining high routine coverage with oral poliovirus vaccine (OPV) in children <5 years old complemented by national immunization days, “mopping-up” vaccination campaigns, or supplementary immunization activities.Citation4 Currently, the GPEI has reduced the global number of polio cases from 350,000 in 1988 to 33 in 2018 and the number of polio-endemic countries from over 125 in 1988 to just 3 (Afghanistan, Nigeria, and Pakistan) by now.Citation1
Although OPV has played a very important role in the GPEI, it is made from a live attenuated vaccine virus and might result in vaccine-associated paralytic poliomyelitis (VAPP) and spread of vaccine-derived poliovirus (VDPV).Citation5,Citation6 Therefore, it is widely considered that once wild poliovirus has been interrupted globally, the public health benefits of OPV will no longer outweigh the potential risks of it.Citation7 Another polio vaccine, inactivated poliovirus vaccine (IPV), carries no risks of VAPP and VDPV emergence and will completely substitute OPV in polio eradication in the future.Citation8 Indeed, according to the polio eradication and endgame strategic plan 2013–2018, the World Health Organization (WHO) has proposed to introduce at least one dose of IPV in all OPV-using countries in 2015 and replace all trivalent OPV with bivalent OPV in 2016. In addition, after the global certification of total wild poliovirus eradication, bivalent OPV will be also withdrawn from routine use and IPV will be used exclusively.Citation9
More than 60 years ago, IPV was first developed by Dr. Jonas Salk, also called the Salk IPV. It consists of three inactivated wild-type poliovirus strains. The Salk IPV is very well tolerated and there are no serious systemic adverse reactions attributable to it.Citation10 However, due to the high biosafety risk in manufacture of Salk IPV with wild-type polioviruses, there is at least a biosafety level three containment facility required in the production, which significantly increases the cost of Salk IPV and limits the widespread use of it in many developing countries.Citation11,Citation12 Thus, the WHO has no longer recommended the creation of new Salk IPV production facilities, while promoted the emergence of new IPV manufacturers relying on the use of attenuated strains, such as Sabin strains. Production of IPV based on the Sabin strains carries a lower biosafety risk and will increase the availability and affordability of IPV in the developing countries.Citation13
In China, Beijing Minhai Biotechnology Company has developed a Sabin strain-based IPV (Sabin IPV), and conducted phase I and Ib clinical trials to preliminarily assess the safety and immunogenicity of the Sabin IPV. Results from the initial phase I trials indicated that the Sabin IPV showed good safety and immunogenicity in Chinese infants.Citation14 This phase Ⅱ trial was aimed to further estimate the safety and immunogenicity of the Sabin IPV.
Materials and methods
Participants
Study participants were infants aged 60 ~ 90 days, and were recruited from Dafeng and Lianshui Counties, Jiangsu Province, China, between December 2017 and February 2018. Infants were eligible in this study if they were healthy; had been born at full term with normal birth weight; had no prior vaccination of polio; did not have congenital malformation; did not have histories of allergies, epilepsy, or mental illness; did not have immunodeficiency; did not have coagulopathy; did not have serious chronic illness; did not recently have acute febrile disease; did not recently receive any research drug or immunosuppressant; and had not received any subunit or inactivated vaccine within the past 7 days, any live attenuated vaccine within the past 14 days, and any blood-derived product within the past 3 months.
Study design
This was a phase Ⅱ, randomized, controlled trial (Clinical Trials.gov identifier NCT03902054) to evaluate the safety and immunogenicity of a various Sabin IPV preparations developed by Beijing Minhai Biotechnology Company. A total of 600 eligible participants entered this trial and were randomly assigned to one of five different treatment groups at a ratio of 1:1:1:1:1. Participants in groups A, B, and C separately received an experimental Sabin IPV (developed by Beijing Minhai Biotechnology Co. Ltd.) with low, medium, or high D antigen content on a 0-1-2 month schedule. Participants in groups D and E received control Sabin IPV (developed by Chinese Academy of Medical Sciences, CAMS) and control Salk IPV (developed by Sanofi Pasteur), respectively, on the same schedule. For safety assessment, participants were observed and followed up within 30 days of each dose. For immunogenicity assessment, serum samples were collected before the first dose and 30 days after the third dose.
