ABSTRACT
Traditional non-living vaccines are often least effective in the populations that need them most, such as neonates and elderly adults. Vaccine adjuvants are one approach to boost the immunogenicity of antigens in populations with reduced immunity. Ideally, vaccine adjuvants will increase the seroconversion rates across the population, lead to stronger immune responses, and enable the administration of fewer vaccine doses. We previously demonstrated that a cationic liposomal formulation of the commercial influenza split virus vaccine (CCS/C-HA) enhanced cellular and humoral immunity to the virus, increased seroconversion rates, and improved survival after live virus challenge in a preclinical model, as compared to the commercial vaccine as is (F-HA). We now evaluated vaccine efficacy in different strains and sexes of mice and determined the role of innate immunity in the mechanism of action of the CCS/C adjuvant by testing the response of mice deficient in Toll-like receptors or the TLR/IL-1 adaptor protein MyD88 following immunization with CCS/C-HA vs. F-HA. Although TLR2- and TLR4-deficient mice responded to F-HA immunization, F-HA immunization failed to engender a significant immune response in the absence of MyD88. In contrast, immunization with the CCS/C-HA vaccine overcame the requirement for MyD88 in the response to the commercial vaccine and improved the immune responses and seroconversion rates in all strains of mice tested, including those deficient in TLR2 and TLR4.
Introduction
Influenza vaccines are the primary strategy to reduce the substantial health burden from seasonal influenza. Inactivated influenza vaccines have been available since the 1930sCitation1 and are administered via intramuscular (i.m.) injection. Live attenuated, cold-adapted influenza vaccines (LAIV) were licensed in the United States in 2003, and are administered via nasal spray.Citation2-4 The effectiveness of influenza vaccines correlates predominantly to the age and immune competence of the vaccinee and the antigenic relatedness of vaccine strains to circulating strains.Citation5-7 More effective seasonal influenza vaccines are required in order to provide long-lasting immunity and broad protection against strains that differ antigenically from vaccine viruses. Of particular need are vaccine strategies that address immunity in populations at most risk of influenza infection, such as the elderly and neonates.
We previously reported that novel cationic liposomes composed of the polycationic sphingolipid N-palmitoyl d-erythro-sphingosyl-carbamoyl-spermine triacetate salt (CCS) and cholesterol (CCS/C), and including influenza hemagglutinin (HA) antigen, stimulate increased humoral and cellular immune responses in different animal models and protect against influenza virus infectionCitation8-10 A. Joseph, N. Itskovitz-Cooper, S. Samira, O. Flasterstein, H. Eliyahu and D. Simberg et al., A new intranasal | Cited By in Scopus (32). In mice, intranasal (i.n.) administration of the standard commercial trivalent influenza vaccine formulated in CCS/C liposomes evokes strong systemic (serum) and local mucosal (nose and lungs) antibody responses as well as cellular responses and protective immunity.Citation11 The CCS/C-influenza vaccine is significantly more immunogenic than the standard vaccine given i.m. or i.n. High antibody titers and protective immunity to viral challenge are sustained for >9 months following immunization.Citation8,Citation10 Furthermore, the CCS/C-adjuvanted vaccine is highly efficacious following a single dose (i.m. or i.n.) in both young (2 mo) and old (18 mo) mice, and it elicits high titers of strain cross-reactive Hemagglutination Inhibiting (HI) antibodies.Citation11 In ferrets, the i.n. CCS/C-influenza vaccine elicits up to 10-fold higher HI antibody titers compared to the conventional i.m. vaccine. In rats, the CCS/C influenza vaccine is significantly more immunogenic by the i.m. or i.n. route than conventional vaccine.Citation10 Hence, CCS/C has the potential to be a valuable adjuvant.
Most adjuvants in clinical or experimental use enhance immunity by activating innate immune signaling pathways in antigen-presenting cells.Citation12 Since innate immunity is diminished in populations most at risk of infection,Citation13,Citation14 the reliance of adjuvants on innate signaling pathways is a potential limitation. In the current study, we further elucidate the mechanism of action of the CCS/C influenza vaccine by testing it in mice deficient in receptors or signaling molecules of the innate immune system. Toll-Like Receptors (TLRs) in humans and mice respond to a broad array of evolutionarily conserved microbial molecules.Citation15 TLR4 is required for the response to lipopolysaccharide from most gram-negative bacteria, whereas TLR2 governs the response to peptidoglycan and lipopeptides from most gram-positive bacteria.Citation16 Stimulation of TLRs by microbial structures leads to the activation of transcription factors that drive cytokine expression, proliferation, survival, and inflammatory mediator expression. MyD88 is a central adaptor protein that assembles a protein complex at the intracellular portion of all TLRs except TLR3, and members of the IL-1 receptor family, enabling the recruitment of kinases.Citation17 Therefore, much of innate immune signaling converges on MyD88, and MyD88 deficiency in mice models a state of severe compromise in innate immunity.Citation15,Citation18
We now demonstrate that the CCS/C liposomes as adjuvant improve the immune response of TLR2- and TLR4-deficient mice, to the influenza vaccine. Furthermore, whereas MyD88 is required for an effective response to the commercial influenza vaccine, incorporation of this vaccine within the CCS/C liposomes overcomes this requirement.
