ABSTRACT
The interleukin 6 (IL6) family of proteins regulate important cellular processes and act through a variety of signaling pathways via a shared gp130 receptor. In the liver, there is a large body of evidence showing a protective and pro-regenerative role for IL6 cis and trans signaling. While a few studies suggest a pathological role for IL6 trans-signaling in the liver. IL11 is often thought of as similar to IL6 and redundancy has been inferred. However, recent studies reveal that IL6R and IL11RA are expressed on dissimilar cell types and these cytokines actually have very different roles in biology and pathology. In the liver, IL6R is mostly expressed on immune cells, whereas IL11RA is highly expressed on hepatocytes and hepatic stellate cells, both of which exhibit autocrine IL11 activity. In contrast to the beneficial effects of IL6 in the liver, IL11 causes liver disease and its expression in stromal and parenchymal cells leads to fibrosis, inflammation, steatosis and hepatic failure. In this review, we address IL6 and IL11 in the context of liver function. We end by discussing the possibility of IL6 gain-of-function versus IL11 inhibition as therapeutic approaches to treat liver disease.Citation1,Citation2
Introduction
The interleukin 6 (IL6) family of cytokines are characterized by their common use of the widely expressed signal-transducing receptor, glycoprotein 130 (gp130). To date, the IL6 family has 10 members: IL6, IL11, IL27, oncostatin M (OSM), leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), cardiotrophin 1 (CT1), cardiotrophin-like cytokine factor 1 (CLCF1), and two recently added cytokines, IL35 and IL39.1–Citation4 Specificity for IL6 family member signaling is established by binding of the individual cytokines to their cognate, high-affinity ‘alpha’ receptors (i.e. IL6 to IL6R, IL11 to IL11RA, CNTF to CNTFR), which show cell-specific expression patterns.Citation5 These cytokine:receptor complexes then bind to gp130 to differentially initiate downstream signaling pathways, including JAK/STAT or MEK/ERK.
IL6 has been reported to play a pathological role across a range of conditions including heart failure, inflammatory diseases (e.g. asthma, rheumatoid arthritis, systemic lupus erythematosus), and cancers.Citation3,Citation6 In contrast to IL6, IL11 is little studied and the roles of IL11 in disease, aside from its effect in cancer, are only now being appreciated (). The IL11 field is also confounded by assumptions that IL11 has cytoprotective, anti-fibrotic, and anti-inflammatory activity based on previous studies of effects of recombinant human IL11 (rhIL11) in mouse models of disease.Citation7 Only very recently, have experiments shown a central role for endogenous and species-matched IL11 in the pathology of fibro-inflammatory diseases such as inflammatory bowel disease (IBD), cardiorenal and lung fibrosis, and acute and chronic liver disease.Citation5,Citation8-11
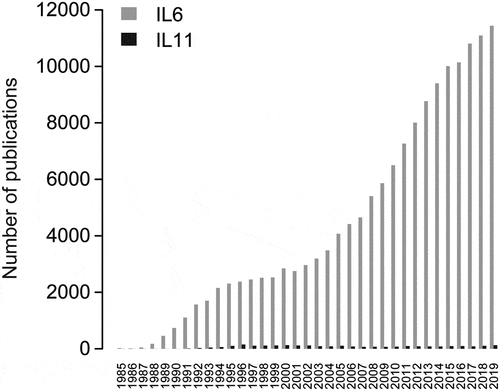
Figure 1. Number of publications for IL6 (gray) or IL11 (black) by year (1985–2019). The R package Pubmedwordcloud was used to generate these plots using case insensitive keywords ‘il6ʹ, ‘il-6ʹ, ‘interleukin-6ʹ, ‘interleukin 6ʹ for IL6 and ‘il-11ʹ, ‘interleukin-11ʹ and ‘interleukin 11ʹ for IL11
In this review, we examine the roles IL6 and IL11 in the liver and discuss the therapeutic opportunities these cytokines may provide for liver disease. While the older literature proposed these two cytokines have overlapping and redundant roles in the liver, we discuss recent data that challenges long-held assumptions.