Ethics statement
This trial was approved by the ethical review committee of Jiangsu Provincial Center for Disease Control and Prevention. Parent or guardian of each participant in this trial was requested to provide written informed consent before enrollment.
Randomization and masking
SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used to generate the random sequence according to the preset block size, by independent biostatisticians. The experimental and control vaccines were numbered with the random sequence (each vaccine had a unique number), and then were masked by using the same packaging which marked the unique number and other contents. When enrolled in the study, participants were randomly assigned a unique number by the researchers and received the vaccine marked with the identical number. In this study, the participants, study designers, safety evaluators, data managers, and laboratory staff were all blinded to the vaccine assignment.
Vaccine and vaccination
The experimental Sabin IPVs with low, medium, and high D antigen content were produced by Beijing Minhai Biotechnology Company, containing three attenuated poliovirus master seeds: for type 1 poliovirus, Sabin SO+1; for type 2, Sabin SO+1; and for type 3, RSO1. Working virus seeds were inoculated into Vero cells and then were cultivated, harvested, concentrated, and purified. After inactivation of the purified virus suspension by formaldehyde, three groups of vaccines were prepared according to the D antigen content detected by an enzyme-linked immunosorbent assay. Low dose group of vaccine (group A) were 10 (for type 1 poliovirus), 30 (for type 2), and 30 (for type 3) D-antigen units per dose (DU/dose); medium dose group (group B) were 15, 45, and 45 DU/dose; and high dose group (group C) were 22, 65, and 65 DU/dose. The experimental Sabin IPVs were all manufactured in the facility that has been certified to comply with the Chinese GMP by the Chinese State Food and Drug Administration (CFDA).
The control Sabin IPV (group D) licensed by the CFDA in 2015 was manufactured by CAMS, containing 30, 32, and 45 DU/dose for type 1 (Sabin SO+1), 2 (Sabin SO+1), and 3 (RSO1) polioviruses, respectively.
The control Salk IPV (group E) was manufactured by Sanofi Pasteur, containing 40, 8, and 32 DU/dose for type 1 (Mahoney), 2 (MEF-1), and 3 (Saukett) polioviruses, respectively.
All the experimental and control vaccines were sterile liquid vaccines (0.5 mL/dose) for intramuscular injection on a 0-1-2 month schedule.
Concomitant immunization
During the study period, subjects could be routinely immunized with other subunit or inactivated vaccines at least 7 days apart from the trial vaccines, and also could be routinely immunized with other live attenuated vaccines at least 14 days apart from the trial vaccines. In addition, subjects could be vaccinated emergently with rabies vaccines or tetanus vaccines when necessary.
Immunogenicity assessment
Whole blood sample was collected from each participant before the first dose and 30 days after the third dose. After centrifuged, serum specimens were separated, frozen, and stored at −20°C at the study site before transferring to the National Institute for Food and Drug Control (NIFDC) for detection of anti–poliovirus neutralizing antibodies (NA). A WHO-recommended microneutralization assay, using Sabin strains to detect the poliovirus type-specific NA, was performed by NIFDC.Citation15,Citation16 The primary endpoint was seroconversion rate of NA on day 30 after 3 doses. The secondary endpoints were seropositive rate, geometric mean titer (GMT), and geometric mean fold increase (GMFI) of NA. A titer of 1:8 was considered to be positive. Seroconversion was defined as a post-vaccination antibody titer ≥1:8 (when baseline titer was <1:8) or as a fourfold increase in titer from pre- to post-vaccination values (when baseline titer was ≥1:8).
