ABSTRACT
Background: Older people (≥60 years old) are particularly vulnerable to influenza virus infection, and vaccine is effective in reducing the disease burden in this population. However, it remains obscure whether their antibody response is lower than those of younger adults (18–60 years old). Thus, this meta-analysis was performed to compare the immunogenicity of influenza vaccines and understand their association with real-world vaccine effectiveness (VE) between these two age groups.
Methods: A systematic literature search was conducted to identify relevant studies from Jan 01, 2008 to Nov 10, 2018. These are randomized controlled trials that included older adult samples, which assessed the immunogenicity of inactivated quadrivalent influenza vaccines produced in embryonated eggs. We excluded the studies focused only in children or adults. The outcomes were seroprotecton rate (SPR) and seroconversion rate (SCR).
Results: Six studies were eventually included in the present meta-analysis (7,976 participants). For the SPR, the pooled risk ratio (RR) was 0.92 (95% CI: 0.90–0.94, I2 = 66%, P < .0001) for A/H1N1 and 0.94 (95% CI: 0.90–0.98, I2 = 91%, P = .002) for B/Victoria, and the antibody responses of A/H3N2 and B/Yamagata were similar in the two age groups. For the SCR, the pooled RR was 0.85 (95% CI: 0.76–0.94, I2 = 93%, P = .003), 0.77 (95% CI: 0.66–0.91, I2 = 94%, P = .002), and 0.83 (95% CI: 0.71–0.96, I2 = 94%, P = .02) for A/H1N1, B/Victoria and B/Yamagata, respectively, and the antibody responses of A/H3N2 were similar in the two groups. Some variations were found in the antibody responses across virus types and subtypes after influenza vaccination.
Conclusion: The SPR and SCR of older adults were lower than those in younger adults for A/H1N1 and B/Victoria, while the two age groups had similar antibody responses for A/H3N2. The antibody responses to vaccines were not significantly associated with real-world VE, indicating that antibody response might not fully reflect the vaccine effectiveness of A/H3N2.
Introduction
The elderly are disproportionately affected by influenza-related diseases and complications, and influenza vaccines are effective in reducing disease burden even in this population.Citation1,Citation2 Frailty is one of the main factors attenuating antibody titer and avidity upon vaccination.Citation3 However, whether older adults produce lower antibody responses than younger adults with seasonal influenza virus strains after vaccination remains largely obscure.
In order to assess the efficacy of influenza vaccines, hemagglutination inhibition (HAI) assay has been employed as a surrogate to evaluate whether an influenza vaccine could be approved, utilizing standardized reagents (e.g.: standard sera) to quantify influenza-specific antibody titers, which is solely based on antibody responses.Citation4 However, the results of antibody response (HAI) are not always able to accurately predict the vaccine effectiveness (VE) in subsequent seasons or continuous seasons. The test-negative design (TND) case-control study emerged as a valid approach to estimate influenza vaccine effectiveness.Citation5 Accumulating evidence suggests that substantial variation does exist in VE across virus types and subtypes. The subtype with highest VE is A/H1N1, whereas the lowest VE is A/H3N2 in adults (aged ≥18). For the A/H3N2 strains, the VE of older adults is confirmed to be 7% lower than adults. For A/H1N1 or B strains, there are no significant differences between older adults and adults.Citation6–14 A meta-analysis has reported that QIV has similar antibody responses for the three common strains of A/H1N1, A/H3N2 and B lineage included in the TIV.Citation15 Thus, there is an intriguing question how antibody response is associated with the real-world VE, and how this is affected by aging.
