ABSTRACT
Little is known regarding Chinese mothers’ intention to vaccinate their daughters against human papillomavirus (HPV) since the HPV vaccine was approved for use in China in 2016. The aim was to explore maternal HPV vaccination acceptance, preference for 2-, 4- or 9-valent HPV vaccine and acceptance of domestically manufactured HPV vaccines. Study participants were mothers of primary school children in Southeastern region of Fujian. An online cross-sectional survey was undertaken between June and August 2019. Among the total of 3,586 completed responses (response rate 28.5%), the intention to vaccinate daughter against HPV was high (83.3%). Higher maternal education and perceived benefit and barriers were associated with greater intention to vaccinate. Among mothers who did not intend to vaccinate their daughters, the three most common reasons were daughter being too young to receive HPV vaccination (40.6%), fear of side effects (31.9%) and vaccine price is too high (16.0%). The largest proportion (41.4%) preferred their daughter to be vaccinated with the 9-valent HPV vaccine (9vHPV). Greater preference for 9vHPVwas strongly associated with higher maternal education level and annual household income. The majority of mothers expressed a preference for imported HPV vaccine (56.3%). Our result indicates that lower intentions to vaccinate daughters against HPV among less educated and lower-income mothers may lead to significant social inequalities in HPV vaccine uptake in the country.
Introduction
Cervical cancer is a major cause of death in women, with an estimated 310,000 annual deaths globally caused by the disease, making it the fourth most common cancer killer of women.Citation1 Being the largest developing country, more cases of cervical cancer are diagnosed in China per year than in any other country, accounting for around 20% of all estimated cervical cancers diagnosed worldwide in 2018.Citation2,Citation3 The development of the prophylactic vaccines against the most important oncogenic HPV types is a major step toward reducing cervical cancer rates and the ultimate elimination of cervical cancer. Most national HPV vaccination programmes target adolescent and pre-adolescent girls before the initiation of sexual activity, though some countries have implemented gender-neutral programmes. Despite the efficacy and availability of prophylactic HPV vaccines, their uptake has been limited due to a number of barriers faced by many countries worldwide, namely: high cost; difficulty in effectively reaching HPV vaccine target populations; cultural issues related to the fact that HPV infection is a sexually transmitted infection; limited awareness of cervical cancer and its relationship to HPV infection; and concerns about HPV vaccination with respect to safety.Citation4 To overcome some of these obstacles, many countries in the world have implemented free HPV vaccination programmes to improve vaccine coverage.Citation4
A decade after the United States Food and Drug Administration licensure in 2006, the first HPV vaccine was approved in China in 2016. GlaxoSmithKline’s bivalent vaccine (2vHPV), Cervarix®, and Merck’s quadrivalent HPV vaccine (4vHPV), Gardasil®, were approved for use in women in China by the China Food and Drug Administration in July 2016 and April 2017, respectively. A 9-valent HPV vaccine (9vHPV), Gardasil®9, was recently licensed in China in May 2018. To date, the 4vHPV (Chinese Yuan [CNY] 798/shot; 1 USD = 7 CNY) cost is slightly higher than that of the 2vHPV (CNY580/shot). 9vHPV, however, is nearly double the price of the 4vHPV (CNY1298/shot). Currently, there is no national HPV vaccination programme in China that provides free or discounted HPV vaccines.
Similarly to many countries worldwide, China has faced many obstacles in the introduction of HPV vaccination, such as the high cost of vaccines, shortage of HPV vaccine, the requirement of cold-chain delivery, and vaccination attitudes and concerns.Citation5,Citation6 Parental acceptance of HPV vaccination for daughters is known as one of the most important challenges to HPV vaccine uptake. Among the main factors that influence parental HPV vaccination acceptance are confidence in the safety and effectiveness of vaccines in general, recommendation of HPV vaccination by a physician, low education level and awareness of HPV infection and the related diseases.Citation7 Also, because HPV is a sexually transmitted infection, some parents have expressed unfounded concerns that HPV vaccination may promote high-risk sexual behavior.Citation8 In emerging economies in Asia (e.g. Malaysia, Thailand) as well as high-income economic regions (e.g. Hong Kong, Taiwan), cervical screening and HPV vaccination are still hampered by embarrassment surrounding sexually transmitted infections (STIs) and gynecological examination.Citation9–13
To the best of our knowledge, no study on Chinese mothers’ HPV vaccination acceptance was carried before the vaccine became available in ChinaCitation14 No study has assessed parental HPV vaccination acceptance in China since the HPV vaccine became available on the Chinese market. The only published study on HPV vaccination acceptance after the introduction of the HPV vaccine in China was conducted on college students and young adult women.Citation15Evaluation of the level of HPV acceptance of parents can help to provide insight into improving vaccination rates. Thus, it is critical to address this research gap as parental consent and support are essential to increase HPV vaccine uptake in adolescent girls. Thus, the main aim of the present study was to determine maternal intention to vaccinate daughters against HPV. The influence of knowledge and health beliefs in HPV vaccination intention has been well established in many studies.Citation16 Therefore, the second objective of this study is to investigate the association between HPV vaccination intention and both the awareness health beliefs (based on the Health Belief Model [HBM]) associated with HPV vaccination. To date, three globally licensed HPV vaccines are offered in China, as such, the preference for 2-, 4- or 9-valent HPV vaccine was also queried in this study. Understanding preferences is particularly important for designing more effective vaccine-promotion programs.