Safety assessment
After each vaccination, the subjects must be observed in the observation room for 30 min and the researchers must record the local/systemic adverse events occurring ≤30 min for each subject. The local/systemic adverse events occurring >30 min to ≤30 days after each vaccination were observed and recorded on a diary card by the parent or guardian of each subject. Within 30 days after each vaccination, the safety assessors should conduct face-to-face or telephone interviews with the parent or guardian regularly to review the safety record for each subject. The solicited local adverse reactions included pain, redness, swelling, induration, rash, and pruritus. The solicited systemic adverse reactions included fever, crying, vomiting, diarrhea, cough, activity decline, and inappetence. Any adverse event was graded according to the guidelines issued by the CFDACitation17 and determined whether related to the vaccination by the investigators. Data on serious adverse events (SAEs) were also collected and verified by the investigators throughout the trial.
Statistical analysis
The sample size of our study was determined on the basis of the following aspects: first, we referred to the sample size in the phase Ⅱ trial of CAMS;Citation11 second, we consulted the technical guideline for a phase Ⅱ clinical trial issued by the CFDA;Citation18 third, we considered the potential for withdrawal of participants. Finally, we set a total sample size of 600 infants, with 120 in each treatment group.
SPSS Statistics standard 23.0 was used for statistical analysis. Participants who received at least one dose and had at least one safety evaluation were included in the safety set. Participants who received all three doses, had the effective pre- and post-immunization NA test results, and did not critically deviate from the protocol were included in the per-protocol set. Safety assessment was performed based on the safety set and immunogenicity assessment was performed based on the per-protocol set. An arbitrary value of 1:4 was given to the sample with NA titer <1:8 for statistical calculation. The NA titers were transformed into log10 titers for the calculation of the GMTs and GMFIs with 95% confidence intervals (CIs). Proportions and 95% CIs were calculated using the Clopper–Pearson method.Citation19,Citation20 Pearson χ2 test or Fisher’s exact test was used for the analysis of binary outcomes (e.g., sex, incidence of adverse events, seroconversion rate, seropositive rate, or frequency of concomitant vaccination), and one way ANOVA or Wilcoxon rank-sum test was used for the analysis of continuous outcomes (e.g., age, GMT, or GMFI). Using 2-sided hypothesis test and P-value <0.05 was considered statistically significant.
Results
Study participants
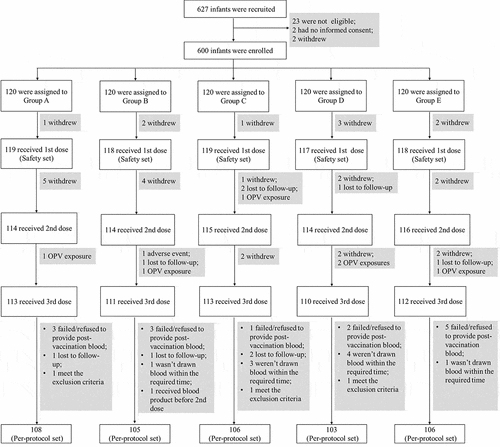
From December 2017 to February 2018, a total of 627 infants were recruited, and 600 infants met the inclusion and did not meet the exclusion criteria entered the study. Participants were interviewed intensively for four times by the investigators and the last intensive interview was completed in May 2018. Of these 600 infants, 119 in group A (low-dose of investigational Sabin IPV), 118 in group B (medium-dose of investigational Sabin IPV), 119 in group C (high-dose of investigational Sabin IPV), 117 in group D (control Sabin IPV) and 118 in group E (control Salk IPV) received at least one dose and were included in the safety set. After eliminating the infants who withdrew or could not follow the study protocol, there were a total 528 (group A: 108, group B: 105, group C: 106, group D: 103, group E: 106) infants were finally fitted into the per-protocol set (). The basic characteristics of participants in the safety and per-protocol sets are shown in and there was no statistically significant difference in age or sex among any of groups.