Standard-dose quadrivalent influenza vaccine (QIV) is the only available vaccine that could cover all four seasonal influenza strains in circulation (A/H1N1, A/H3N2, B/Victoria and B/Yamagata). Various advisory bodies have suggested that high-dose vaccines or adjuvant vaccines may provide better protection for the elderly age group, when compared to a standard-dose of influenza vaccine, but these two types of vaccines are not always available in some countries such as China.Citation16–19Recent data showed that cell-cultured QIV was significantly more effective than egg-based QIV, high-dose and adjuvanted trivalent vaccines in preventing influenza-related office visits.Citation20 Since increasing number of countries and regions have recently gained access to quadrivalent inactivated influenza vaccines, it becomes feasible to assess the differences between these two age groups in response to vaccination of QIV. Remarkably, the subjects of standard-dose TIV regarded as control group in randomized controlled trials (RCTs) of QIV would be compared to estimate the differences of antibody responses between the two age groups. In this study, based on meta-analysis of RCTs of QIV, we have comparatively investigated antibody responses and their association with real-world VE between older and adults. Although there are different definitions of old age across various studies, in order to ensure the rigor of age grouping of subjects included in this study, we defined people aged ≥60 as older adults and people aged 18–60 as younger adults.
Methods
Search strategy, selection criteria and data extraction
The present study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria, Citation21 and the study protocol was registered in the PROSPERO international prospective register of systematic reviews (CRD42018117088).
A systematic literature search was conducted on electronic databases (Medline, Cochrane Library [Wiley], Web of Science) to identify relevant studies between January 01, 2008 and November 10, 2018. ClinicalTrials.gov was manually searched for the relevant registered clinical trials. The keywords we searched included “influenza vaccine,” “quadrivalent influenza vaccine,” “randomized control trial,” “frail elderly.” Studies included in the aggregate data meta-analysis are RCTs, which included older adults, assessed the immunogenicity of inactivated QIV produced in embryonated eggs, and all vaccines used in the study were standard-dose vaccines. Studies only focused on children or younger adults were excluded. The Cochrane Collaboration’s Risk of Bias Tool built in RevMan software was employed to assess the quality of the included studies.Citation22
Two reviewers (JR Shi and W Zhao) independently performed data extraction and assessment of potential risk of bias. Disagreements between the two reviewers were settled by discussion, and a third reviewer (ZY Meng) would arbitrate when the discussion did not resolve the disputed points. Extracted data included demographics of the participant population, interventions and vaccine strains, type of study, and vaccine manufacturers.
Definitions and outcomes
The seroprotection rate (SPR) and seroconversion rate (SCR) were employed to measure the immunogenicity of A/H1N1, A/H3N2, B/Victoria and B/Yamagata in both the quadrivalent and trivalent influenza vaccine groups. However, the primary outcome in this meta-analysis was the different pooled antibody responses (SPR and SCR) between the two groups. The secondary outcome was the association of pooled immunogenicity data (SPR and SCR) to real-world VE, and this was used to determine whether the trend of antibody response of virus types and subtypes was similar to VE (highest in A/H1N1 and lowest in A/H3N2).Citation6–14
The experimental group and the control group were also different according to the exploration factors. When comparing the differences of antibody differences between older adults and adults, the SPR/SCR data of immunogenicity of adults were the references group. When comparing the differences between different subtypes after vaccination, for example, to explore the differences between A/H1N1 and A/H3N2, the data of A/H3N2 strains were the references group.
Statistical analysis
Chi-square tests were first performed on each of the included studies to determine how far these different studies differed in the responses to the same virus types. The pooled data are expressed by risk ratios (RRs) that can be quantified as significant (RR value: ≤0.5 or ≥2.0).Citation23 Then, the pooled RRs were conducted for SPR and SCR, and their corresponding 95% confidence intervals (95% CI). A random-effects model (DerSimonian-Laird method) was used when there was high heterogeneity in the data.Citation24 Otherwise, fixed-effects model was chosen.
