ABSTRACT
Inactivated influenza vaccines are known to be less immunogenic in human elderly in regards to serologic antibody response induced by vaccination. Accumulating evidence, however, points to a comparable effectiveness of influenza vaccines in the young and the elderly individuals. In the current study, we assessed immunogenicity and effectiveness of trivalent inactivated vaccine FluLaval in young and aged cotton rats Sigmodon hispidus and found that while serologic response to immunization was indeed reduced in older animals, comparable protection against influenza infection was afforded by prime-boost vaccination in both young and aged cotton rats. Both hemagglutination inhibition (HAI) titers and seroconversion rates were lower in the aged animals compared to the young ones. Reduction of viral load in the lung and nose, however, was comparable between young and aged animals vaccinated twice. One-time immunization with FluLaval was less efficacious at protecting the nose of aged animals, indicating that boosting of preexisting immunity can be particularly important for nasal protection in the elderly. Coincidentally, a one-time immunization with FluLaval had a detrimental effect on pulmonary pathology in the young animals, suggesting that boosting of immunity is essential for the young as well. Overall, these results suggest that reduced antibody response to and sufficient efficacy of influenza vaccines in the elderly are not two irreconcilable phenomena and that incomplete immunity to influenza can be detrimental at any age.
Introduction
Influenza infection is associated with increased morbidity and hospitalizations in the elderly.Citation1–3 Four to five times greater rates of influenza-associated hospitalizations have been reported for individuals ≥65 years of age compared to the general population in the U.S. alone.Citation4 Annual vaccination against influenza is the primary method of prevention and has been recommended for the elderly since the 1960s.Citation5,Citation6
Influenza vaccines have been in use since the early 1940s. Today, the most commonly distributed influenza vaccines include inactivated split-virion (detergent-disrupted) and purified subunit vaccines that come in trivalent or quadrivalent forms. Trivalent inactivated influenza vaccines (TIV) were first licensed in the U.S. in 1978Citation7 and remained the only type of licensed influenza vaccine in the U.S. for 25 years until the first live attenuated vaccine (FluMist) was approved in 2003. The period between 2003 and 2015 signifies the time when an increasing number of new types of influenza vaccines have been licensed. The list includes high-dose TIV for individuals over the age of 65 (2009), quadrivalent inactivated and live-attenuated vaccines (2012–2013), cell-based influenza vaccines (2012–2013), and trivalent MF59-adjuvanted vaccine for use in individuals over the age of 65 (2015).Citation7 A number of considerations drove a rapid increase in new influenza vaccine technologies, fueled in large part by a concern over effectiveness of influenza vaccination, particularly the ability of vaccines to induce sufficient immune response in the elderly population.
Older people were shown to have reduced antibody response to influenza vaccination. Fold-rise of hemagglutination inhibition (HAI) antibody titer and geometric mean titers (GMTs) in human elderly are reduced compared to younger adults in response to the same vaccine formulation.Citation8,Citation9 Increasing the antigen dose of TIV fourfold significantly improved antibody response in adults 65 years of age and olderCitation10,Citation11 and resulted in the approval of the high-dose TIV FluZone (Sanofi Pasteur) for the elderly. The use of adjuvants was also shown to be beneficial for improving immunogenicity of TIV in the elderly.Citation12,Citation13 MF59-adjuvanted TIV Fluad (Seqirus) has been licensed and together with the high-dose TIV FluZone (Sanofi Pasteur) represents the two currently recommended influenza vaccines for adults ≥65 years of age.Citation6
While reduced antibody response to influenza vaccines in the elderly is a generally accepted phenomenon, the extent of vaccine effectiveness in older vs. younger individuals is a subject of considerable debate (seeCitation14 for review). Data from the U.S. Flu Vaccine Effectiveness (VE) network established by the CDC indicate that VE in the elderly vs. younger adults may vary by season, with some seasons showing reduced VE in adults over 65 years of ageCitation15,Citation16 and other seasons showing no reduction.Citation17,Citation18 A recently published pooled analysis of VE data collected over 5 seasons (from 2012–2013 to 2015–2016) determined that the overall levels of protection afforded by influenza vaccination are comparable between adults age 18–49 years and adults ≥65 years old.Citation19 That study concluded that between 2012 and 2016 VE against A(H1N1)pdm09 was 49% and 48% for adults ≥65 and 18–49 years, respectively. The same report provided estimates of VE against influenza B viruses as 62% and 55%, and VE against A(H3N2) viruses as 14% and 21% for older and younger adults, respectively. A conclusion was drawn that the levels of protection were not influenced by advanced age, but that protection was lower in both age categories against influenza A(H3N2) virus.Citation19 Similarly, the European I-MOVE (Influenza Monitoring Vaccine Effectiveness) network, established to monitor seasonal and pandemic influenza VE in the European Union and European Economic Area, reported that in the 2008–2009 season VE of TIV against laboratory-confirmed influenza in community-dwelling elderly was 65.4% in subjects 65–74 year-old,Citation20 supporting the notion that advanced age does not significantly affect VE.