Most recently, the bivalent Innovax’s HPV vaccine has just been approved by the China National Medical Products Administration (NMPA) in December 31, 2019.Citation17–20 Study of the immunogenicity, safety, and efficacy of the vaccine had been conducted, and the vaccine was well-tolerated and highly efficacious against HPV-16/18–associated high-grade genital lesions and persistent infection in women.Citation19 It is timely that the present study also investigated participants’willingness to accept domestically manufactured HPV vaccines. The country’s locally made HPV vaccines may be less expensive and also help to counteract vaccine shortages in China.
Materials and methods
Study participants and survey design
Study participants were mothers of primary school children in the Yongding district of Longyan City, Southestern of Fujian. A total of 78 primary schools agreed to assist in the data collection. A total of 27,756 students are enrolled in these schools, of which 12,586 were females. Principals of schools were briefed about the study and school administrators were requested to assist in sending out an online survey link to all the mothers of female students in the school record. In an attempt to reach a more comprehensive recipient coverage, the link to the survey was also sent to parents’ social media groups or chats.
Instruments
The first section of the questionnaire assessed participants’ knowledge using a series of questions regarding HPV infection, the relationship of HPV infection with the development of cervical cancer and genital warts, and HPV vaccination (23-item scale). To date, there is no established measurement for assessing HPV-related knowledge. The questionnaire used in this study was adapted from our previous study.Citation15 The response options were ‘true’, ‘false’ or ‘don’t know’. A correct response was given a score of 1 and an incorrect or ‘don’t know’ response was scored 0. The total possible knowledge score ranged from 0 to 23, with higher scores representing higher levels of knowledge.
The second section assessed health beliefs using HBM-derived items to measure the participants’ beliefs about HPV vaccination.Citation21,Citation22 The questions probed perceived susceptibility to HPV (three items), perceived severity of HPV infection (three items), perceived benefits of HPV vaccine (three items), perceived barriers to getting a vaccination against HPV (four items), and cue to action (1 item). Perceived susceptibility queried participants about their risk in contracting HPV, genital and cervical/vulva cancer in their lifetime. Perceived severity assessed participants’ perception of the harm of HPV infection, genital warts, and cervical/vulva cancer. Questions evaluating perceived benefits queried participants their views about the benefit of the HPV vaccines in preventing their daughters against HPV infection, genital warts and cervical/vulva cancer. Perceived cues to action questioned participants about their exposure to HPV vaccination promotion on the mass media. Response options were ‘agree’ and ‘disagree’.
A five-point scale was also used for questions about intention to vaccinate daughter against HPV, namely ‘yes, definitely accept’, ‘yes, the chances are quite high’, ‘no, only a moderate chance’, ‘no, the chances are quite low’, and ‘no, definitely not accept’. Mothers who responded ‘no, only a moderate chance’, and ‘no, definitely not accept’ were asked to provide a reason for not accepting the HPV vaccination. Participants who indicated acceptance of HPV vaccine were subsequently asked for their preferences for the three different HPV vaccines, 2-, 4- and 9-valent. A note indicating the prices of the vaccine, and that HPV vaccines have been recommended as a three-dose series, given over six months, was also enclosed. The last question queried the participants regarding the acceptance of domestically manufactured HPV vaccine, if available on the market.
The last section of the questionnaire assessed participants’ demographic characteristics such as age, birthplace, current residing location, marital status, highest education level, occupational types, and annual household income. Participants were also asked if they ever knew anyone has had cervical cancer. Participants’ daughter information such as age, history of chronic diseases, and experience of serious side effects in any previous vaccinations were also obtained.
Statistical analyses
The reliability of knowledge items was evaluated by assessing the internal consistency of the items representing the knowledge score. Multivariable logistic regression analyses were performed to investigate factors influencing 1) level of HPV-related knowledge, 2) intention to vaccinate daughter against HPV, and 3) acceptance of locally manufactured HPV vaccine. All variables found to have a statistically significant association (two-tailed, p-value < 0.05) with intention to take HPV vaccination and vaccine acceptability in the univariate analyses were entered into multivariable logistic regression analyses via forced-entry methodCitation23was used. Odds ratios (OR), 95% confidence intervals (95% CI) and p-values were calculated for each independent variable. The model fit was assessed using the Hosmer−Lemeshow goodness of fit test. A significant test indicates that the model is not a good fit.Citation24
Multinomial logistic regression was used to determine factors influencing the preferences for 2-,4- or 9-valent HPV vaccine. The 2-valent HPV vaccine served as the reference group. A P-value of <0.05 was selected for determining which variables were selected for the multinomial logistic regression and to determine the level of significance of variables.