The heterogeneity between studies was assessed by using I2 statistics, and be quantified as low (≤25%), moderate (25%-50%), and high (>50%), Citation25,Citation26 and subgroup analyses were performed by region (Europe, Asia, and USA), manufacturer (Sanofi Pasteur, Jiangsu GDK, Abbott Biologicals B.V and M/s Cadila Healthcare Limited, India) and study time (2013, 2017, and 2018). A sensitivity analysis was performed by the time of collecting blood samples. A study that collected blood samples at 28 days after the inoculation, Citation27 with all the other included studies collected blood samples at 21 days after the vaccination. P < .05 was set as the threshold for statistical significance. Chi-square tests were performed using IBM SPSS 22.0, and all meta-analysis were conducted using the RevMan 5.3 software by the Cochrane Collaboration.Citation28
Results
Risk assessment, literature search and characteristics of the eligible studies
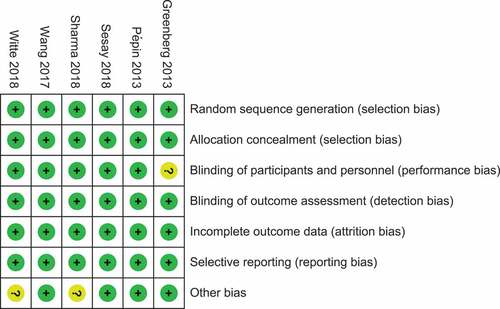
The flow diagram for the selection of studies and the quality of the included literatures are summarized in and supplement data (figure S1), and all of them are controlled high-quality clinical study. A total of 309 unduplicated publications were identified, and eight studiesCitation26,Citation29–35 met the predetermined inclusion criteria, namely those RCTs studies assessed the antibody responses of inactivated standard-dose QIV produced in embryonated eggs, and the research objects included older adults. Regretfully, there were two studies that the required information was not included in their original publications. But we did not receive responses after contacting the authors.Citation32,Citation33 Hence, these studies were eventually excluded from the aggregate data meta-analysis. Furthermore, one study lacks the data of TIV.Citation32 All the six studies (7,976 participants) include in this meta-analysis were conducted in the northern hemisphere: three studies originated from Europe, two studies came from Asia (China and India), and one study hailed from North America ().
Table 1. Summary of study characteristics
Meta-analysis of immunogenicity
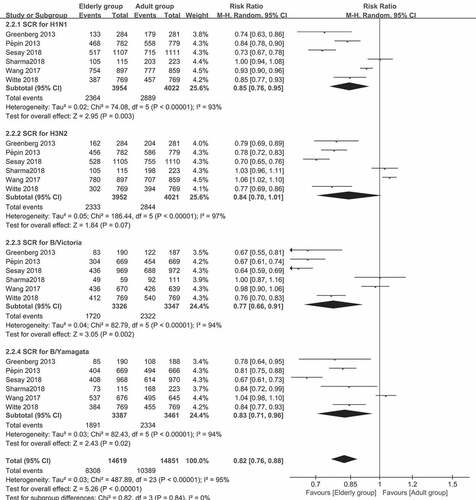
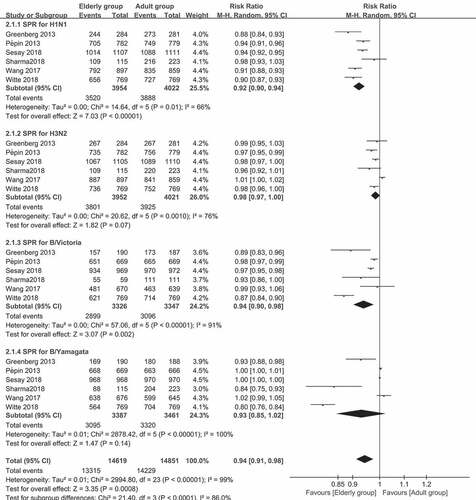
The results for the pooled RRs of SPR and SCR for the four strains in the two age groups are presented in and . For the SPR of the A/H1N1 strain, the pooled SPR RR was 0.92 (95% CI: 0.90–0.94, I2 = 66%, P < .0001). For the B/Victoria lineage, the pooled SPR RR was 0.94 (95% CI: 0.90–0.98, I2 = 91%, P = .002). For the A/H3N2 strain and B/Yamagata lineages, the SPR (P = .07 and 0.14, respectively) was similar in the two age groups. For SCR of the comparison between older (≥60 years old) and younger adults (18–60 years old), the pooled SCR RRs was 0.85 (95% CI: 0.76–0.94, I2 = 93%, P = .003) for the A/H1N1 strain, 0.77 (95% CI: 0.66–0.91, I2 = 94%, P = .002) for the B/Victoria lineage, and 0.83 (95% CI: 0.71–0.96, I2 = 94%, P = .02) for the B/Yamagata lineage. For the A/H3N2 strain, the pooled data in the two groups were similar (p = .07). Interestingly, the RRs ranged from 0.77 to 0.94, indicating that there was no significant association between aging and antibody responses.