Table 1. Baseline characteristics of infants participating in this study in China, 2018
Immunogenicity
Before vaccination, no significant differences of GMTs or seropositive rates were observed among groups for all the three poliovirus types (P > .05 for all comparisons).
After three doses, the seropositive rates of groups A–E were all 100% for all the 3 poliovirus types. The type-1 seroconversion rates of groups A–E were 99.1%, 100.0%, 99.1%, 99.0%, and 93.4%, respectively; type-2 seroconversion rates were 93.5%, 97.1%, 98.1%, 95.1%, and 91.5%, respectively; and type-3 seroconversion rates were 95.4%, 98.1%, 98.1%, 95.1%, and 100.0%, respectively. The differences of type-2 and type-3 seroconversion rates were not significant (all P > .05) among groups, while the type-1 seroconversion rate of group E was significantly lower than that of the other groups (P = .004).
After vaccination, the type-1 GMTs of groups A–E were 3609.9, 3694.9, 5252.9, 3894.0, and 693.6, respectively; the type-2 GMTs were 691.2, 801.7, 890.2, 259.4, and 175.5, respectively; and the type-3 GMTs were 908.2, 1030.1, 1347.7, 556.9, and 559.2, respectively. Significant differences of GMTs for all the three poliovirus types were observed among groups (all P < .001). In addition, the GMFIs of groups A–E ranged from 37.2 to 301.3 for type 1 poliovirus, 25.3 to 127.2 for type 2 poliovirus and 85.1 to 237.8 for type 3 poliovirus, and these differences were all significant among groups (all P < .001) ().
Table 2. Seropositivity rates, seroconversion rates, GMTs and GMFIs of poliovirus type-specific neutralizing antibody in infants after three doses of vaccine in China, 2018
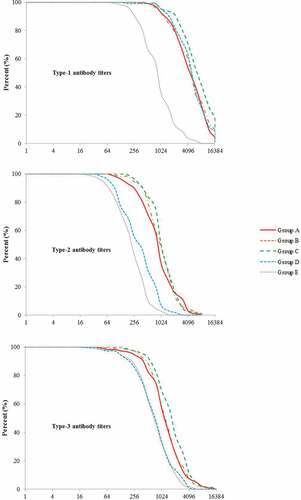
The distributions of post-vaccination antibody titers for all the three poliovirus types are shown in . In groups A–C, the antibody levels for all the three types of poliovirus increased with the increasing antigen dose of the investigational Sabin IPVs. For type-1 antibody titers, groups A–D were significantly higher than group E, and group D was between the group A/B and C. For type-2 and type-3 antibody titers, groups A–C were significantly higher than groups D and E.
Safety
Overall adverse events are shown in . The frequencies of SAEs (A: 5.0%, B: 2.5%, C: 1.7%, D: 4.3%, E: 2.5%, P = .592), unsolicited adverse reactions (A: 0.0%, B: 2.5%, C: 0.8%, D: 0.9%, E: 0.0%, P = .170) and solicited adverse reactions (A: 69.8%, B: 71.2%, C: 72.3%, D: 70.1%, E: 62.7%, P = .536) all showed no statistically significant differences among groups. In addition, there were also no significant differences in the prevalence of ≥grade 3 unsolicited and solicited adverse reactions among the groups (all P > .05). Both the unsolicited and solicited adverse reactions were considered to be related to the vaccine, while no SAEs were determined related to the vaccination.