Ethical considerations
This study was approved by the Medical Ethics Committee at the Fujian Medical University, Fuzhou, China. Respondents were informed that their participation was voluntary, and consent was implied on the completion of the questionnaire. They were also informed that all responses were collected and analyzed without identifiers.
Results
Study participants
Between June and August 2019, a total of 3,586 completed responses were received. In the assumption that the survey link reached all the 12,586 female students’ mothers, the response rate is 28.5%. A summary of the characteristics of the respondents is provided in the first column of . A large proportion of the respondents were age 36 − 40 years (36.0%). The majority (55.1%) reported an annual household income of below CNY50,000. Only 9.1% reported that their daughter had ever experienced serious side effects from previous vaccinations.
Table 1. Factors associated with intention to vaccinate daughters with HPV vaccine (N = 3586)
HPV-related knowledge
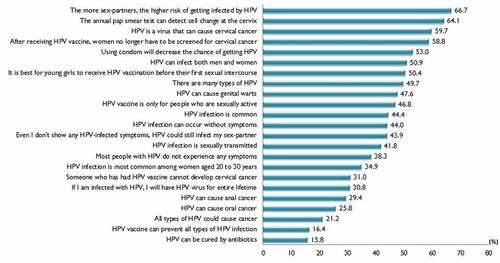
shows the proportion of correct responses to all the 23 knowledge items. The 23 knowledge items in the study sample had a reliability (Kuder−Richardson 20) of 0.954. Among the items with a low proportion of correct responses were ‘HPV can be cured by antibiotics’, ‘HPV vaccine can prevent all types of HPV infection’ and ‘All types of HPV could cause cancer’. There was a high proportion of correct responses to the statement ‘The more sex partners, the higher the risk of getting infected by HPV’, whereby two-thirds correctly responded to the statement. The mean total knowledge score of the overall respondents was 9.7 (SD ± 6.5). The median knowledge score was 10, and the knowledge scores of the study participants ranged from 0 to 23. Knowledge scores were categorized high or low based on the median split; as such, a total of 1753 (48.9%) were categorized as having a high score (11 to 23) and 1833 (51.1%) had a low score (0 to 10). The multivariable logistic analysis revealed educational level, occupational types and annual household income as significant predictors of having a high knowledge score. In the multivariate regression model, mothers whose highest education was secondary school (OR 1.86, 95%CI 1.36 − 2.55), high school/technical school (OR 2.63, 95%CI 1.88 − 3.67), and university level (OR 3.01, 95%CI 2.01 − 4.50), had a greater likelihood of having a high knowledge score than mothers whose highest educational level was primary school. Mothers who were professional and managerial occupational types (OR 1.52, 95%CI 1.08 − 2.13) had a higher likelihood of having a higher knowledge score than mothers who were housewives. An annual household income of CNY50,000 − 120,000 (OR 1.24, 95%CI 1.06 − 1.44), and above CNY120,0000 (OR 1.43, 95%CI 1.11 − 1.85) also had a higher likelihood of having a high knowledge score, than those with an annual income below CNY50,000.
Health beliefs regarding HPV and HPV vaccination
The second column of shows the proportion of agreement with HBM-derived items. A high proportion of mothers believed that women, in general, have a high risk of getting HPV infection (78.4%) and cervical cancer (75.9%). The vast majority perceived HPV infection, genital warts, and cervical cancer to be severely harmful (91.4%, 90.9%, and 94.0%, respectively). Most mothers also perceived HPV vaccination as beneficial, viewing it as effective in preventing HPV infection, genital warts, and cancers (84.6%, 77.5%, and 82.4%, respectively). On the whole, the majority of mothers perceived few barriers to HPV vaccination. Of all the perceived barrier items, the highest proportion was reported on the perception that it was not worth spending money to have daughter vaccinated against HPV (29.5%) followed by the perception that HPV vaccination leads to increased juvenile sexual acts (25.2%). For the cues-to-action item, only 29.8% reported having been often exposed to HPV vaccination promotion for adolescents on mass media.