Materials and methods
Animals
Mouse experiments were performed in the animal facility of the Hebrew University, Jerusalem, Israel. Specific pathogen-free (SPF) female and male BALB/c mice, female C3 H/HeNHsd mice, and female C57BL/6JOlaHsd mice (8–9 weeks old) were obtained from Harlan (Jerusalem, Israel). C57/BL10 wild-type (used for experiments shown in and ) and C57/BL10ScN (TLR4lps-del), a spontaneous TLR4 knock-out strain, were from the Jackson Laboratory (JAX stock #003752, Maine, USA). Myd88−/- and Tlr2−/-, mice backcrossed to the C57BL/6JOlaHsd background were kindly provided by Prof. S. Akira (Osaka University, Japan) to GN. All animal studies were approved by the Institutional Animal Care and Use Committees of the Hebrew University.
Table 1. Proliferative response and cytokine production by spleen cells following two doses of F-HA vs. CCS/C-HA vaccination in WT, Tlr2-/-, and Tlr4-/- mice
Influenza antigens (HA)
A commercial trivalent split vaccine (Vaxigrip, Aventis Pasteur or Sanofi Pasteur) from several vaccination seasons, based on availability, was used throughout the study. The three strains of the vaccine were those recommended by the World Health Organization for the respective influenza season: A/Solomon Islands/3/2006 (H1N1)-like virus; A/Wisconsin/67/2005 (H3N2)-like virus; B/Malaysia/2506/2004-like virus (season 2007/2008, used for the mice strains and gender comparison studies). A/Brisbane/59/2007 (H1N1) – like strain IVR-148 derived from A/Brisbane/59/2007, A/Brisbane/10/2007 (H3N2) – like strain NYMC X-175 C derived from A/Uruguay/716/2007 and B/Florida/4/2006 – like strain B/Florida/4/2006 (season 2008/2009, used for the TLR/MyD88 knock-out mouse experiments). The level of HA is based on HA quantification as described in the commercial vaccine insert. In all seasonal vaccines, the HA content was ~50% of total split vaccine protein (quantified by Lowry methodCitation19).
Lipids
The polycationic sphingolipid N-palmitoyl D-erythro sphingosyl carbamoyl-spermine (CCS) was obtained from Biolab Ltd (Jerusalem, Israel). High purity (HP) cholesterol was obtained from Minakem, France. Cholesterol was used as “helper lipid”. Helper lipids such as cholesterol are neutral lipids that contribute to the stability and delivery efficiency of cationic lipids. All lipids used were ≥98% pure.
CCS/C-HA vaccine preparation and physicochemical characterization
Vaccine was formulated as described previouslyCitation11 using a 3:2 CCS:C molar ratio in PBS containing Vaxigrip at the desired HA:lipid weight ratio. The physical characterization of the CCS/C-based vaccine including the level of association of the commercial split vaccine proteins to the CCS/C liposomes, mean particle size, size range, and zeta potential were determined as described previously.Citation10 As determined by the Lowry method after the separation between the liposomes and their medium, >85% and in most cases ~95% of the commercial split protein is associated with the cationic liposomes. The CCS/C-based vaccine mean particle size, and size range were determined using a particle size analyzer (BeckmanCoulter LS I3 320). This instrument combines two methods of size distribution analyses: multi-wave light diffraction and polarization intensity differential scattering (PIDS polarization of the light). This combination allows the determination of broad size distributions in the range of 40 nm to 2 mm and therefore is more suitable for particles out of the nano-size range such as the cationic liposomes used in this study. The vaccine liposomes are a mixture of uni-, oligo- and multi-lamellar liposomes (lipid vesicles) of various shapes and broad size distribution with a reproducible mean size of 1.0 µm, and >85% of the particles are in the range of 0.1 to 3.0 µm. As determined by using a Zetasizer Nano Series ZEN2600 F (Malvern Instruments, Malvern, UK) these liposomes are cationic having a positive zeta potential measured in PBS (0.01 M phosphate buffer in 0.145 M sodium chloride, pH 7.2) of ~33 mV (for more details on the characterization see ref. 10).