Table 3. Overall adverse events in infants after three doses of vaccine in this study in China, 2018
The solicited adverse reactions included local adverse reactions and systemic adverse reactions. In terms of the local adverse reactions, the most common reaction was redness, frequencies of which were significantly higher for experimental vaccines relative to control groups (A: 21.9%, B: 23.7%, C: 29.4%, D: 16.2%, E: 12.7%, P = .016). The incidences of injection-site swelling (A: 6.7%, B: 6.8%, C: 5.0%, D: 0.0%, E: 1.7%, P = .007) and pain (A: 5.0%, B: 6.8%, C: 7.6%, D: 0.0%, E: 0.9%, P = .001) were also significantly higher for experimental vaccines relative to control groups. In terms of the systemic adverse reactions, the most common reaction was fever, frequencies of which showed no statistically significant difference among groups (A: 50.4%, B: 50.8%, C: 47.1%, D: 48.7%, E: 39.0%, P = .351). Also, there were no significant differences in prevalence of the other systemic adverse reactions among groups (all P > .05). Most of the local and systemic adverse reactions were mild or moderate in severity, except those for four infants in groups A and B, who experienced grade 3 redness and swelling and two infants in group E, who separately experienced grade 3 fever and crying. There were no significant differences among groups in the severity of both local and systemic adverse reactions (all P > .05).
Concomitant immunization
The incidences of concomitant immunization in groups A–E were 80.7%, 68.6%, 68.9%, 71.8%, 75.4%, respectively (). In the five groups, most of the subjects concomitantly received DTaP vaccine during the trial, and only a few subjects concomitantly received some other vaccines, such as Hib vaccine, Hep-B vaccine, 13-valent pneumonia vaccine, meningococcal polysaccharide vaccine, or OPV. The incidences of concomitant vaccination for all the vaccines showed no significant difference among groups (all P > .05).
Table 4. Concomitant vaccination of the trial participants in this study in China, 2018
Discussion
In this study, the investigational Sabin IPVs in groups A, B, and C, the control Sabin IPV in group D and the control Salk IPV in group E all showed good immunogenicity. The seropositive rates were all 100% and the seroconversion rates were all more than 90% in groups A-E against the three poliovirus types after three doses. For type 1 poliovirus, the seroconversion rates, GMTs and GMFIs of the investigational Sabin IPVs were mainly higher than those of the control Salk IPV; while for type 2 and 3 polioviruses, the GMTs and GMFIs of the investigational Sabin IPVs were higher than those of both the control Sabin and Salk IPVs. Thus, those results also indicated that the investigational Sabin IPVs might have better immunogenicity compared with the other two control vaccines.
With the increasing D antigen content, there was a rising trend for the post-vaccination GMTs in the low-, medium-, and high-dose groups of the Sabin IPVs in our study. But different from the phase Ⅱ trials of CAMSCitation11 and Sinovac Biotech,Citation21 there were not much differences of the post-vaccination GMTs among groups of the experimental Sabin IPVs with low, medium, and high D antigen content in this study. The possible explanation was that the D antigen content difference of the experimental Sabin IPVs in this study (low: 10:30:30 DU/dose, medium: 15:45:45 DU/dose, high: 22:65:65 DU/dose) was not as great as that in other two studies (CAMS, low: 15:16:22.5 DU/dose, medium: 30:32:45 DU/dose, high: 45:64:67.5 DU/dose; Sinovac Biotech, low: 7.5:22.5:22.5 DU/dose, medium: 15:45:45 DU/dose, high: 22.5:67.5:67.5 DU/dose).
This phase Ⅱ trial also demonstrated that the low-, medium-, and high-dose Sabin IPVs were all well tolerated in infants aged 60 ~ 90 days. There were no significant differences in the overall frequencies of SAEs, unsolicited and solicited adverse reactions among groups. No SAEs were detected related to the vaccination by investigators, and most adverse reactions were mild or moderate in severity.
In this study, the frequencies of injection-site redness, swelling, and pain in low-, medium-, and high-dose groups of experimental Sabin IPVs were all higher than those in groups of control IPVs, indicated that our experimental Sabin IPVs might be more reactogenic than the control IPVs. However, it was noticeable that most local reactions were mild or moderate and nearly all the local reactions were recovered within 7 days of vaccination (data were not shown), no matter in experimental or control groups. Therefore, our experimental Sabin IPVs might be generally safe although being more reactogenic than the control vaccines.