HPV vaccination intention
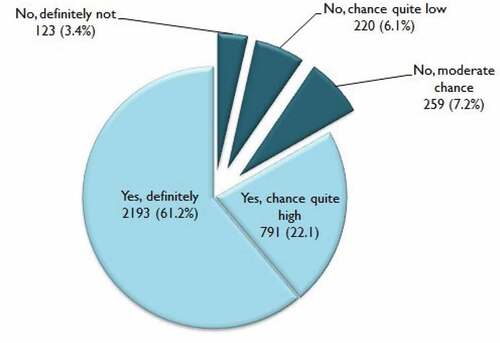
In total, 83.3% (n = 2,984) reported ‘Yes, definitely/chances are quite large’ and 16.7% (n = 602) reported ‘No, moderate chance/chances are quite low/definitely not’ (). As shown in , variables that were significantly associated with intention to vaccinate daughters against HPV in the univariate analyses were mother’s age, educational level, annual household income, HPV-related knowledge, perceived susceptibility, perceived severity, perceived benefit of vaccination, perceived barriers to vaccination (not having time), and cues-to-action (mass media exposure). Results from the multivariable logistic regression model showed that mothers’ educational level and perceived benefits were the most influential predictors of intention to vaccinate daughters against HPV (). Mothers with university education level had over twofold higher odds of intention to vaccinate their daughters (OR = 2.15, 95% CI: 1.34–3.46). The odds of intention to vaccinate among mothers who perceive the benefit of HPV vaccines as being effective in preventing HPV infection were also twofold higher (OR = 2.02, 95% CI: 1.50–2.98). Another significant factor associated with intention to vaccinate daughters against HPV was having no barriers in taking time off for daughter vaccination (OR = 1.37, 95% CI: 1.01 − 1.72).
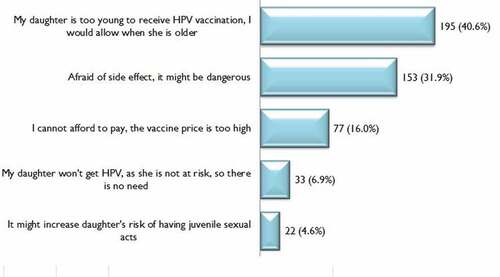
Many reasons were provided for not accepting HPV vaccination. shows that among mothers who reported they were not willing to vaccinate their daughters, the three most common reasons, in descending order, were the daughter being too young to receive HPV vaccination (40.6%), fear of side effects (31.9%) and vaccine price being too high (16.0%).
Preferences for 2-,4- or 9-valent HPV vaccine
Among mothers who reported having intention to vaccinate daughters against HPV, the majority of mothers preferred to have their daughters vaccinated with 9vHPV (n = 1258, 41.4%) followed by 2vHPV (n = 1025, 33.7%). shows the result of the multinomial logistic regression for preferences for 2-,4- or 9-valent HPV vaccine (9vHPV vs 2vHPV and 4vHPV vs 2vHPV). Preference for 9vHPV over 2vHPV was associated with higher education levels and higher annual household income. Mothers who reported knowing someone who has cervical cancer also had a higher preference for 9vHPV over 2vHPV. Mothers who perceived the harm of cervical or vulvar cancers as severe, that their daughter would not have severe side effects after receiving HPV vaccination, and that receiving the HPV vaccination would not increase the daughter’s risk of juvenile sexual acts expressed a higher preference for 9vHPV over 2vHPV. Mothers of higher annual household income who perceived that harm from cervical or vulvar cancers is severe, and who had no barriers in taking time off for the daughter’s HPV vaccination expressed a higher preference for 4vHPV over 2vHPV.
Table 2. Multinomial logistic regression model for factors associated with preference for 2-,4- or 9-valent HPV vaccine (N = 3041)
Acceptance of domestically manufactured HPV vaccine
The majority of mothers expressed a preference for imported HPV vaccine (n = 2019, 56.3%) (). Only 21.7% (n = 777) preferred a domestically manufactured HPV vaccine and 22% (n = 790) expressed no preference. Multivariable logistic regression analysis showed that mothers aged 23–35 years were more likely (OR = 1.32, 95% CI:1.09–1.61) than those over 40 years to have a preference for imported HPV vaccine rather than having preference domestically manufactured vaccine or not having a preference. Mothers whose birthplace was in an urban area were also more likely to prefer imported HPV vaccine than those whose birthplace was in a rural area (OR = 1.26, 95% CI:1.04 − 1.52). Mothers with an annual household income above CNY120,000 (OR = 1.57, 95% CI:1.22 − 2.01) and CNY50,000- CNY120,000 (OR = 1.19, 95% CI:1.03 − 1.38) were more likely to prefer imported HPV vaccines than those with an annual household income below CNY50,000. Other factors, such as age, daughter’s side effect experience in previous vaccination, and having a daughter with chronic illness did not influence the preference of imported vaccines.
Table 3. Factors associated with preference of HPV vaccine (N = 3586)
Discussion
The mean total knowledge score at the midpoint of the scale implies that mothers in this study have moderate HPV-related knowledge. It is important to note that imperative knowledge, such as adolescent girls should receive HPV vaccination before their first sexual intercourse, that after receiving HPV vaccination women should continue to be screened for cervical cancer, that HPV infection can occur without symptoms and awareness of HPV-associated diseases were barely known by half of the mothers in this study. The broad spectrum of knowledge deficits found in this study provides important insights for future targeted awareness-raising education messages for mothers in China. Continuous public education campaigns are needed to improve knowledge of HPV, HPV-associated diseases and HPV vaccination among mothers in China.