Most commonly used animal models of influenza infection, ferrets and mice, have not been able so far to help reconcile the differences between reduced antibody response to influenza vaccines in human elderly and comparable VE in the elderly and younger individuals. Mice have been used rather extensively to study the effect of aging on influenza pathogenesis.Citation21–25 However, in influenza vaccine studies, both HAI responses and protection against influenza disease in aged mice are reduced compared to young animals,Citation21–24 which contradicts findings in humans summarized above. Influenza infection studies in mice are usually carried out as lethal challenge with mouse-adapted influenza strains, precluding assessment of antiviral efficacy against naturally-circulating influenza viruses. The use of adapted strains is necessitated by the lack of human-like repertoire of sialic acid receptors in common laboratory strains of mice.Citation26,Citation27 In contrast, ferrets have sialic acid receptor repertoire similar to that of humans and are susceptible to infection with unadapted influenza viruses.Citation28 Young ferrets are invaluable for influenza pathogenesis and transmission studies.Citation29,Citation30 These animals are widely used for serological assessment of a match between influenza vaccines and circulating viral strains.Citation31–33 However, the use of these animals for vaccine efficacy studies is complicated, as ferrets are outbred, and a significant variability exists in regards to influenza disease severity, particularly in the aged animals.Citation34 Inbred cotton rats Sigmodon hispidus are susceptible to infection with unadapted human influenza viruses and carry a human-like repertoire of sialic acid receptors.Citation35–37 Efficacy of human influenza vaccines and therapeutics has been confirmed in the cotton rat model.Citation38,Citation39 Cotton rats accurately reproduce differences between mild disease caused by seasonal influenza and morbid disease caused by highly pathogenic influenza viruses or influenza-bacterial coinfections.Citation37,Citation40 These animals reproduce the effect of advanced age and immunosenescence on respiratory viral infections.Citation41–44 In this study, we compared antibody response to and efficacy of inactivated split-virion influenza vaccine FluLaval in young and aged cotton rats Sigmodon hispidus. Cotton rats over 9 months of age were used to model human elderly, based on the earlier demonstration of immunosenescent antiviral responses in animals of that age.Citation41–44 Results of this work allow us to reconcile some of the seemingly contradictory findings of reduced immunogenicity and comparable efficacy of influenza vaccines in the elderly.
Materials and methods
Animals
Inbred cotton rats Sigmodon hispidus were obtained from a colony maintained at Sigmovir Biosystems, Inc. Animals were housed in large polycarbonate cages and were fed a standard diet of rodent chow and water. The colony was monitored for antibodies to adventitious respiratory viruses and other common rodent pathogens, and no such antibodies were found. All studies were conducted under applicable laws and guidelines and after approval from the Sigmovir Biosystems, Inc. Institutional Animal Care and Use Committee (IACUC).
Viruses
The seed of influenza A/California/07/2009(H1N1) (A/California) was obtained from the CDC and grown in eggs in house. The stocks of virus containing 2 × 108 TCID50/ml and 2 × 106 TCID50/ml on MDCK cells were used for the studies on FluLaval 2016–2017 and FluLaval 2012–2013, respectively. The egg-grown stock of influenza A/Victoria/361/2011 (H3N2) (A/Victoria) used in preliminary studies on FluLaval dose-range assessment was kindly provided by the laboratory of Dr. Perez at the University of Maryland (currently at Georgia State University) and had a titer of 104 TCID50/ml on MDCK cells.