Figure 2. (a) The seroprotection rate (SPR) of older vs. younger adults for the four virus strains after vaccination (SPR was defined as the percentage of participants with a HAI titer of ≥40). (b) The seroconversion rate (SCR) of older vs. younger adults for the four strains after vaccination (SCR was defined as the percentage of those with either a pre-vaccination HAI titer of <10 and a post-vaccination HAI titer of ≥40, or a pre-vaccination HAI titer of ≥10 and a ≥ fourfold increase in HAI titer after vaccination)
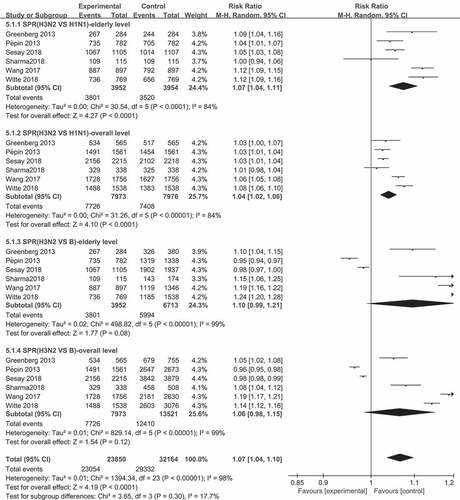
The association between antibody responses and real-world vaccine effectiveness
Interestingly, mild variations were found in SPR and SCR across virus types and subtypes after influenza vaccination, which was different to the real-world VE.Citation6 Especially for the A/H3N2 strain, compared to the A/H1N1 strain, the pooled SPR RR was 1.04 (95% CI: 1.02–1.06, I2 = 84%, P < .0001) in the overall population (all subjects), and 1.07 (95% CI: 1.04–1.11, I2 = 84%, P < .0001) in the elderly population (), indicating that the SPR of the A/H3N2 strain was slightly higher, when compared to that of the A/H1N1 strain, which mismatch with the real-world VE. Furthermore, the P-values of the antibody responses (SPR and SCR) of all other comparisons (such as the SPR and SCR of A/H1N1 vs. B and A/H3N2 vs. B, and the SCR of A/H3N2 vs. A/H1N1) were greater than 0.05. Hence, these had similar antibody responses (data not shown).
Subgroup analysis and sensitivity analysis
Chi-square test revealed that after vaccination, the antibody responses (SPR and SCR) in elderly people were not always the same as those produced by younger adults in different studies. For example, for A/H3N2 strains, no significant differences were found in the SPR reported by GreenbergCitation29 and Wang, Citation26 which hints that the titer of antibody produced by the older adults group was similar to that produced by the younger adults group. Furthermore, there was a significant difference in the results of Pepin, Citation30 Sesay, Citation31 Sharma,Citation33 and WitteCitation32 (Table S1).
Subgroup analyses revealed that the differences of regions, manufactures or study time are likely not the factors causing heterogeneity (data not shown). As shown in table S1, there were always differences for the same virus strains among different studies. Remarkably, similar to data in table S1, the antibody responses also vary for the same strains among different studies with different vaccine manufacturers (table S2). However, the sensitivity analysis revealed that the sampling time was a heterogeneity source. For the SPR of the A/H3N2 strain, after removing a study, Citation26 I2 decreased from 66% to 0%, and the P-value decreased from 0.15 to <0.001, becoming statistically significant. For SCR, the change in A/H3N2 was similar to SPR, and there were few effects on the other comparison.