Maternal acceptance of HPV vaccination was extremely high in the present study, with approximately eight out of 10 mothers expressing intent to accept HPV vaccination for their daughters. On a promising note, mothers in this study expressed higher acceptance compared to only 36% acceptance in a previous study conducted before the HPV vaccine was available on the market.Citation14 The finding of a significantly higher acceptance of HPV vaccination among mothers with higher educational qualifications in the multivariable analysis is a likely indication that the more educated mothers are generally more exposed to information on HPV infection and vaccination against it from various sources, just as previously reported in other studies.Citation25,Citation26 Given that the intention to accept HPV vaccination is generally higher among high-income and more educated populations, it may be important to target socio-economically disadvantaged groups with interventions to achieve higher HPV vaccination.
Although knowledge is not significantly associated with intention to vaccinate daughters against HPV in the multivariable model, mothers with higher educational qualifications were shown to have a significantly higher level of HPV-related knowledge. Therefore, an action to improve HPV knowledge among mothers might increase HPV vaccine coverage of adolescents in China. The HBM parameters, perceived benefits (potential advantages) and perceived barriers, also significantly influenced the intention to vaccinate. This suggests that intentions to vaccinate their daughters were higher among participants who thought that the HPV vaccination was effective. Therefore, beliefs in the benefits of HPV vaccination for cervical cancer prevention seem to be a key factor influencing mothers’ intentions to vaccinate their daughters, which is likewise found in many studies.Citation27,Citation28 As such, educational strategies and marketing programmes that emphasize recognizing and responding to potential benefits of HPV vaccination should be developed to shape a more positive perception about HPV vaccination among mothers in China.
On the other hand, the perceived barrier regarding taking time off for a daughter’s vaccination was negatively related to vaccination intention. Difficulty in taking time off for appointments is a crucial factor affecting immunization coverage and it is important to address this issue, particularly for HPV vaccination.Citation29 The 3-dose HPV vaccine series poses an additional challenge for mothers having to take time off work to complete the series. In this regard, a school-based approach for effectively delivering HPV vaccine would be useful, as recommended by the World Health Organization (WHO) in countries that have fairly high enrollment of girls in schools.Citation30 The 2-dose schedule for HPV vaccination (age 9–14 yr) was shown to be noninferior to 3 doses,Citation31,Citation32 which prompted the U.S. Advisory Committee on Immunization Practices (ACIP) to recommend a 2-dose schedule for HPV vaccination in 2016,Citation33 echoing an earlier recommendation by the WHO. Contemplating a 2-dose HPV vaccine schedule with an interval of 6–12 months between doses for girls aged 14 years and below would greatly reduce the logistic barriers of administrating a 3-dose vaccination schedule.Citation34 Parents in the U.S. were very receptive to the idea of a 2-dose schedule and viewed this option as less expensive, more convenient and thought it would facilitate series completion.Citation35
In our study, concern regarding the safety and side effects of the HPV vaccine for young adolescents was the main reason why mothers were not accepting HPV vaccination. Our result is supported by the findings in many other studies. It has been widely reported that having trustworthy evidence of the safety of the HPV vaccine for young adolescents was associated with parental acceptance of vaccination for their children in both Western and Asian countries.Citation7,Citation26,Citation36 As such, it may be helpful for health-care providers to emphasize the safety record of the HPV vaccines, such as providing easily understood information that HPV vaccination has an excellent safety profile and that there is no evidence of vaccine-related serious adverse events among Chinese women or among women globally.Citation37–39 It is also important to inform parents that their children are not too young for the HPV vaccine and that there is evidence showing that immunization at a younger age is associated with increasing vaccine effectiveness, as younger adolescents have a better immunogenic response than their older counterparts.Citation40 In this study, the high costs of HPV vaccines in China represented an important barrier to vaccination and has been likewise reported by many.Citation6,Citation12,Citation41 In order to achieve adequate HPV vaccine coverage, particularly among those who are socio-economically disadvantaged, it will be essential that the provision of low- or no-cost HPV vaccination be considered by the Chinese health authorities concerned.
We also investigated mothers’ preferences for 2-,4- or 9-valent HPV vaccine in view of the availability of three different types of HPV vaccines on the Chinese market. The newly available 9vHPV has the potential to prevent approximately 90% of cervical cancers and HPV-related vulvar, vaginal, and anal cancers, 70%–85% of cervical precancerous lesions, and 90% of anogenital warts.Citation42,Citation43 Our study found that over 40% of mothers prefer to vaccinate their daughter with 9vHPV. By demographics, we found that mothers with higher education and socio-economic status were likely to prefer the 9-valent HPV vaccine, implying price is an important factor for the affordability of high-valency HPV vaccine. Having family members or friends with cervical cancer has been found in a previous study to be associated with higher HPV vaccination acceptance.Citation44 In this study, mothers who know someone with cervical cancer were likely to prefer the 9vHPV, which gives a greater HPV-type coverage than the lower valency HPV vaccines.