Vaccines
FluLaval 2012–2013 and 2016–2017 (GSK) and FluMist 2012–2013 (MedImmune) were obtained from commercial sources at the time of their distribution to public (2012 and 2016, respectively) and used in animal studies within the period prior to the indicated expiration date. FluLaval is a split-virion, inactivated influenza virus vaccine prepared from influenza viruses propagated in the allantoic cavity of embryonated eggs. Each virus is grown individually and inactivated by UV light, followed by formaldehyde treatment, purified by centrifugation, and disrupted with sodium deoxycholate. FluLaval 2012–2013 was a trivalent vaccine that contained the following 3 strains (in the amount corresponding to 15 μg of hemagglutinin (HA) of each strain, or 45 μg total HA, in each 0.5 ml vaccine dose): A/California/7/2009 NYMC X-179A (H1N1), A/Victoria/361/2011 IVR-165 (H3N2), and B/Hubei-Wujiagang/158/2009 NYMC BX-39 (a B/Wisconsin/1/2010-like virus). FluLaval 2016–2017 was a quadrivalent vaccine that contained the following 4 strains (in the amount corresponding to 15 μg of HA of each strain, or 60 μg total HA, in each 0.5 ml vaccine dose): A/California/7/2009 NYMC X-179A (H1N1), A/Hong Kong/4801/2014 (H3N2) NYMC X-263B, B/Phuket/3073/2013, and B/Brisbane/60/2008. Both FluLaval 2012–2013 and 2016–2017 formulations included thimerosal as a preservative. FluMist 2012–2013 contained live attenuated influenza virus reassortants of each of the following 3 strains: A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010. Each 0.2 mL FluMist vaccine dose contained 106.5−7.5 FFU (fluorescent focus units) of each influenza strain.
Animal studies
For studies on FluLaval dose-range optimization, young (6–8 weeks old) cotton rats were immunized intramuscularly (i.m.) with FluLaval 2012–2013 at 0.005–0.3 μg HA (each strain) per 100 μl/100 g animal and boosted with the same formulation three weeks later. Three weeks after the boost, serum was collected from all animals for HAI and IgG ELISA assay. An additional group of animals was infected intranasally (i.n.) with influenza A/Victoria (103 TCID50 per animal), and blood was collected 6 weeks later. Unimmunized and uninfected animals were used as negative controls.
For comparative immunogenicity studies of influenza vaccines in cotton rats, young (6–8 weeks old) and aged (≥9 months old) animals were immunized i.m. with FluLaval 2012–13 (during 2012–2013 season) or 2016–2017 (during 2016–2017 season) at 0.02 or 0.3 μg HA per 100 μl/100 g animal and boosted 3 weeks later. Blood samples were collected prior to boost and 3 weeks after the boost for analysis of serum HAI titers against influenza A/California, a strain whose antigens were included in both 2012–2013 and 2016–2017 FluLaval, or the corresponding FluLaval formulation itself. Three weeks after the boost, animals were infected with influenza A/California at 105 TCID50 (for FluLaval 2012–2013 studies) or 105 TCID50 (for FluLaval 2015–2016 studies) per 100 μl/100 g animal and sacrificed 1 and 4 days later for analysis of influenza load in the lungs and nose and histopathology in the lungs. The upper lobe of the left lung and nose were collected for viral titration and homogenized in EMEM with 10% sucrose stabilizing media. A lingular lobe of the left lung was used for RNA extraction and qPCR analysis. The right lung was inflated with 10% buffered formalin for histopathology analysis. Control animals were infected with A/California twice with an interval of 3 weeks, or mock-immunized with PBS i.n., infected with A/California once, and sacrificed on the corresponding day(s) after infection. An additional group of animals in the 2012–2013 studies was inoculated i.n. with 1:5 dilution of FluMist 2012–2013 in 100 μl/100 g animal, boosted three weeks later, and infected with A/California in another 3 weeks. Samples were collected for analysis as described above. Studies on one-time immunization included animals vaccinated with FluLaval 2016–2017 once, infected with A/California 3 weeks later, and sacrificed on days 1 and 4 post-infection. Secondary infection control animals for these studies were infected with A/California twice with an interval of 3 weeks and sacrificed on days 1 and 4 after the second infection.
A/California and FluLaval HAI assays
Cotton rat serum samples were diluted 3:1 in receptor destroying enzyme (RDE) II (Seiken, Hardy Diagnostics) and incubated at 37°C for 20 hours. Samples were heat inactivated at 56°C for 30 minutes, further diluted with 6 volumes of PBS, and frozen at −20°C until the day of the assay. Chicken red blood cells (RBCs) in Alsever’s solution (Lampire Biological Laboratories) were washed with PBS, diluted to 10% in PBS and stored at 4°C. Serum samples were thawed and serially diluted twofold for 10 dilutions, starting with an original dilution of 1:40. Twenty-five μl aliquots of each sera dilution were mixed in duplicates with 25 μl of solution containing 4 HAU A/California or FluLaval and incubated at room temperature for 30 minutes. Fifty μl of 0.5% dilution of chicken RBCs was added to the wells, gently mixed, and incubated 30–60 min at room temperature. Results were read and recorded. The HAI titer of each sample was expressed as the inverse of the last dilution where agglutination was not observed. HAI geometric mean titer ± standard error for all samples in a group per time point was calculated. Limit of assay detection (HAI = 40) was used as a baseline for evaluating seroconversion which was defined as a fourfold increase in HAI titer.