Discussion
There were three important findings in the present meta-analysis. Firstly, the antibody responses (SPR and SCR) of older adults were found to be lower than those of younger adults after influenza vaccination for A/H1N1 strains and B/Victoria lineages. Furthermore, the elderly had a lower SCR for B/Yamagata lineages, and the two age groups had similar antibody responses for the A/H3N2 strain. Secondly, limited variations were found in antibody responses across virus types and subtypes after influenza vaccination, which was a different trend compared to that of real-world VE. Finally, in the present meta-analysis, the pooled RRs ranged from 0.77 to 0.94, revealing no significant association between aging and antibody responses. This might explain the inevitability of why different studies often have different results for the same virus in the four virus strains.
The antibody responses of the A/H3N2 strain were found to be not below or even above the A/H1N1 strain and B lineage (B/Victoria and B/Yamagata), but the real-world VE of A/H3N2 was the lowest.Citation9–14,Citation16,Citation17 These different trends hint that antibody responses to vaccine were not significantly associated with real-world VE, which is consistent with previous studies.Citation36–38 In addition, studies that revealed that vaccinated elderly subjects, who developed laboratory-confirmed influenza illness due to A/H3N2 strain infection, had similar A/H3N2-specific antibody titers following vaccination, when compared to subjects who did not develop laboratory-confirmed influenza illness.Citation39–41 Furthermore, a meta-analysis included 5,210 participants showed that there were markedly different VE between A/H1N1 and B lineage.Citation12 However, the antibody responses between A/H1N1 and B lineage were similar in our findings. Those indicate that the antibody responses measured by antibody responses only might not fully reflect the real-world VE of A/H3N2 strains. Some studies have reported that the immune function gradually declined with age, including the reduction in antibody response after immunization and more reliance on T-cell mediated response.Citation42–44 However, this did not conflict with the present findings that no significant association between aging and antibody responses were derived from the overall population of studies.
A high heterogeneity was observed in the meta-analysis. Hence, a random effects model was used. The reason for the heterogeneity in the present meta-analysis may be that the vaccines had different virus strains with different production processes, and there were variations in the HAI assay responses of subjects due to the different historical exposures to natural infection or vaccination. For the sensitivity analysis, after carefully reviewing the studyCitation26 and its chi-square test results, it was found that the antibody responses (SPR and SCR) of A/H3N2 in elderly people might be enhanced over time. Furthermore, the plateau for antibody responses in older adults may occur later, when compared to younger adults, which indicates that the postponement sampling time from day 21 to day 28 might be a useful tip to increase the odds of success in clinical trials for influenza vaccines in older adults. Nevertheless, the major findings of the present meta-analysis are unlikely as a result of the heterogeneity and bias, since the magnitude and direction of any bias would be similar for each virus strain, permitting valid comparisons to be conducted among them.
A few potential limitations of the present meta-analysis should be noted. First, the reporting bias was not conducted due to the insufficient number of studies included. Furthermore, to our knowledge, some companies did not publish the relevant data for reasons of confidentiality or being unknown reasons, and not all potential data contributors shared their complete data. That is, the investigators consider that a reporting bias exists. Furthermore, all the included studies were conducted in the northern hemisphere in the present aggregate data meta-analysis, and this insufficient coverage might limit the universality of the present findings. Finally, the source of the samples was volunteers, instead of the natural population. This means that there was a certain selection bias.