Immunization of mice
In mouse experiments, the un-adjuvanted commercial Vaxigrip split vaccine (referred to as free HA [F-HA]) and the CCS/C-HA vaccine were used at 0.5–1.0 μg HA/strain/dose and 0.15–3 mg CCS/C/dose (HA:CCS/C weight ratio 1:100) and administered once or twice (28 d apart) intramuscularly (i.m.) in 50 μl volumes.
Evaluation of humoral immune responses in mice
Mice were bled from the facial vein, and sera were prepared as described previously.Citation11 In the case of one immunization, mice were bled and sacrificed on d30 after vaccination. In the case of two immunizations, mice were bled on d 26, re-vaccinated on d 28, and then bled again 9 or 10 d later. HI antibodies were determined on individual serum samples by the standard HI assay, starting at a 1:20 sample dilution.Citation20 HI titer was tested against the most immunogenic strain from the commercial vaccine used in each experiment, as indicated in the figure legend. Mice with HI titer ≥40 (considered a protective titer in humans) were defined as seroconverted.
Assessment of cellular responses in mice
Mice were sacrificed by cervical dislocation under deep anesthesia, and spleens were isolated. Splenocytes were tested for proliferative responses and for the production of IFNγ, IL-2, and IL-5 following in vitro stimulation with antigen. Cell cultures were performed as described previously.Citation10 Briefly, to measure proliferation 0.5 × 106 cells per well were incubated in triplicate, with or without the antigen (0.05–0.5ug/ml), in a final volume of 0.2 ml. After 96 hr, cultures were pulsed with 1 μCi 3 H-thymidine ON. Con A was used as positive control (0.25 μg/ml). Data shown in represent the stimulation index (SI) = mean cpm + antigen/mean cpm without the antigen. To measure cytokine production, 2.5 × 106 cells per well were incubated in duplicates as above. Con A was used as positive control (0.25 μg/ml), in a final volume of 1 ml. The supernatants were collected 72 hr later and tested by ELISA (BioLegend Max Set Deluxe, according to manufacturer’s instructions). Cytokine levels were calculated after subtraction of background (cells incubated with medium alone). Data in represent a single experiment with 3–5 mice per group.
Table 2. Proliferative response and cytokine production by spleen cells following vaccination with one or two doses of F-HA vs. CCS/C-HA vaccine in WT and Myd88-/- mice
Statistical analysis
In all studies, the differences between groups were analyzed using a two-tailed Student’s t-test when two means were compared, and analysis of variance (ANOVA) when more than two means were compared. P-values <0.05 were considered significant.
Results
CCS/C-HA adjuvant effect in different mouse strains
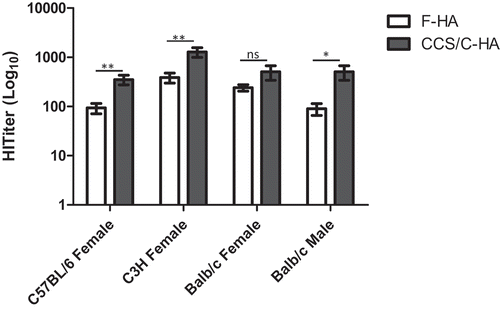
Genetic background influences both murine and human responses to vaccines and vaccine adjuvants through effects on antigen presentation and co-stimulatory signals, including the types and levels of cytokines that are produced during T and B cell activation.Citation21-24 We therefore compared the immunogenicity of the F-HA native vaccine and the CCS/C-HA adjuvanted vaccine in three inbred mouse strains differing in MHC haplotype and in the character of their immune responses.Citation25-27 Vaccine efficacy was compared in female mice of all strains, and in male Balb/c mice. HI titers differed among strains in response to both vaccines (). For both vaccines independently, one-way ANOVA across the four groups of mice demonstrated that C3 H mice responded significantly better than the other strains, and that differences observed between the other strains did not reach significance. In C3 H and C57BL/6 female mice, and in Balb/c male mice, the CCS/C-HA vaccine induced significantly higher HI antibody titers than the F-HA vaccine (). The effect of CCS/C-HA vaccination on HI titer across strains was highly significant compared to F-HA vaccination (P < .0001, 2-way ANOVA).