IL6 and IL11 – different children from the same family
Since the discovery and cloning of IL6 in 1986,Citation12 more than 30 y of extensive research has been carried out on this key cytokine. Analysis of the published literature shows that IL6 is the 6th most studied gene of all time.Citation13 The same cannot be said for IL11, which was first cloned not long after IL6 in 1990Citation14 (). IL11 remains poorly studied and based on some recent data, it appears that some of the assumed IL11 functions may have been misinterpreted.Citation7
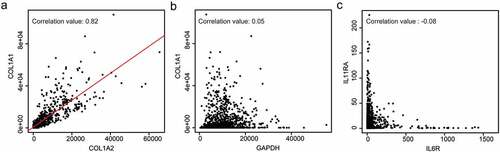
IL6 consists of 183 amino acids and IL11 is a 178 amino acid protein but these cytokines share limited (~20%) sequence homology. Crystal structures show that IL11 is dissimilar to IL6 and the key gp130 residues required for their respective hexameric signaling complex formation also differ.Citation15,Citation16 While these structural and binding properties suggest differences in IL6 and IL11 signaling, perhaps the starkest example of their dissimilarity is apparent in their receptor distribution, which verges on mutual exclusivity (). While IL6R is expressed most highly on immune cells, IL11RA is expressed in stromal cells, such as fibroblasts and hepatic stellate cells, and also on parenchymal cells, including hepatocytesCitation5,Citation10,Citation18,Citation19. Hence, it may be expected that IL6 biology relates mostly to immune functions whereas IL11 activity is more closely linked to the stromal and parenchymal biology. Another intriguing dissimilarity is that in healthy humans IL6 is highly expressed across tissues whereas IL11 is barely detectable (https://gtexportal.org/home/index.html).
Figure 2. IL11RA is expressed on different cell types as compared to IL6R. Graphs showing example data for (a) correlated gene expression (COL1A1 vs COL1A2), (b) unrelated gene expression (COL1A1 vs GAPDH), and (c) IL11RA vs IL6R gene expression, which appears largely exclusive and in disparate cell types. The expression values are normalized counts obtained from the FANTOM consortium.Citation17
These biological pointers suggest distinct roles for IL6 and IL11 and this is apparent when we look at the phenotypes of individuals with naturally occurring genetic loss-of-function (LOF) mutations in the general population. IL6R LOF in humans leads to recurrent infections, inflammatory derangement, eczema and eosinophilia.Citation20 In contrast, IL11RA LOF is associated with craniosynostosis, delayed tooth eruption and joint laxity.Citation21 LOF mutations in gp130, the shared partner of IL6R and IL11RA, cause a combined phenotype of craniosynostosis and immune dysfunction, consistent with loss of both IL6 and IL11 signaling.Citation22
In summary, IL6 is an established pro-inflammatory factor that plays an important role in human inflammatory and immune diseases (e.g. rheumatoid arthritis and cytokine storm), where its therapeutic inhibition is established as beneficial.Citation23 While we know much less about IL11, it has emerged as an important pro-fibrotic factor across organs and, more recently, as a hepatotoxin.Citation5,Citation8-11
IL6R and IL11RA expression in hepatocytes and hepatic stellate cells
IL6, originally termed B cell growth/stimulatory factor II (BSF2),Citation24 was initially shown to stimulate acute phase response from HepG2 cells and rat hepatocytes.Citation25 Subsequent studies showed that IL6 can be produced from and act on the liver and IL6 is a well-established and important determinant of the acute phase response, which involves secretion of CRP, serum amyloid A, hepcidin, among other factors from hepatocytes. Thus, it is abundantly clear that IL6 stimulates hepatocytes, directly or indirectly.
The published literature states that hepatocytes express the IL6RCitation26,Citation27 and this would fit with a direct effect of IL6 on hepatocytes. However, recent studies of primary human and mouse hepatocytes have struggled to detect IL6R expression at the transcriptional, translational or protein levels whereas gp130 and IL11RA are abundantly detected.Citation28 This suggests that IL6 could possibly act indirectly on hepatocytes, perhaps via Kupffer cells, infiltrating immune cells, in trans, via very low levels of receptor expression or through other yet-to-be-determined mechanisms. These conflicting data present a conundrum that requires further study but differences may reflect, at least in part, the variable characteristics of primary hepatocytes used in the recent studies as compared to HepG2 or AML12 cell lines, often used in the previous literature.