Seasonal influenza vaccination is associated with a significant reduction in influenza-specific hospitalizations, especially in elderly people with underlying chronic diseases, Citation45 and improving the influenza vaccine coverage rates remains a very important goal for this age group. At present, the vaccine coverage for a population of over 65 years old was still below the 75-80% target, Citation46 even in some highly developed countries or regions.Citation47–50 Furthermore, egg-based influenza vaccine production cannot simply satisfy the global demand. Although high-dose and adjuvant influenza vaccines have been preferentially recommended, Citation1–3 the contents of antigen in high-dose vaccines were 4-folds of a standard-dose vaccine, and the MF-59 adjuvant had patent restrictions, which would inevitably limit their coverage. A meta-analysis of five RCTs concluded that QIV has equivalent efficacy against the shared three strains in TIV.Citation15 Therefore, the QIV may be the best option for many regions, and the present meta-analysis provides significant references for many advisory bodies and drug evaluation and approval agencies.
Further studies are needed to determine whether the postponement of sampling time from 21 to 28 days could increase the antibody responses in the elderly people. This might be helpful to increase the odds of success in clinical trials for influenza vaccines in the elderly population. Furthermore, it was found that the antibody responses only measured by HAI assay (antibody response) might not fully reflect the VE of A/H3N2 strains. Therefore, adding valuable additional information, such as virus neutralization assayCitation51 or the ratio of interferon (IFN)-γ (pro-inflammatory) to interleukin (IL)-10 (anti-inflammatory) of peripheral blood mononuclear cells, Citation39,Citation52 may be practical to further improve the accuracy of the HAI assay.
Disclosure of potential conflicts of interest
The authors declare no competing financial interests.
Supplemental Material
Download MS Word (28.6 KB)Acknowledgments
We sincerely thank Ze Chen (Shanghai Institute of Biological Products), Shuo Shen (Wuhan Institute of Biological Products), and Qiuwei Pan (Department of Gastroenterology and Hepatology, Erasmus MC-University Medical Center, Rotterdam) for providing the critical revision of the manuscript, and Serge van de Witte (Abbott Healthcare Products B.V.) for providing the data of vaccine strains.
Supplementary Materials
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1747375
Additional information
Notes on contributors
Xiaoming Yang
ZY Meng, JY Zhang and XM Yang designed the study, JR Shi retrieved the literatures, and screened and abstracted publications with W Zhao. Li Cheng contacted the authors, and ZY Meng and JY Zhang analyzed the data. ZY Meng wrote the manuscript, with editorial contributions from XY Huang, JY Zhang and XM Yang. All authors reviewed the manuscript for accuracy and scientific content.
References
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. doi:10.1016/S0140-6736(17)33293-2.
- Nichols MK, Andrew MK, Hatchette TF, Ambrose A, Boivin G, Bowie W, Chit A, Dos Santos G, ElSherif M, Green K, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: A pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network).. Vaccine. 2018;36(16):2166–75. doi:10.1016/j.vaccine.2018.02.093.
- Andrew MK, Bowles SK, Pawelec G, Haynes L, Kuchel GA, McNeil SA, McElhaney JE. Influenza vaccination in older adults: recent innovations and practical applications. Drugs Aging. 2019;36(1):29–37. doi:10.1007/s40266-018-0597-4.
- Tauraso NM, O’Brien TC, Seligman EJ. Problems of influenza virus vaccine standardization. Bull World Health Organ. 1969;41:497–506.
- Skowronski DM, Gilbert M, Tweed SA, Petric M, Li Y, Mak A, McNabb G, De Serres G. Effectiveness of vaccine against medical consultation due to laboratory-confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004-2005. Can Commun Dis Rep. 2005;31(18):181–91.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. doi:10.1016/S1473-3099(16)00129-8.
- Darvishian M, van den Heuvel ER, Bissielo A, Castilla J, Cohen C, Englund H, Gefenaite G, Huang W-T, la Bastide-van Gemert S, Martinez-Baz I, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med. 2017;5(3):200–11. doi:10.1016/S2213-2600(17)30043-7.
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–30. doi:10.1016/S0140-6736(11)61051-9.
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–27. doi:10.1093/cid/cit736.
- Russell K, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018;36(10):1272–78. doi:10.1016/j.vaccine.2018.01.045.