Figure 1. Effect of mouse strain and gender on immunogenicity of F-HA vs. CCS/C-HA
Involvement of TLR2 and TLR4 in the response to F-HA and CCS/C-HA vaccination
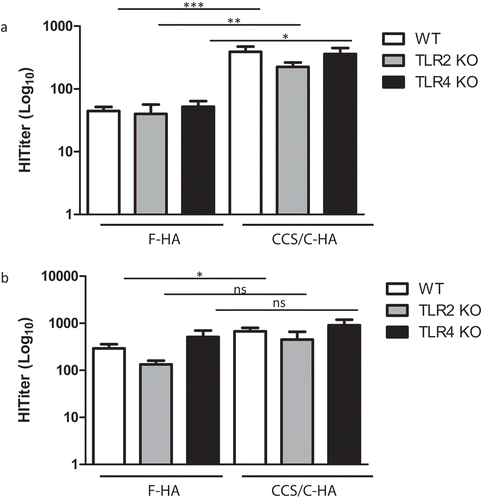
Innate immune signaling pathways enhance MHC-directed antigen presentation and co-stimulatory molecule expression and influence the phenotype and strength of the response through induction of an inflammatory cytokine milieu during the encounter between APCs and responder T cells.Citation12 TLRs 2 and 4 govern the response to a broad array of microbial molecules and host damage associated ligands generated at the immunization site.Citation28 We therefore tested the effect of one or two immunizations with F-HA vs. CCS/C-HA in mice deficient in TLR2 or TLR4. For both vaccines, HI titers were similar between WT, TLR2 knock-out (KO), and TLR4 KO mice in response to one (), or two (), immunizations. After primary immunization, the CCS/C-HA vaccine led to significantly higher titers in all strains (). However, after the second immunization, the CCS/C-HA vaccine showed a significant improvement in HI titer in WT mice (P = .01), but not in either of the KO strains (). Splenic T cell responses (proliferation and IFNγ cytokine secretion) were enhanced by immunization with CCS/C adjuvant in WT mice in a similar manner to our previous findings. In both TLR-deficient strains the CCS/C adjuvant greatly enhanced the splenocyte response to HA, with a similar pattern to that in WT mice – proliferation and production of IFNγ a Th1-type cytokine, were upregulated but IL-5, a Th2-type cytokine, was not impacted (). Together, the results indicate that TLR2 and TLR4 play a very minimal role in the adjuvant effect of CCS/C liposomes.
Figure 2. TLR2 and TLR4 do not contribute to antiviral immunity induced by F-HA and CCS/C-HA
The CCS/C adjuvant overcomes the requirement for MyD88 which is required for the immune response to F-HA
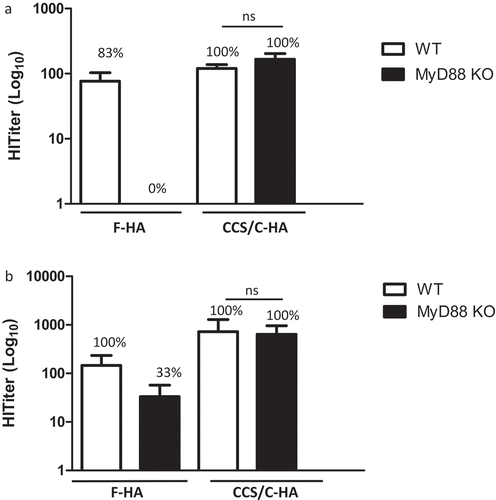
Mice genetically deficient in MyD88 lack most TLR signaling,Citation29 but also lack responsiveness to major pro-inflammatory cytokines that contribute to the maturation and phenotype of T cells such as IL-1 and IL-18. To explore the role of these pathways in the antibody response to HA immunization and to test the augmenting role of the liposomal adjuvant in the response to HA antigen, we immunized WT vs. Myd88−/- mice. Mice were immunized once (D 0, Group A) or twice (D 0 and 28, Group B). Blood samples were collected on d 30 in Group A, and on d 26 and 36 in Group B. Samples were tested for HI antibody titers, and after sacrificing the animals on d 30 (Group A) and 36 (Group B), the spleens were harvested and cells were tested for cellular responses, including cytokine release and proliferative responses.