This study found that the overall safety and immunogenicity were similar among the low-, medium- and high-dose of experimental Sabin IPVs. Although the high-dose of experimental vaccine may elicit higher GMTs after immunization, the financial cost of it is higher because of more antigen content needed in its production. Therefore, the low- or medium-dose will be an appropriate dose for further studies.
Similar to some other studies,Citation11,Citation21,Citation22 the limitations of this study were that non-inferiority test could not be conducted due to the small sample size and the cross-neutralization assays were not conducted as well. Further study will increase the sample size to provide a more reliable estimate of the safety and immunogenicity of the experimental Sabin IPV, and the cross-neutralizing capacity of the experimental Sabin IPV against wild-strain virus will be also examined in our future study. In addition, due to the results of the immunogenicity analyses in this study were not adjusted for the effect of maternal antibodies (pre-vaccination antibodies), the immunogenicity of the studied IPVs might be underestimated.Citation15 However, there were no significant differences of pre-vaccination GMTs or seropositive rates among groups for all three poliovirus types, indicated a well-balanced immune level before vaccination. Thus, the maternal antibodies might have limited effect on the comparisons of the post-vaccination immunogenicity among groups.
Conclusion
In summary, the experimental Sabin IPVs with low-, medium-, and high-D antigen content all revealed good safety and immunogenicity profiles although being more reactogenic than the control vaccines.
Abbreviations
| IPV | = | inactivated poliovirus vaccine |
| OPV | = | oral poliovirus vaccine |
| GPEI | = | Global Polio Eradication Initiative |
| VAPP | = | vaccine-associated paralytic poliomyelitis |
| VDPV | = | vaccine-derived poliovirus |
| GMTs | = | geometric mean titers |
| GMFIs | = | geometric mean fold increases |
| CIs | = | confidence intervals |
| SAEs | = | serious adverse events |
| CAMS | = | Institute of Medical Biology, the Chinese Academy of Medical Sciences in Kunming; NA, neutralizing antibodies |
| CFDA | = | Chinese State Food and Drug Administration |
| NIFDC | = | National Institute for Food and Drug Control |
Author contributions
Q.L, Y.H, and G.L designed the trial and the study protocol. R.T, C.Z, H.Z, J.Z, J.W, Q.L, and Y.H led and participated in the site work. C.L committed to assay the antibody titers for IPVs. R.T and G.L contributed to the data collection, data management, and statistical analysis and wrote the paper.
Disclosure of potential conflicts of interest
Guifan Li is employed by Beijing Minhai Biotechnology Co. Ltd. All other authors have no conflicts.
Additional information
Funding
References
- Global Polio Eradication Initiative. Polio prevention. [accessed 2019 July 3]. http://polioeradication.org/polio-today/polio-prevention/.
- Tang R, Chu K, Hu Y, Chen L, Zhang M, Liu S, Ma H, Wang J, Zhu F, Hu Y, et al. Effect of maternal antibody on the infant immune response to inactivated poliovirus vaccines made from Sabin strains. Hum Vaccin Immunother. 2019;15(5):1160–66. doi:10.1080/21645515.2019.1572410.
- World Health Assembly: Global Eradication of Poliomyelitis by the Year 2000. Geneva (Switzerland): World Health Organization; 1988.
- Li R, Li C, Wang H, Luo H, Li Y, Wang J, Ying Z, Yu W, Shu J, Wen N, et al. Immunogenicity of two different sequential schedules of inactivated polio vaccine followed by oral polio vaccine versus oral polio vaccine alone in healthy infants in China. J Pediatric Infect Dis Soc. 2016 Sep;5(3):287–96. doi:10.1093/jpids/piv017.
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. 2014 Nov 1;210(Suppl 1):S380–9. doi:10.1093/infdis/jiu184.
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210(Suppl 1):S283–93. doi:10.1093/420infdis/jiu295.
- Vaccines & Biologicals Department: Report of the interim meeting of the Technical Consultative Group (TCG) on the Global Eradication of Poliomyelitis, Geneva, 13–14 November 2002. Geneva (Switzerland): World Health Organization; 2004. WHO/V&B/03.04.