Another important cell type in the liver is the hepatic stellate cell (HSC). Activated HSCs are the main drivers of liver fibrosis and a therapeutic target cell in nonalcoholic steatohepatitis (NASH)Citation29–31 . In disease, HSCs undergo a cellular transition to become invasive, collagen secreting and pro-inflammatory myofibroblasts.Citation32 This phenomenon is similar to fibroblast-to-myofibroblast transformations in other organs, which is dependent on IL11 signaling.Citation7 Profiling of primary human HSCs at the protein level shows high expression of IL11RA and gp130 but no (or very low) detectable IL6R.Citation10 As with hepatocytes, HSCs are known to be IL6-responsive and are a source of IL6 themselves.Citation33,Citation34 However, based on the receptor expression data,Citation10 HSCs do not appear to be a direct target for IL6, unless direct binding of IL6 to gp130 in the absence of the alpha receptor was possible, which has been reported.Citation35,Citation36 In contrast, IL11 can act directly on HSCs through binding to its cognate IL11RA receptor and this effect can be either paracrine or autocrine, as HSCs themselves secrete large amounts of IL11 when stimulated by disease factors.Citation10
IL6 gain-of-function could be a therapeutic approach for treating liver disease
There is a large body of data showing that, overall, the effects on IL6 on liver health – separate to its role in the acute phase response – are beneficial and that IL6 activity in the liver promotes cytoprotection, regeneration and is metabolically advantageous.Citation26,Citation37,Citation38 In addition to cis-signaling, IL6 can also bind to soluble IL6R (sIL6R), which is generated from membrane-bound IL6R expressing cells by ADAM-mediated proteolysis. In this way, IL6:sIL6R complexes can signal in trans to activate cells that express gp130 but do not have their own IL6R. An artificial fusion protein construct of IL6 coupled to sIL6R, called Hyper-IL6, has been shown to potently activate cells, including hepatocytes, independent of IL6R.Citation26,Citation39
Given the limited evidence for IL6R expression on primary human hepatocytes or HSCs, IL6 trans-signaling may be of specific importance for liver health. Indeed, while IL6 knockout mice have impaired liver regeneration following partial hepatectomy or chronic injury,Citation40,Citation41 only Hyper-IL6 (not IL6) reverses D-galactosamine-mediated liver toxicity and promotes hepatocyte proliferation and survival.Citation42 A similar phenotype was observed in mice subjected to hepatectomy in that only Hyper-IL6 (but not IL6) accelerated liver regeneration.Citation43 Exacerbation of acute liver damage in mice following blockade of trans-signaling using soluble gp130 (sgp130) further supports a role for IL6 trans signaling in promoting liver health.Citation44,Citation45 Hence, Hyper-IL6 treatment could potentially be beneficial for patients following liver damage by boosting hepatocyte regeneration. Recently, an approach of transplanting Hyper-IL6-pretreated hepatic progenitor cells into the injured livers of mice was shown to promote liver regeneration.Citation46 While the weight of experimental data points to a beneficial effect of IL6 cis and trans signaling in the liver, hepatic IL6 trans signaling has also been suggested as maladaptive.Citation26,Citation37,Citation47-49 However, specific therapeutic inhibition of IL6 trans-signaling using gp130 decoy constructs was found to have no beneficial effect in two independent studies of murine NASH.Citation28,Citation50
Overall, the manipulation of IL6 signaling for patient benefit in liver disease is hard to envision in the near future. While the majority of the data point to IL6 gain-of-function (in cis or trans) as beneficial, the literature is discordant and administration of Hyper-IL6-like therapies may have untoward effects. In the clinic, therapies targeting IL6 (sarilumab) or IL6R (tocilizumab) are approved for inflammatory conditions (e.g. rheumatoid arthritis, cytokine storm) but may possibly be associated with hepatocellular injury.Citation51 Outside the liver, clinical trials investigating the therapeutic potential of IL6 inhibition with therapeutic antibodies or decoy molecules and of JAK/STAT inhibition with small molecules are underway for cancer as well as for additional inflammatory and autoimmune diseases.Citation51