F-HA immunization was unable to induce HI antibodies in MyD88-deficient mice following a single immunization (n = 6) (). Strikingly, the CCS/C-HA vaccination overcame the dependency of F-HA immunization on MyD88-signaling. In MyD88-deficient mice, one vaccination with CCS/C-HA led to 100% seroconversion with HI titers equivalent to the titers in WT mice vaccinated twice with the F-HA vaccine (). Boost-vaccination of Myd88−/- mice with CCS/C-HA led to high titer HI antibodies in all mice tested. In WT mice, the CCS/C-HA vaccine outperformed F-HA immunization, consistent with our previous results ( andCitation8). We next tested the antigen-specific proliferative response of splenic T cells from WT and Myd88−/- mice immunized with F-HA vs. CCS/C-HA (). We observed variability in the magnitude of the response of WT mice between vaccination experiments (e.g. IFNγ level between ), and the CCS/C-HA failed to improve WT splenocyte IFNγ production in group A (). As expected, F-HA failed to elicit proliferative responses and cytokine production by anti-HA T cells in the absence of MyD88. In stark contrast, splenic T cells from Myd88−/- mice immunized with CCS/C-HA proliferated and secreted IL-2, indicating activation in response to HA. Interestingly, IFNγ production increased in the Myd88−/- cultures, but these cells also showed enhanced production of IL-5, a Th2-type cytokine.
Figure 3. Adjuvant effect of CCS/C-HA does not require MyD88
Discussion
Liposomes remain at the forefront of drug and vaccine design owing to their well-documented abilities to act as delivery vehicles. Whereas most research has focused on the ability of liposomes to deliver genes, drugs, and vaccine antigens, more recently liposomes have been shown to function as vaccine adjuvants by enhancing the immunogenicity of peptide and protein antigens. We previously demonstrated that the CCS/C liposomal adjuvant/carrier vaccine exhibits several advantages for influenza immunization. In contrast to immunization with non-adjuvanted HA antigen, a single i.m. immunization with the CCS/C-HA vaccine elicits potent and long-term serum HI titers, and characteristics of both Th1- and Th2-T cell helper responses in mice, rats, and ferrets.Citation8,Citation10,Citation11
In North America, annual influenza vaccination is recommended for all individuals >6 months old,Citation30 although the vast majority of influenza-related hospitalizations and deaths occur in individuals >65 y old.Citation31,Citation32 Vaccination of the elderly with higher doses of HA per strain improved the prevention of influenza,Citation33 however, retrospective cohort studies that examined the effectiveness of high-dose vs. standard vaccination in the > 65-y-old population differed in their findings.Citation34,Citation35 Alternatively, vaccine adjuvants can improve immunogenicity in high-risk groups,Citation36 and the FDA recently approved an influenza vaccine containing the adjuvant MF59 for seasonal influenza vaccination in individuals >65 y old.Citation37 Adjuvants may be particularly important for pandemic vaccines in order to shorten the response time and broaden cross-reactivity.Citation38 MF59 is an oil in water emulsion of squalene oil that increases immune responses when mixed with the trivalent vaccine. However, the MF59-adjuvanted vaccine is not recommended over other available vaccines due to the lack of evidence to support its efficacy compared to standard vaccination.Citation30
To improve the success of vaccination in the elderly, the effects of age on innate and adaptive immunity must be addressed. Importantly, most adjuvants boost immunity by recruiting innate immune cells and enhancing innate signaling pathways leading to more potent antigen presentation.Citation12 Some adjuvants are naturalCitation39 or syntheticCitation40 Toll-like receptor (TLR) ligands that target specific TLR pathways, such as the LPS derivative Monophosphoryl lipid A.Citation41 Others, such as the approved MF59, also depend on the innate immune TLR/IL-1 R signaling protein MyD88 for their adjuvant effect.Citation42,Citation43 However, a quandary arises since much evidence demonstrates impaired innate immunity in both aged mice, and elderly individuals.Citation13,Citation14,Citation44,Citation45 Aging reduces the number of antigen-presenting cells and their endogenous expression of MHC molecules, co-stimulatory molecules, and TLRs.Citation46,Citation47 Activation of innate immune cells from the elderly results in significantly lower cytokine and chemokine expression compared to the response of cells from younger individuals.Citation47 This “immunosenescence” of the innate immune system presents a barrier to the efficacy of vaccines and vaccine adjuvants that rely on canonical innate signaling pathways to enhance adaptive immunity.