Since the 9vHPV became available, it has become the most popular and sought-after HPV vaccine in China. However, currently, the supply of 9vHPV is not sufficient to meet the high demand. In the future, if there is a sufficient supply to meet the demand for HPV vaccine, having HPV vaccine advocates sharing their stories over the media might help in the acceptance of a higher valency HPV vaccine, as well as HPV vaccine uptake. Other messages that highlight the severity of cervical cancer and counter the misperceptions regarding side effects and HPV vaccination leading to adolescent sexual activity should be imparted in the future awareness campaign. In view of the fact that the current shortage of 9vHPV in the market is forseen to persist for a considerable time, mothers should be provided with adequate information, particularly about the risk protection differences between the 9-valent and the 2-and 4 valent HPV vaccines for them to make a decision based on the age of their child and the vaccines’ affordability.
The availability of domestically produced HPV vaccine in China is much anticipated as it is likely to offer more widespread use and curb the current shortage and high price of imported HPV vaccine.Citation5 Nonetheless, acceptance of the domestically produced HPV vaccine remains a huge challenge, largely due to public lack of trust in local vaccine manufacturers. Repeated vaccine scandals have eroded public trust and caused a surge in demand for imported vaccines, including HPV vaccines.Citation45 Thus, it is extremely important for the country to restore public trust in domestically produced HPV vaccines, once available on the market. In this study, over half of the mothers expressed a preference for imported HPV vaccine. The young age and higher socio-economic status mothers who expressed a higher likelihood of favoring imported HPV vaccine may be important groups to target for future intervention to restore confidence in the domestically produced HPV vaccine. Above all, the government needs to reform the country’s vaccine regulatory processes and more stringent regulations should be put in place to ensure the country’s vaccine safety. Pending the availability of the domestic HPV vaccine, the Chinese government needs to work with a broad range of international stakeholders to negotiate for lower vaccine prices, and scaling up the vaccine supply by manufacturers to meet the country’s needs are essential.
Limitations
This study has several limitations. First, this study used a cross-sectional study design, which precluded the evaluation of the temporality and causality of the observed relationships. Second, data were collected from participants’ self-reports; thus, these may be subjected to socially desirable responses. Third, it should be noted that the intention to receive the vaccine does not necessarily result in actual receipt of vaccine; therefore, results should be interpreted with caution. Last is the limitation in the sampling method, which involved recruiting participants only from primary schools in the Fujian province, thus the result of the study may not be generalizable to the population as a whole. Further, this study achieved only a 28.5% response rate. Despite these limitations, the sample was large, with diverse sociodemographic backgrounds reflective of mothers in the general population in China. Another limitation of this study is that the assessment of knowledge was done prior to vaccination intention, thus may potentially influence participants’ responses in vaccination intentions.
Conclusions
The present study showed high HPV vaccination acceptance among Chinese mothers, which is of great importance for a country with low cervical cancer screening coverage and high cervical cancer burden. Our results show that mothers favor 9vHPV, which provides the highest possible coverage for their daughters. While the prospect of domestically manufactured HPV vaccine is encouraging and will help to counteract the shortage of HPV vaccine in China, nevertheless it remains a challenge to gain the public’s trust. To some degree, maternal socio-economic status, perceived susceptibility, barriers and benefits, and social norms influence intention to vaccinate daughters against HPV and the preference for high valency and domestically manufactured HPV vaccine. Of note, a lower intention to vaccinate daughters against HPV among less educated and lower-income mothers may possibly lead to significant social inequalities in HPV vaccine uptake in the country in the future. Additionally, continuous education campaigns are needed to improve knowledge of HPV and HPV-associated diseases among mothers in China and to promote the benefits of HPV vaccination to adolescents.
Disclosure of potential conflicts of interest
Dr Zimet has received honoraria from Sanofi Pasteur for his work on the Adolescent Immunization Initiative and an honorarium and travel support from Merck & Co., Inc. to present at an HPV vaccine symposium.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. doi:10.3322/caac.21492.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016 Mar;66(2):115–32. doi:10.3322/caac.21338.
- Malagón T. Reasons for optimism about eliminating cervical cancer in China. Lancet Public Health. 2019 Sep 1;4(9):e434–5. doi:10.1016/S2468-2667(19)30157-4.
- Ladner J, Besson MH, Hampshire R, Tapert L, Chirenje M, Saba J. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC Public Health. 2012 Dec;12(1):370. doi:10.1186/1471-2458-12-370.
- Wong LP, Han L, Li H, Zhao J, Zhao Q, Zimet GD. Current issues facing the introduction of human papillomavirus vaccine in China and future prospects. Hum Vaccin Immunother. 2019 Apr 25;15(7–8):1533–40. doi:10.1080/21645515.2019.1611157.