Inhibiting IL11 signaling to treat liver disease
Most of the published literature on IL11 in the liver suggests that IL11 gain-of-function is beneficial for liver health. For instance, rhIL11 was shown to be protective in mouse models of ischemia/reperfusion injury, acetaminophen (APAP)-induced liver injury (AILI), acute endotoxemia, and Concanavalin A-induced T cell-mediated hepatotoxicity.Citation52–57 Based on the premise that the beneficial effects of rhIL11 in mice infer the true biology of IL11, a single clinical trial using rhIL11 was performed in patients with chronic Hepatitis C.Citation58
However, Widjaja et.al recently showed that species-matched IL11 is in fact hepatotoxic and induces reactive oxygen species (ROS)-dependent hepatocyte cell death via c-Jun N-terminal kinase (JNK) while also inhibiting liver regeneration.Citation8 The discrepancy of these newer findings with the published literature, where a high dose of rhIL11 was injected to rodents, may be explained by the fact that while rhIL11 binds to mouse IL11RA, it does not activate the same signaling pathways as endogenous IL11. As such, rhIL11 injection to the mouse inhibits physiologically relevant IL11 signaling (i.e. rhIL11 is an antagonist of murine IL11 signaling in the mouse). This has a large implication for our understanding of IL11 biology in the liver and other organs.
Using a mouse model, Widjaja et al. showed that therapeutically targeting IL11 signaling using neutralizing IL11RA antibodies 10 hours following acetaminophen-induced liver damage, reverses hepatic failure, promotes liver regeneration and improves survival. This study also suggested the translational potential of anti-IL11 therapies as an adjunctive approach to the current standard of care (N-Acetyl Cysteine (NAC)) for patients suffering from liver damage due to acetaminophen poisoning.
A central importance of IL11 in NASH has also recently been described.Citation10 In this study, a specific effect of IL11 on HSC-to-myofibroblast transformation was shown and inhibition of IL11 signaling genetically or with antibodies reduced liver fibrosis, inflammation and hepatocyte damage. Most recently, a role for IL11 in steatohepatitis has also been observed, inferring a role for pathological IL11 signaling in hepatocytes themselves in the early stages of metabolic liver disease.Citation28
Based on data from preclinical models, targeting Transforming Growth Factor β1 (TGFβ1) as a therapeutic strategy for treating acute and chronic liver disease has been proposed.Citation59,Citation60 However, systemic and long-term inhibition of TGFβ1 provokes inflammation and autoimmune diseases, in addition to increasing risk of neoplasia and cardiovascular problems.Citation61,Citation62 While IL11 acts downstream of TGFβ1 (and many other disease factors) in hepatocytes and HSCs,Citation10 it is important to recognize that the safety profile for inhibiting IL11, rather than TGFβ1 upstream, is promising. Humans lacking TGFβ1 have severe childhood onset IBD,Citation63 whereas loss of IL11RA function in humans has mild effect, as discussed above. Furthermore, long-term treatment of mice with high dose (10 mg/kg) of anti-IL11 therapy is well tolerated both in healthy mice and in models of liver disease over many months.Citation10 Whether or not anti-IL11 therapy translates to the clinic for treating human liver disease has yet to be tested.
Concluding remarks
Here we reviewed the biology of IL6 as compared to IL11 in the context of liver health, disease and regeneration. There is a substantial body of work relating to IL6 function in the liver and, overall, this shows that IL6 cis and trans signaling to be beneficial for liver function and regeneration. In contrast, the literature on IL11 in the liver is limited and the earlier studies that suggested IL11 is beneficial for the liver were likely misinterpreted due to a reliance on the use of rhIL11 in mouse models of liver disease. Thus, paradoxically, inhibiting IL11 activity, rather than potentiating it, may be as a therapeutic approach for treating liver disease.