We therefore examined the immunogenicity of the CCS/C-HA vaccine compared to the standard vaccine in the setting of MHC differences, and deficiencies in innate immunity. Whereas CCS/C-HA improved immune responses across strains, the most significant finding was that the CCS/C adjuvant induces immunity (HI titers) independently of MyD88. In contrast, the standard trivalent vaccine was highly dependent on MyD88, and even two immunizations of Myd88−/- mice were unable to induce significant seroconversion and HI titers. MyD88 is an adaptor protein that assembles a signaling complex at the intracellular domains of both TLRs and receptors of the IL-1 superfamily,Citation17 suggesting that immunization with the trivalent vaccine requires activation of one or both, of these systems. The fact that immunogenicity induced by the trivalent vaccine was not significantly impaired in TLR2 or TLR4-deficient mice compared to WT mice does not rule out the involvement of other TLRs, especially TLR7 that recognizes single-stranded viral RNA and signals through MyD88.Citation40,Citation48 The cytokine profile of splenic T cells from MyD88-deficient mice immunized with CCS/C-HA secreted a mixed pattern of Th1 and Th2 cytokines. Using pooled sera, we found that the CCS/C-HA vaccine-induced primarily an IgG1 response in MyD88-deficient mice (data not shown), suggestive of Th2-type immunity. Others have shown a shift to Th2-immunity following immunization or infection of MyD88-deficient mice,Citation49,Citation50 and we demonstrated that pharmacologic inhibition of MyD88 induces a shift to Th2-type immunity.Citation51 Of note, Alum, an adjuvant approved for human use, also induces Th2-immunity.Citation52 Since the NLRP3 inflammasome was shown to mediate the adjuvant effect of alum,Citation53 future studies should determine the role of NLRP3 and other inflammasomes in the mechanism of action of the CCS/C adjuvant.
There are several limitations to the current study, including, but not limited to, small sample sizes and lack of repeat comparisons for some of the experiments, and the use of surrogate measures of vaccine efficacy (HI titer). We also used the most immunogenic strain to determine the HI titer of the immunized mice; however, to further the development of this adjuvant for human use, the HI titer against all strains, especially the least immunogenic, should be determined.
Our previous findings established that the CCS/C-HA vaccine is effective in young and aged mice, enables administration of fewer vaccine doses, and is protective in ferrets.Citation8,Citation10,Citation11 The current study adds to the benefit profile of the CCS/C-HA influenza vaccine by demonstrating its ability to induce immunity in the setting of impaired innate immune signaling. Future studies should focus on the protective efficacy of the CCS/C-HA vaccine in animal models of influenza infection, in particular in the setting of impaired immunity.
Abbreviations
| APC | = | Antigen Presenting Cells |
| C | = | Cholesterol |
| CCS | = | Ceramide Carbamoyl Spermine |
| F-HA | = | free HA (non-liposomal associated) |
| HA | = | Hemagglutinin |
| HI | = | hemagglutination inhibition |
| i.m. | = | intramuscular |
| INFγ | = | interferon γ |
| IL-2/5 | = | interleukin 2/5 |
| SPF | = | specific pathogen free |
| KO | = | knockout |
| MyD88 | = | myeloid differentiation factor 88 |
| TLR | = | Toll Like Receptor |
| TNFα | = | tumor necrosis factor α. |
Disclosure of potential conflicts of interest
All authors declare that they have no competing interests to disclose.
Acknowledgments
This work was supported by the Israel Science Foundation (grants 1396/12 and 1391/17), and by the Barenholz Research Fund (the Barenholz Research Fund was established by Professor Barenholz from royalties obtained from various Barenholz licensed patents, and these funds are dedicated to support research in the Barenholz Lab). OE was supported by a Hebrew University scholarship for outstanding students.
References
- Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine. 2008;26(Suppl 4):D5–9. doi:https://doi.org/10.1016/j.vaccine.2008.05.076.
- Belshe RB RB, Mendelman PM PM, Treanor J, King J, Gruber WC WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338(20):1405–12. doi:https://doi.org/10.1056/NEJM199805143382002.
- Boyce TG, Poland GA. Promises and challenges of live-attenuated intranasal influenza vaccines across the age spectrum: a review. Biomed Pharmacother. 2000;54(4):210–18. doi:https://doi.org/10.1016/S0753-3322(00)89027-7.
- De Villiers PJ, Steele AD, Hiemstra LA, Rappaport R, Dunning AJ, Gruber WC, Forrest BD. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine. 2009;28(1):228–34. doi:https://doi.org/10.1016/j.vaccine.2009.09.092.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–81. doi:https://doi.org/10.1056/NEJMoa070844.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Jan;12(1):36–44. doi:https://doi.org/10.1016/S1473-3099(11)70295-X.
- Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respi Viruses. 2011;5(2):67–75. doi:https://doi.org/10.1111/j.1750-2659.2010.00183.x.
- Even-or O, Joseph A, Itskovitz-Cooper N, Samira S, Rochlin E, Eliyahu H, Goldwaser I, Balasingam S, Mann AJ, Lambkin-Williams R. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine. 2011;29(13):2474–86. doi:https://doi.org/10.1016/j.vaccine.2011.01.009.