- Zhao F, Qiao Y. Cervical cancer prevention in China: a key to cancer control. Lancet (London, England). 2019 Mar 9;393(10175):969. doi:10.1016/S0140-6736(18)32849-6.
- Newman PA, Logie CH, Lacombe-Duncan A, Baiden P, Tepjan S, Rubincam C, Doukas N, Asey F. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018 Apr 1;8(4):e019206. doi:10.1136/bmjopen-2017-019206.
- Perez S, Zimet GD, Tatar O, Stupiansky NW, Fisher WA, Rosberger Z. Human papillomavirus vaccines: successes and future challenges. Drugs. 2018;78(14):1385–96. doi:10.1007/s40265-018-0975-6.
- Graham JE, Mishra A. Global challenges of implementing human papillomavirus vaccines. Int J Equity Health. 2011 Dec;10(1):27. doi:10.1186/1475-9276-10-27.
- Siu JY, Fung TK, Leung LH. Social and cultural construction processes involved in HPV vaccine hesitancy among Chinese women: a qualitative study. Int J Equity Health. 2019 Dec;18(1):1–8. doi:10.1186/s12939-019-1052-9.
- Juntasopeepun P, Davidson PM, Suwan N, Phianmongkhol Y, Srisomboon J. Human papillomavirus vaccination intention among young women in Thailand. Asian Pac J Cancer Prev. 2011 Jan 1;12(12):3213–19. PMID:22471456.
- Wong LP. Knowledge and attitudes about HPV infection, HPV vaccination, and cervical cancer among rural southeast Asian women. Int J Behav Med. 2011 Jun 1;18(2):105–11. doi:10.1007/s12529-010-9104-y.
- Cheung T, Lau JT, Wang JZ, Mo P, Siu CK, Chan RT, Ho J. The Acceptability of HPV vaccines and perceptions of vaccination against HPV among physicians and nurses in Hong Kong. Int J Environ Res Public Health. 2019 Jan;16(10):1700. doi:10.3390/ijerph16101700.
- Zhang SK, Pan XF, Wang SM, Yang CX, Gao XH, Wang ZZ, Li M, Ren ZF, Zhao FH, Qiao YL. Perceptions and acceptability of HPV vaccination among parents of young adolescents: A multicenter national survey in China. Vaccine. 2013 Jul 11;31(32):3244–49. doi:10.1016/j.vaccine.2013.05.046.
- Lin Y, Lin Z, He F, Hu Z, Zimet GD, Alias H, Wong LP. Factors influencing intention to obtain the HPV vaccine and acceptability of 2-, 4-and 9-valent HPV vaccines: A study of undergraduate female health sciences students in Fujian, China. Vaccine. 2019 Oct 16;37(44):6714–23. doi:10.1016/j.vaccine.2019.09.026.
- Santhanes D, Yong CP, Yap YY, San Saw P, Chaiyakunapruk N, Khan TM. Factors influencing intention to obtain the HPV vaccine in South East Asian and Western Pacific regions: A systematic review and meta-analysis. Sci Rep. 2018 Feb 26;8(1):1–1. doi:10.1038/s41598-018-21912-x.
- Ferrer HB, Audrey S, Trotter C, Hickman M. An appraisal of theoretical approaches to examining behaviours in relation to Human Papillomavirus (HPV) vaccination of young women. Prev Med. 2015 Dec 1;81:122–31. doi:10.1016/j.ypmed.2015.08.004.
- Hofman R, van Empelen P, Richardus JH, de Kok IM, De Koning HJ, van Ballegooijen M, Korfage IJ. Predictors of HPV vaccination uptake: a longitudinal study among parents. Health Educ Res. 2013 Sep 16;29(1):83–96. doi:10.1093/her/cyt092.
- China National Medical Products Administration (NMPA). First domestic recombinant human papilloma virus vaccine approved in China [Internet] 2020 [cited 1 Jan, 2020. http://www.nmpa.gov.cn/WS04/CL2056/373049.html
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2019 May 11;pii: djz074. doi:10.1093/jnci/djz074.
- Becker MH. The health belief model and personal health behavior. Health Educ Monogr. 1974;2(4):324–508. doi:10.1177/109019817400200407.
- Champion V, Skinner CS. The health belief model. In: Glanz K, Rimer B, Viswanath K editors. Health behavior and health education. Vol. 4. San Francisco (CA): Jossey-Bass; 2008. p. 45–65. –65.
- Katz MH. Multivariable analysis: a practical guide for clinicians and public health researchers (3rd ed.). Cambridge, NY: Cambridge university press; 2011.
- Hosmer Jr. DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression (3rd ed.) Hoboken, NJ: John Wiley & Sons; 2013.
- Baumann A, Andersen B, Østergaard L, Larsen MB. Sense & sensibility: decision-making and sources of information in mothers who decline HPV vaccination of their adolescent daughters. Vaccine. 2019 Aug 9;2:100020. doi:10.1016/j.jvacx.2019.100020.