In the case of IL6, while its inhibition is highly effective for treating inflammatory diseases (), its activity overall is beneficial for liver function. Delivering an IL6 gain-of-function therapeutic (e.g. Hyper-IL6) might be envisaged in liver disease but this approach could be pro-inflammatory and there remains the possibility of IL6 trans signaling being hepatotoxic. There are also potential issues with manufacturing, pharmacokinetics and immunogenicity for alien protein constructs like Hyper-IL6. On the other hand, there is strong genetic evidence in humans and mice of an acceptable safety profile for IL11 inhibition. Thus, therapeutic antibodies against IL11 or IL11RA offer an accepted therapeutic approach for targeting liver diseases with an established mechanism of action in both acute and chronic liver diseases ().
Table 1. Overview of established and hypothetical uses of IL6 or IL11 therapies across organs and diseases
Disclosure of potential conflicts of interest
S.A.C. and A.A.W. are co-inventors on a number of patent applications relating to the role of IL11 in human diseases that include the published patents: WO/2017/103108, WO/2017/103108 A2, WO/2018/109174 A2, WO/2018/109170 A2, WO/2019/073057. S.A.C. is co-founder and shareholder of Enleofen Bio PTE LTD, a company that developed anti-IL11 therapeutics.
Acknowledgments
The authors thank Dr. Owen Rackham for his support with FANTOM database.
Additional information
Funding
References
- Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13(3):290–99. doi:10.1038/ni.2227.
- Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, Chen G, Hou C, Ma N, Shen B, et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol. 2016;46(6):1343–50. doi:10.1002/eji.201546095.
- Rose-John S. Interleukin-6 family cytokines [Internet]. Cold Spring Harb Perspect Biol. 2018;10(2):a028415. doi:10.1101/cshperspect.a028415.
- Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50(4):812–31. doi:10.1016/j.immuni.2019.03.027.
- Schafer S, Viswanathan S, Widjaja AA, Lim -W-W, Moreno-Moral A, DeLaughter DM, Ng B, Patone G, Chow K, Khin E, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552(7683):110–15. doi:10.1038/nature24676.
- Garbers C, Scheller J. Interleukin-6 and interleukin-11: same same but different [Internet]. Biol Chem. 2013;394(9):1145–61. doi:10.1515/hsz-2013-0166.
- Cook SA, Schafer S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med. 2020;71(1):263–76. doi:10.1146/annurev-med-041818-011649.
- Widjaja AA, Dong J, Adami E, Viswanathan S, Ng B, Singh BK, Lim WW, Zhou J, Pakkiri LS, Shekeran SG, et al. Redefining Interleukin 11 as a regeneration-limiting hepatotoxin [Internet]. https://www.biorxiv.org/content/10.1101/830018v1.
- Ng B, Dong J, D’Agostino G, Viswanathan S, Widjaja AA, Lim -W-W, Ko NSJ, Tan J, Chothani SP, Huang B, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med [Internet]. 2019;11(511):eaaw1237. doi:10.1126/scitranslmed.aaw1237.
- Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D’Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology. 2019;157:777–92.e14. doi:10.1053/j.gastro.2019.05.002.
- Lim -W-W, Ng B, Widjaja A, Xie C, Su L, Ko N, Lim S-Y, Kwek X-Y, Lim S, Cook SA, et al. Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice. PLoS One. 2020;15:e0227505. doi:10.1371/journal.pone.0227505.
- Kinashi T, Harada N, Severinson E, Tanabe T, Sideras P, Konishi M, Azuma C, Tominaga A, Bergstedt-Lindqvist S, Takahashi M, et al. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II [Internet]. Nature. 1986;324:70–73. doi:10.1038/324070a0.
- Dolgin E. The most popular genes in the human genome [Internet]. Nature. 2017;551:427–31. doi:10.1038/d41586-017-07291-9.
- Paul SR, Bennett F, Calvetti JA, Kelleher K, Wood CR, O’Hara RM Jr, Leary AC, Sibley B, Clark SC, Williams DA. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990;87:7512–16. doi:10.1073/pnas.87.19.7512.