- Even-or O, Samira S, Ellis R, Kedar E, Barenholz Y. Adjuvanted influenza vaccines. Expert Rev Vaccines. 2013;12(9):1095–108. doi:https://doi.org/10.1586/14760584.2013.825445.
- Even-or O, Samira S, Rochlin E, Balasingam S, Mann AJ, Lambkin-Williams R, Spira J, Goldwaser I, Ellis R, Barenholz Y, et al. Immunogenicity, protective efficacy and mechanism of novel CCS adjuvanted influenza vaccine. Vaccine. 2010;28(39):6527–41. doi:https://doi.org/10.1016/j.vaccine.2010.04.011.
- Joseph A, Itskovitz-Cooper N, Samira S, Flasterstein O, Eliyahu H, Simberg D, Goldwaser I, Barenholz Y, Kedar E. A new intranasal influenza vaccine based on a novel polycationic lipid–ceramide lipid—ceramide carbamoyl-spermine (CCS)I. Immunogenicity and efficacy studies in mice. Vaccine. 2006;24(18):3990–4006. doi:https://doi.org/10.1016/j.vaccine.2005.12.017.
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi:https://doi.org/10.1016/j.immuni.2010.10.002.
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–701. doi:https://doi.org/10.4049/jimmunol.169.9.4697.
- Metcalf TU, Cubas RA, Ghneim K, Cartwright MJ, Grevenynghe JV, Richner JM, Olagnier DP, Wilkinson PA, Cameron MJ, Park BS. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 2015;14(3):421–32. doi:https://doi.org/10.1111/acel.12320.
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24(1):353–89. doi:https://doi.org/10.1146/annurev.immunol.24.021605.090552.
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–51. doi:https://doi.org/10.1016/S1074-7613(00)80119-3.
- Cohen P. The TLR and IL-1 signalling network at a glance. J Cell Sci. 2014;127(11):2383–90. doi:https://doi.org/10.1242/jcs.149831.
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005 2004;17(1):1–14. doi:https://doi.org/10.1093/intimm/dxh186.
- Ben-Yehuda A, Joseph A, Zeira E, Even-Chen S, Louria-Hayon I, Babai I, Zakay-Rones Z, Greenbaum E, Barenholz Y, Kedar E. Immunogenicity and safety of a novel liposomal influenza subunit vaccine (INFLUSOME-VAC) in young adults. J Med Virol. 2003;69(4):560–67. doi:https://doi.org/10.1002/jmv.10345.
- Babai I, Barenholz Y, Zakay-Rones Z, Greenbaum E, Samira S, Hayon I, Rochman M, Kedar E. A novel liposomal influenza vaccine (INFLUSOME-VAC) containing hemagglutinin-neuraminidase hemagglutinin–neuraminidase and IL-2 or GM-CSF induces protective anti-neuraminidase antibodies cross-reacting with a wide spectrum of influenza A viral strains. Vaccine. 2001;20(3–4):505–15. doi:https://doi.org/10.1016/S0264-410X(01)00326-7.
- de Bruyn G. Cofactors that may influence vaccine responses. Curr Opin HIV AIDS. 2010;5(5):404–08. doi:https://doi.org/10.1097/COH.0b013e32833d1fca.
- Haralambieva IH, Kennedy RB, Ovsyannikova IG, Whitaker JA, Poland GA. Variability in humoral immunity to measles vaccine: new developments. Trends Mol Med. 2015;21(12):789–801. doi:https://doi.org/10.1016/j.molmed.2015.10.005.
- Kraal G, Weissman IL, Butcher EC. Genetic control of T-cell subset representation in inbred mice. Immunogenetics. 1983;18(6):585–92. doi:https://doi.org/10.1007/BF00345966.
- Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1(12):1279–83. doi:https://doi.org/10.1038/nm1295-1279.
- Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro.. J Exp Med. 1995;181(2):713–21. doi:https://doi.org/10.1084/jem.181.2.713.
- Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic ML, Pejnovic N. Differential Immunometabolic phenotype in Th1 and Th2 dominant mouse strains in response to high-fat feeding. PLoS One. 2015;10(7):e0134089. doi:https://doi.org/10.1371/journal.pone.0134089.
- Daugelat S, Ladel CH, Kaufmann SH. Influence of mouse strain and vaccine viability on T-cell responses induced by mycobacterium bovis bacillus calmette-guerincalmette-guérin. Infect Immun. 1995;63(5):2033–40. doi:https://doi.org/10.1128/IAI.63.5.2033-2040.1995.
- Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-antigen ligand–antigen conjugate vaccines. Ther Deliv. 2012;3(6):749–60. doi:https://doi.org/10.4155/tde.12.52.