- Wong CK, Man KK, Ip P, Kwan M, McGhee SM. Mothers’ preferences and willingness to pay for human papillomavirus vaccination for their daughters: a discrete choice experiment in Hong Kong. Value Health. 2018 May 1;21(5):622–29. doi:10.1016/j.jval.2017.10.012.
- Gamble HL, Klosky JL, Parra GR, Randolph ME. Factors influencing familial decision-making regarding human papillomavirus vaccination. J Pediatr Psychol. 2009 Dec 4;35(7):704–15. doi:10.1093/jpepsy/jsp108.
- Thomas TL, Strickland OL, DiClemente R, Higgins M, Williams B, Hickey K. Parental human papillomavirus vaccine survey (PHPVS): nurse-led instrument development and psychometric testing for use in research and primary care screening. J Nurs Meas. 2013;21(1):96–109. PMID: 23786137. doi:10.1891/1061-3749.21.1.96.
- Cartmell KB, Young-Pierce J, McGue S, Alberg AJ, Luque JS, Zubizarreta M, Brandt HM. Barriers, facilitators, and potential strategies for increasing HPV vaccination: A statewide assessment to inform action. Papillomavirus Res. 2018 Jun 1;5:21–31. doi:10.1016/j.pvr.2017.11.003).
- WHO. Human papillomavirus vaccines: WHO position paper, May 2017–Recommendations. Vaccine. 2017;35(43):5753–55. doi:10.1016/j.vaccine.2017.05.069.
- Bhatla N, Nene BM, Joshi S, Esmy PO, Poli UR, Joshi G, Verma Y, Zomawia E, Pimple S, Prabhu PR, et al. Are two doses of human papillomavirus vaccine sufficient for girls aged 15–18 years? Results from a cohort study in India. Papillomavirus Res. 2018 Jun 1;5:163–71. doi:10.1016/j.pvr.2018.03.008.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Azurah AG, Fong SM, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Jama. 2016 Dec 13;316(22):2411–21. doi:10.1001/jama.2016.17615.
- Meites E. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. MMWR. 2016:65. doi:10.1111/ajt.14206.
- WHO. Human papillomavirus vaccines: WHO position paper, October 2014-Recommendations. Vaccine. 2015 Aug 26;33(36):4383–84.doi:10.1016/j.vaccine.2014.12.002.
- Fontenot HB, Domush V, Zimet GD. Parental attitudes and beliefs regarding the nine-valent human papillomavirus vaccine. J Adolesc Health. 2015 Dec 1;57(6):595–600. doi:10.1016/j.jadohealth.2015.09.003).
- Jaspers L, Budiningsih S, Wolterbeek R, Henderson FC, Peters AA. Parental acceptance of human papillomavirus (HPV) vaccination in Indonesia: a cross-sectional study. Vaccine. 2011 Oct 13;29(44):7785–93. doi:10.1016/j.vaccine.2011.07.107.
- Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, Pan QJ, Zhang WH, Zhao FH, Zhang CF, et al. Efficacy, immunogenicity and safety of the AS04‐HPV‐16/18 vaccine in Chinese women aged 18‐25 years: end‐of‐study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019 Oct;8(14):6195–211. doi:10.1002/cam4.2399.
- Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, Wang S, Liao X, Shou Q, Qiu Y, et al. Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine. 2019 Jun 12;37(27):3617–24. doi:10.1016/j.vaccine.2018.08.009.
- Arbyn M, Xu L, Simoens C, Martin-Hirsch PPL. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors (Review). Cochrane Database Syst Revi. 2018;(5). Art. No.: CD009069. doi:10.1002/14651858.CD009069.pub3.
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, et al. HPV study group for adult women: immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27(4):581–87. doi:10.1016/j.vaccine.2008.10.088.
- Yin Y. HPV vaccination in China needs to be more cost-effective. Lancet. 2017 Oct 14;390(10104):1735–36. doi:10.1016/S0140-6736(17)32606-5.
- Garland SM, Pitisuttithum P, Ngan HY, Cho CH, Lee CY, Chen CA, Yang YC, Chu TY, Twu NF, Samakoses R, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian countries. J Infect Dis. 2018 May 15;218(1):95–108. doi:10.1093/infdis/jiy133.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, Bosch FX, de Sanjosé S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012 Dec;7(1):38. doi:10.1186/1750-9378-7-38.
- Ogilvie G, Anderson M, Marra F, McNeil S, Pielak K, Dawar M, McIvor M, Ehlen T, Dobson S, Money D, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: parental factors associated with HPV vaccine receipt. PLoS Med. 2010 May 4;7(5):e1000270.
- Pan XF, Li R, Pan A, Larson H. Human papillomavirus vaccine approval in China: a major step forward but challenges ahead. Lancet Infect Dis. 2016 Dec 1;16(12):1322–23. doi:10.1016/S1473-3099(16)30450-9.