- Gu Z-J, Wijdenes J, Zhang X-G, Hallet -M-M, Clement C, Klein B. Anti-gp130 transducer monoclonal antibodies specifically inhibiting ciliary neurotrophic factor, interleukin-6, interleukin-11, leukemia inhibitory factor or oncostatin M [Internet]. J Immunol Methods. 1996;190:21–27. doi:10.1016/0022-1759(95)00232-4.
- Putoczki TL, Dobson RCJ, Griffin MDW. The structure of human interleukin-11 reveals receptor-binding site features and structural differences from interleukin-6. Acta Crystallogr D Biol Crystallogr. 2014;70:2277–85. doi:10.1107/S1399004714012267.
- (dgt) TFCATRPAC, The FANTOM Consortium and the RIKEN PMI and CLST (DGT). A promoter-level mammalian expression atlas [Internet]. Nature. 2014;507(7493):462–70. doi:10.1038/nature13182.
- Lokau J, Agthe M, Flynn CM, Garbers C. Proteolytic control of Interleukin-11 and Interleukin-6 biology. Biochim Biophys Acta Mol Cell Res. 2017;1864:2105–17. doi:10.1016/j.bbamcr.2017.06.008.
- Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, Zhao X-Y, Ji Y, Li C, Guo L, et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75:644–60.e5. doi:10.1016/j.molcel.2019.07.028.
- Spencer S, Köstel Bal S, Egner W, Lango Allen H, Raza SI, Ma CA, Gürel M, Zhang Y, Sun G, Sabroe RA, et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses [Internet]. J Exp Med. 2019;216(9):1986–98. doi:10.1084/jem.20190344.
- Brischoux-Boucher E, Trimouille A, Baujat G, Goldenberg A, Schaefer E, Guichard B, Hannequin P, Paternoster G, Baer S, Cabrol C, et al. IL11RA-related Crouzon-like autosomal recessive craniosynostosis in 10 new patients: resemblances and differences. Clin Genet. 2018;94(3–4):373–80. doi:10.1111/cge.13409.
- Schwerd T, Twigg SRF, Aschenbrenner D, Manrique S, Miller KA, Taylor IB, Capitani M, McGowan SJ, Sweeney E, Weber A, et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis [Internet]. J Exp Med. 2017;214(9):2547–62. doi:10.1084/jem.20161810.
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295.
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi:10.1038/324073a0.
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells [Internet]. PNAS. 1987;84(20):7251–55. doi:10.1073/pnas.84.20.7251.
- Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–15. doi:10.1016/j.jhep.2016.02.004.
- Geisterfer M, Richards C, Baumann M, Fey G, Gywnne D, Gauldie J. Regulation of IL-6 and the hepatic IL-6 receptor in acute inflammation in vivo. Cytokine. 1993;5(1):1–7. doi:10.1016/1043-4666(93)90017-Y.
- Dong J, Adami E, Chothani SP, Viswanathan S, Ng B, Lim WW, Singh BK, Zhou J, Ko NSJ, Shekeran SG, et al. Autocrine IL11 cis-signaling in hepatocytes is an initiating nexus between lipotoxicity and non-alcoholic steatohepatitis [Internet]. https://www.biorxiv.org/content/10.1101/2020.03.11.986802v1.full.
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72. doi:10.1152/physrev.00013.2007.
- Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis [Internet]. Adv Drug Deliv Rev. 2017;121:27–42. doi:10.1016/j.addr.2017.05.007.
- Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22. doi:10.1038/s41591-018-0104-9.
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411. doi:10.1038/nrgastro.2017.38.
- Kim Y, Fiel MI, Albanis E, Chou HI, Zhang W, Khitrov G, Friedman SL. Anti-fibrotic activity and enhanced interleukin-6 production by hepatic stellate cells in response to imatinib mesylate. Liver Int. 2012;32(6):1008–17. doi:10.1111/j.1478-3231.2012.02806.x.
- Xiang D-M, Sun W, Ning B-F, Zhou T-F, Li X-F, Zhong W, Cheng Z, Xia M-Y, Wang X, Deng X, et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67(9):1704–15. doi:10.1136/gutjnl-2016-313392.