- Dominguez A, Soldevila N, Toledo D, Godoy P, Espejo E, Fernandez MA, Mayoral JM, Castilla J, Egurrola M, Tamames S, et al. The effectiveness of influenza vaccination in preventing hospitalisations of elderly individuals in two influenza seasons: a multicentre case–control study, Spain, 2013/14 and 2014/15. Euro Surveill. 2017;22(34). doi:10.2807/1560-7917.ES.2017.22.34.30602.
- Darvishian M, van den Heuvel ER, Bissielo A, Castilla J, Cohen C, Englund H, Gefenaite G, Huang W-T, la Bastide-van Gemert S, Martinez-Baz I, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med. 2017;5(3):200–11
- Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, McElhaney JE, Ambrose A, Boivin G, Bowie W, et al. The Importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216(4):405–14. doi:10.1093/infdis/jix282.
- Cowling BJ, Feng S, Finelli L, Steffens A, Fowlkes A. Assessment of influenza vaccine effectiveness in a sentinel surveillance network 2010-13, United States. Vaccine. 2016;34(1):61–66. doi:10.1016/j.vaccine.2015.11.016.
- Moa AM, Chughtai AA, Muscatello DJ, Turner RM, MacIntyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials. Vaccine. 2016;34(35):4092–102. doi:10.1016/j.vaccine.2016.06.064.
- Government of Canada. Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2018–2019. An Advisory Committee Statement (ACS). national advisory committee on immunization (NACI). [ accessed 2019 Jan 16]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2018-2019.html#5.22018
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2018–19 Influenza Season. MMWR Recomm Rep. 2018;67(RR–3):1–20. doi:10.15585/mmwr.rr6703a1.
- Australian Government, Department of Health Influenza: the Australian immunizations handbook. Adults aged ≥65 years are strongly recommended to receive either high-dose or adjuvanted influenza vaccine every year. [ accessed 2019 Jan 3]. https://immunisationhandbook.health.gov.au/recommendations/adults-aged-65-years-are-strongly-recommended-to-receive-either-high-dose-or
- European Centre for Disease Prevention and Control. Types of seasons influenza vaccine. [ accessed 2019 Jan 3]. https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017-2018. J Infect Dis. 2019;220(8):1255–64. doi:10.1093/infdis/jiy716.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials[J]. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
- Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76(7):e857–e861. doi:10.4088/JCP.15f10150.
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. doi:10.1016/j.cct.2015.09.002.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
- Wang S-Y, Liu S-Z, Chu K, Zhao Y, Zhu F-C, Hu Y-M, Meng F-Y, Li J-X, Luo L, Yang J-Y, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccines in participants >/=3 years of age: a double-blind, randomized, parallel-controlled phase III clinical trial in China. Expert Rev Vaccines. 2017;16(11):1155–69. doi:10.1080/14760584.2017.1374181.
- Melsen WG, Bootsma MC, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–29. doi:10.1111/1469-0691.12494.
- Review Manager (RevMan) [Computer program]. Version 5.3. London (UK): The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31(5):770–76. doi:10.1016/j.vaccine.2012.11.074.
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013;31(47):5572–78. doi:10.1016/j.vaccine.2013.08.069.
- Sesay S, Brzostek J, Meyer I, Donazzolo Y, Leroux-Roels G, Rouzier R, Astruc B, Szymanski H, Toursarkissian N, Vandermeulen C, et al. Safety, immunogenicity, and lot-to-lot consistency of a split-virion quadrivalent influenza vaccine in younger and older adults: A phase III randomized, double-blind clinical trial. Hum Vaccin Immunother. 2018;14(3):596–608. doi:10.1080/21645515.2017.1384106.
- van de Witte S, Nauta J, Montomoli E, Weckx J. A Phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine. 2018;36(40):6030–38. doi:10.1016/j.vaccine.2018.04.043.