- John TJ. The final stages of the global eradication of polio. N Engl J Med. 2000 Sep 14;343(11):806–07. doi:10.1056/NEJM200009143431111.
- Global Polio Eradication Initiative. Polio eradication and endgame strategic plan 2013-2018. [accessed 2019 July 3]. http://polioeradication.org/who-we-are/strategic-plan-2013-2018/.
- Global Polio Eradication Initiative. IPV. [accessed 2019 July 3]. http://polioeradication.org/polio-today/polio-prevention/the-vaccines/ipv/.
- Liao G, Li R, Li C, Sun M, Li Y, Chu J, Jiang S, Li Q. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J Infect Dis. 2012;205(2):237–43. doi:10.1093/infdis/jir723.
- Verdijk P, Rots NY, van Oijen MG, Oberste MS, Boog CJ, Okayasu H, Sutter RW, Bakker WAM. Safety and immunogenicity of inactivated poliovirus vaccine based on Sabin strains with and without aluminum hydroxide: a phase I trial in healthy adults. Vaccine. 2013 Nov 12;31(47):5531–36. doi:10.1016/j.vaccine.2013.09.021.
- Okayasu H, Sein C, Hamidi A, Bakker WA, Sutter RW.Development of inactivated poliovirus vaccine from Sabin strains: a progress report. Biologicals. 2016 Nov. 44(6):581–87. doi:10.1016/j.biologicals.2016.08.005.
- Tang R, Liang Q, Li G, Zhi H, Wang J, Hu Y. Safety and immunogenicity of an inactivated poliovirus vaccine made from Sabin strains. Chin J Public Health. in press.
- Hu Y, Wang J, Zeng G, Chu K, Jiang D, Zhu F, Ying Z, Chen L, Li C, Zhu F, et al. Immunogenicity and safety of a Sabin strain–based inactivated polio vaccine: a phase 3 clinical trial. J Infect Dis. 2019 Oct 8;220(10):1551–57. doi:10.1093/infdis/jiy736.
- World Health Organization. WHO expanded program on immunization, manual for the virologic investigation of poliomyelitis. Geneva (Switzerland): World Health Organization; 1990.
- Chinese State Food and Drug Administration (CFDA). The guidance principle of grade standard for adverse reactions that occur in clinical trial of prevention vaccines. Register no. 493. Beijing (China): CFDA; 2005.
- Chinese State Food and Drug Administration (CFDA). Technical guidelines for vaccine clinical trials. Register no. 575. Beijing (China): CFDA; 2004.
- Liao G, Li R, Li C, Sun M, Jiang S, Li Y, Mo Z, Xia J, Xie Z, Che Y, et al. Phase 3 trial of a Sabin strain-based inactivated poliovirus vaccine. J Infect Dis. 2016;214(11):1728–34. doi:10.1093/infdis/jiw433.
- Verdijk P, Rots NY, Bakker WA.Clinical development of a novel inactivated poliomyelitis vaccine based on attenuated Sabin poliovirus strains. Expert Rev Vaccines. 2011 May. 10(5):635–44. doi:10.1586/erv.11.51.
- Chu K, Ying Z, Wang L, Hu Y, Xia J, Chen L, Wang J, Li C, Zhang Q, Gao Q, et al. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, dose-finding trial. Vaccine. 2018 Oct 29;36(45):6782–89. doi:10.1016/j.vaccine.2018.09.023.
- Hu Y, Xu K, Han W, Chu K, Jiang D, Wang J, Tian X, Ying Z, Zhang Y, Li C, et al. Safety and immunogenicity of Sabin strain inactivated poliovirus vaccine compared with salk strain inactivated poliovirus vaccine, in different sequential schedules with bivalent oral poliovirus vaccine: randomized controlled noninferiority clinical trials in China. Open Forum Infect Dis. 2019 Aug 26;6(10):ofz380. doi:10.1093/ofid/ofz380.