- Pietzko D, Zohlnhöfer D, Graeve L, Fleischer D, Stoyan T, Schooltink H, Rose-John S, Heinrich PC. The hepatic interleukin-6 receptor. Studies on its structure and regulation by phorbol 12-myristate 13-acetate-dexamethasone. J Biol Chem. 1993;268:4250–58.
- D’Alessandro F, Colamonici OR, Nordan RP. Direct association of interleukin-6 with a 130-kDa component of the interleukin-6 receptor system. J Biol Chem. 1993;268:2149–53.
- Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, Liedtke C, Wasmuth HE, Trautwein C, Streetz KL. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology. 2010;51(2):463–73. doi:10.1002/hep.23322.
- Yamaguchi K, Itoh Y, Yokomizo C, Nishimura T, Niimi T, Fujii H, Okanoue T, Yoshikawa T. Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab Invest. 2010;90(8):1169–78. doi:10.1038/labinvest.2010.75.
- Fischer M, Goldschmitt J, Peschel C, Brakenhoff JPG, Kallen K-J, Wollmer A, Grötzinger J, Rose-John S. A bioactive designer cytokine for human hematopoietic progenitor cell expansion [Internet]. Nat Biotechnol. 1997;15(2):142–45. doi:10.1038/nbt0297-142.
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274(5291):1379–83. doi:10.1126/science.274.5291.1379.
- Yeoh GCT, Ernst M, Rose-John S, Akhurst B, Payne C, Long S, Alexander W, Croker B, Grail D, Matthews VB. Opposing roles of gp130-mediated STAT-3 and ERK-1/2 signaling in liver progenitor cell migration and proliferation. Hepatology. 2007;45(2):486–94. doi:10.1002/hep.21535.
- Hecht N, Pappo O, Shouval D, Rose-John S, Galun E, Axelrod JH. Hyper-IL-6 gene therapy reverses fulminant hepatic failure [Internet]. Mol Ther. 2001;3(5):683–87. doi:10.1006/mthe.2001.0313.
- Peters M, Blinn G, Jostock T, Schirmacher P, Büschenfelde KMZ, Galle PR, Rose–John S. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration [Internet]. Gastroenterology. 2000;119(6):1663–71. doi:10.1053/gast.2000.20236.
- Drucker C, Rabe B, Chalaris A, Schulz E, Scheller J, Rose-John S. Interleukin-6 trans-signaling regulates glycogen consumption after D-galactosamine-induced liver damage. J Interferon Cytokine Res. 2009;29(11):711–18. doi:10.1089/jir.2008.0095.
- Gewiese-Rabsch J, Drucker C, Malchow S, Scheller J, Rose-John S. Role of IL-6 trans-signaling in CCl4 induced liver damage [Internet]. Biochimica et Biophysica Acta (BBA). 2010;1802(11):1054–61. doi:10.1016/j.bbadis.2010.07.023.
- Lu W-Y, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17(8):971–83. doi:10.1038/ncb3203.
- Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278(13):11281–88. doi:10.1074/jbc.M208470200.
- Klein C, Wüstefeld T, Assmus U, Roskams T, Rose-John S, Müller M, Manns MP, Ernst M, Trautwein C. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115(4):860–69. doi:10.1172/JCI23640.
- Matthews VB, Allen TL, Risis S, Chan MHS, Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53(11):2431–41. doi:10.1007/s00125-010-1865-y.
- Kammoun HL, Allen TL, Henstridge DC, Kraakman MJ, Peijs L, Rose-John S, Febbraio MA. Over-expressing the soluble gp130-Fc does not ameliorate methionine and choline deficient diet-induced non alcoholic steatohepatitis in mice. PLoS One. 2017;12(6):e0179099. doi:10.1371/journal.pone.0179099.
- Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–23. doi:10.1016/j.immuni.2019.03.026.
- Yu J, Feng Z, Tan L, Pu L, Kong L. Interleukin-11 protects mouse liver from warm ischemia/reperfusion (WI/Rp) injury. Clin Res Hepatol Gastroenterol. 2016;40(5):562–70. doi:10.1016/j.clinre.2015.11.009.