- Sharma S, Singh VB, Kumar S, Prajapati V, Patel J, Vukkala R, Jangid SK, Sanmukhani J, Gupta G, Patel P, et al. Immunogenicity and safety of the first indigenously developed Indian tetravalent influenza vaccine (split virion) in healthy adults ≥ 18 years of age: A randomized, multicenter, phase II / III clinical trial. Hum Vaccin Immunother. 2018;14(6):1362–69. doi:10.1080/21645515.2018.1441654.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, Aranza Doniz C, Chandrasekaran V, Dewé W, Liu A, Innis BL, Jain VK. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: A phase III, randomized trial. Vaccine. 2014;32(13):1480–87. doi:10.1016/j.vaccine.2014.01.022.
- Kieninger D, Sheldon E, Lin W-Y, Yu C-J, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013;13(1):343. doi:10.1186/1471-2334-13-343.
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals: A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–65. doi:10.1001/jama.1994.03520210045030.
- Govaert TM, Sprenger MJ, Dinant GJ, Aretz K, Masurel N, Knottnerus JA. Immune response to influenza vaccination of elderly people: A randomized double-blind placebo-controlled trial. Vaccine. 1994;12(13):1185–89. doi:10.1016/0264-410X(94)90241-0.
- McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10(3):379–88. doi:10.1016/j.arr.2010.10.008.
- McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC, et al. Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27(18):2418–25. doi:10.1016/j.vaccine.2009.01.136.
- Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010;28(38):6145–51. doi:10.1016/j.vaccine.2010.07.036.
- Merani S, Kuchel GA, Kleppinger A, McElhaney JE. Influenza vaccine-mediated protection in older adults: impact of influenza infection, cytomegalovirus serostatus and vaccine dosage. Exp Gerontol. 2018;107:116–25. doi:10.1016/j.exger.2017.09.015.
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. doi:10.1016/j.vaccine.2005.08.105.
- Seidman JC, Richard SA, Viboud C, Miller MA. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses. 2012;6(1):52–62. doi:10.1111/j.1750-2659.2011.00268.x.
- McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30(12):2060–67. doi:10.1016/j.vaccine.2012.01.015.
- Leval A, Hergens MP, Persson K, Örtqvist Å. Real-time real-world analysis of seasonal influenza vaccine effectiveness: method development and assessment of a population-based cohort in Stockholm County, Sweden, seasons 2011/12 to 2014/15. Euro Surveill. 2016;21(43). doi:10.2807/1560-7917.ES.2016.21.43.30381.
- World Health Organization: Seasonal Influenza Fact Sheet 2018. [accessed 2019 Jan 20]. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- Centers for Disease Control and Prevention. Early-Season Flu Vaccination Coverage, United States, November 2018. [accessed 2019 Jan 16]. https://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2018.htm
- Public Health Agency of Canada. 2016–2017 seasonal influenza vaccine coverage in Canada. [accessed 2019 Jan 16] https://www.canada.ca/en/public-health/services/publications/healthy-living/2016-2017-seasonal-influenza-flu-vaccine-coverage-survey-results.html
- European Center for Disease Prevention and Control. News & events-> Influenza vaccination coverage rates insufficient across EU Member States. [ accessed 2019 Jan 16]. https://ecdc.europa.eu/en/news-events/influenza-vaccination-coverage-rates-insufficient-across-eu-member-states
- Karki S, Dyda A, Newall A, Heywood A, MacIntyre CR, McIntyre P, Banks E, Liu B. Comparison of influenza vaccination coverage between immigrant and Australian-born adults. Vaccine. 2016;34(50):6388–95. doi:10.1016/j.vaccine.2016.10.012.
- Truelove S, Zhu H, Lessler J, Riley S, Read JM, Wang S, Kwok KO, Guan Y, Jiang CQ, Cummings DAT, et al. A comparison of hemagglutination inhibition and neutralization assays for characterizing immunity to seasonal influenza A. Influenza Other Respir Viruses. 2016;10(6):518–24. doi:10.1111/irv.12408.
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–39. doi:10.4049/jimmunol.176.10.6333.