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–22. doi:https://doi.org/10.1016/S1074-7613(00)80086-2.
- Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015–16 influenza season. 2015.
- Ridenhour BJ, Campitelli MA, Kwong JC, Rosella LC, Armstrong BG, Mangtani P, Calzavara AJ, Shay DK. Effectiveness of inactivated influenza vaccines in preventing influenza-associated deaths and hospitalizations among Ontario residents aged >/= ≥65 years: estimates with generalized linear models accounting for healthy vaccinee effects. PLoS One. 2013;8(10):e76318. doi:https://doi.org/10.1371/journal.pone.0076318.
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;2:CD004876. doi:https://doi.org/10.1002/14651858.CD004876.
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–45. doi:https://doi.org/10.1056/NEJMoa1315727.
- Richardson DM, Medvedeva EL, Roberts CB, Linkin DR. Centers for disease C, prevention epicenter P. comparative effectiveness of high-dose versus standard-dose influenza vaccination in community-dwelling veterans. Clin Infect Dis. 2015;61(2):171–76. doi:https://doi.org/10.1093/cid/civ261.
- Izurieta HS, Thadani N, Shay DK, Lu Y, Maurer A, Foppa IM, Franks R, Pratt D, Forshee RA, MaCurdy T, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15(3):293–300. doi:https://doi.org/10.1016/S1473-3099(14)71087-4.
- Whitaker JA, von Itzstein MS, Poland GA. Strategies to maximize influenza vaccine impact in older adults. Vaccine. 2018;36(40):5940–48. doi:https://doi.org/10.1016/j.vaccine.2018.08.040.
- Food and Drug Administration. FDA approves first seasonal influenza vaccine containing an adjuvant. Hum Vaccin Immunother. 2018; 14(2):386–95. Published online 2018 Jan 3. doi:https://doi.org/10.1080/21645515.2017.1373227.
- Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Hum Vaccin Immunother. 2018;14(3):550–64. doi:https://doi.org/10.1080/21645515.2017.1415684.
- Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29(17):3341–55. doi:https://doi.org/10.1016/j.vaccine.2010.08.002.
- Goff PH, Hayashi T, He W, Yao S, Cottam HB, Tan GS, Crain B, Krammer F, Messer K, Pu M, et al. Synthetic toll-like receptor 4 (TLR4) and TLR7 ligands work additively via MyD88 to induce protective antiviral immunity in mice. J Virol. 2017;91(19):e01050–17.
- Quintilio W, de Freitas FA, Rodriguez D, Kubrusly FS, Yourtov D, Miyaki C, de Cerqueira Leite LC, Raw I. Vitamins as influenza vaccine adjuvant components. Arch Virol. 2016;161(10):2787–95. doi:https://doi.org/10.1007/s00705-016-2994-5.
- Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D’Oro U, Giuliani MM. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011;108(27):11169–74. doi:https://doi.org/10.1073/pnas.1107941108.
- O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 - – an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–48. doi:https://doi.org/10.1016/j.vaccine.2011.09.061.
- van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frolich M, Westendorp RGJ. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39(9):1407–14. doi:https://doi.org/10.1016/j.exger.2004.06.009.
- Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10(1):112. doi:https://doi.org/10.1186/1465-9921-10-112.
- Parodi V, de Florentiis D, Martini M, Ansaldi F. Inactivated influenza vaccines: recent progress and implications for the elderly. Drugs Aging. 2011;28(2):93–106. doi:https://doi.org/10.2165/11586770-000000000-00000.
- Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4(1):9. doi:https://doi.org/10.1186/1742-4933-4-9.
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–28. doi:https://doi.org/10.1038/nri3665.
- Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh I-T, Zhong G. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following chlamydia muridarum infection. J Immunol. 2010;184(5):2602–10. doi:https://doi.org/10.4049/jimmunol.0901593.
- Cohen SJ, Cohen IR, Nussbaum G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88(-/-) Mice. J Immunol. 2010;184(1):212–21. doi:https://doi.org/10.4049/jimmunol.0900296.
- Dishon S, Cohen SJ, Cohen IR, Nussbaum G. Inhibition of myeloid differentiation factor 88 reduces human and mouse T-Cell Interleukin-17 and IFNγ production and ameliorates experimental autoimmune encephalomyelitis induced in mice. Front Immunol. 2017;8:615. doi:https://doi.org/10.3389/fimmu.2017.00615.
- He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11(2):477–88. doi:https://doi.org/10.1080/21645515.2014.1004026.
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi:https://doi.org/10.4049/jimmunol.181.1.17.