- Zhu M, Lu B, Cao Q, Wu Z, Xu Z, Li W, Yao X, Liu F, Das A. IL-11 attenuates liver Ischemia/Reperfusion Injury (IRI) through STAT3 signaling pathway in mice [Internet]. PLoS One. 2015;10(5):e0126296. doi:10.1371/journal.pone.0126296.
- Nishina T, Komazawa-Sakon S, Yanaka S, Piao X, Zheng D-M, Piao J-H, Kojima Y, Yamashina S, Sano E, Putoczki T, et al. Interleukin-11 links oxidative stress and compensatory proliferation. Sci Signal. 2012;5(207):ra5. doi:10.1126/scisignal.2002056.
- Maeshima K, Takahashi T, Nakahira K, Shimizu H, Fujii H, Katayama H, Yokoyama M, Morita K, Akagi R, Sassa S. A protective role of interleukin 11 on hepatic injury in acute endotoxemia. Shock. 2004;21(2):134–38. doi:10.1097/01.shk.0000103386.98235.f6.
- Trepicchio WL, Bozza M, Bouchard P, Dorner AJ. Protective effect of rhIL-11 in a Murine model of acetaminophen-induced hepatotoxicity [Internet]. Toxicol Pathol. 2001;29(2):242–49. doi:10.1080/019262301317052521.
- Bozza M, Bliss JL, Maylor R, Erickson J, Donnelly L, Bouchard P, Dorner AJ, Trepicchio WL. Interleukin-11 reduces T-cell-dependent experimental liver injury in mice. Hepatology. 1999;30(6):1441–47. doi:10.1002/hep.510300616.
- Lawitz EJ, Hepburn MJ, Casey TJ. A pilot study of interleukin-11 in subjects with chronic hepatitis C and advanced liver disease nonresponsive to antiviral therapy. Am J Gastroenterol. 2004;99(12):2359–64. doi:10.1111/j.1572-0241.2004.40047.x.
- Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P. the IT-LIVER Consortium. TGF-β signalling and liver disease [Internet]. Febs J. 2016;283(12):2219–32. doi:10.1111/febs.13665.
- Bird TG, Müller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu W-Y, Jamieson T, Govaere O, Campbell AD, et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence [Internet]. Sci Transl Med. 2018;10(454):eaan1230. doi:10.1126/scitranslmed.aan1230.
- Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RRA, Heier A. Induction of heart valve lesions by small-molecule ALK5 inhibitors [Internet]. Toxicol Pathol. 2011;39(6):916–24. doi:10.1177/0192623311416259.
- Akhurst RJ. Targeting TGF-β signaling for therapeutic gain [Internet]. Cold Spring Harb Perspect Biol. 2017;9(10):a022301. doi:10.1101/cshperspect.a022301.
- Kotlarz D, Marquardt B, Barøy T, Lee WS, Konnikova L, Hollizeck S, Magg T, Lehle AS, Walz C, Borggraefe I, et al. Human TGF-β1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet. 2018;50(3):344–48. doi:10.1038/s41588-018-0063-6.
- Li J, Hu XF, Xing PX. CNTO-328 (Centocor).. Curr Opin Investig Drugs. 2005;6:639–45.
- Rossi J-F, Négrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103(8):1154–62. doi:10.1038/sj.bjc.6605872.
- Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–32. doi:10.1182/blood-2004-12-4602.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi:10.1056/NEJMoa1407222.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377(4):317–28. doi:10.1056/NEJMoa1613849.
- Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M, Miyake S, Aranami T, Yamamura T. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82(15):1302–06. doi:10.1212/WNL.0000000000000317.
- Ogata A, Hirano T, Hishitani Y, Tanaka T. Safety and efficacy of tocilizumab for the treatment of rheumatoid arthritis [Internet]. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:CMAMD.S7371. doi:10.4137/cmamd.s7371.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Klearman M, Musselman D, Agarwal S, Green J, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50. doi:10.1016/S0140-6736(13)60250-0.
- Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, Iwata N, Umebayashi H, Murata T, Miyoshi M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371(9617):998–1006. doi:10.1016/S0140-6736(08)60454-7.
- Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, Nomura A, Yoshida S, Nishimoto N. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis. 2018;77(3):348–54. doi:10.1136/annrheumdis-2017-211878.