FluLaval ELISA for binding IgG
Ninety-six well microtiter plates were coated with FluLaval, 1:250 dilution in Coating Solution (KPL) overnight at 4°C. After blocking with a blocking solution (KPL) for one hour at room temperature, serum samples diluted 1:1,500 in diluent solution (KPL) were loaded in triplicates, and incubated for one hour at room temperature. After removal of samples and washing, rabbit anti-cotton rat IgG (1:4,000) was added to all the wells and incubated for one hour at room temperature. Goat anti-rabbit IgG-HRP (Chemicon International, 1:6,000) was used as the secondary antibody, with the incubation for one hour at room temperature, followed by the addition of TMB substrate (KPL) for 15 minutes at room temperature. TMB-Stop solution (KPL) was added and OD450 was immediately measured. Geometric mean of the optical density was calculated for the triplicate sera samples ± standard error for all samples in a group per time point.
High mobility group box 1 (HMGB1) ELISA
HMGB1 levels were measured in the serum of animals using a commercially available ELISA kit according to the manufacturer’s instructions (IBL International, Canada), as previously described.Citation45
Histopathology
Lungs were prepared for histopathology analysis as previously described.Citation46 Each lung section was analyzed for one of the four parameters of pulmonary inflammatory changes: peribronchiolitis (inflammatory cells surrounding a bronchiole), perivasculitis (inflammatory cells surrounding a small blood vessel), alveolitis (inflammatory cells within alveolar spaces), and interstitial pneumonitis (increased thickness of alveolar walls associated with inflammatory cells).Citation46
qRT-PCR
Total RNA was extracted from homogenized lung tissue using the RNeasy purification kit (QIAGEN). One μg of total RNA was used to prepare cDNA using oligo dT primers and Super Script II RT (Invitrogen). qPCR for influenza M gene was run using the following primers: forward 5ʹ GCAGAGACTTGAAGATGTCTTTGC 3ʹ, reverse 5ʹ GGGCATTTTGGACAAAGCGTCTAC 3ʹ, under conditions previously described.Citation47 Influenza M expression level was normalized by the level of β-actin (“housekeeping gene”) expressed in the corresponding organ and presented as the geometric mean ± the standard error (SEM) for all animals in a group at a given time.
Statistical analysis
Data were analyzed using 2-way ANOVA followed by the Tukey post hoc test. A value of p< .05 was considered to be significant.
Results
Dose-range optimization and efficacy of FluLaval in the cotton rat model
To determine optimum conditions for comparing effectiveness of parenteral influenza vaccines in animals of different ages, dose-range studies were carried out using FluLaval from the 2012–2013 season (). Young (6–8 weeks old) cotton rats were immunized with FluLaval in doses from 0.005 to 0.3 μg FluLaval per 100 g animal (). This range included a FluLaval dose of 0.02 μg HA that would correspond to a standard human dose of 15 μg HA per immunization (based on the weight difference between an average 100 g cotton rat and an average 70 kg person). A fourfold lower dose of HA (0.005 μg), and two fourfold higher doses of HA (0.08 and 0.3 μg) were tested as well. Cotton rats were boosted with the same vaccine dose on day 21, and blood was collected three weeks later for analysis of HAI or binding IgG against the vaccine formulation. Control naïve animals were infected with influenza A/Victoria (H3N2 influenza strain whose antigens were included in the 2012–2013 vaccine formulation) and blood was collected for analysis 6 weeks later. Infection with H3N2 influenza A/Victoria was used as a control for these earlier studies because the choice of the influenza challenge strain for subsequent studies (H1N1 influenza A/California) had not been finalized at that time. Additional control animals were left uninfected and unvaccinated.
Figure 1. Dose-optimization and efficacy studies using the 2012–2013 influenza vaccine formulations in cotton rats. (a,b) Dose–range study of immunogenicity of FluLaval 2012–2013 during the corresponding vaccine season (VSeason: 2012–2013). Young animals were immunized intramuscularly (i.m.) twice (with 3 weeks in between) with the indicated doses of FluLaval or infected intranasally (i.n.) once with influenza A/Victoria (H3N2) (Vict) and blood was collected for analysis of serum HAI titers (a) or binding IgG (b) against FluLaval 2012–2013 three weeks after the last immunization or 6 weeks after A/Victoria infection. Results represent GMT±SE for 8–10 animals per group. Negative control animals (Ctr) were unimmunized and uninfected. (c) Efficacy of FluLaval 2012–2013 and FluMist 2012–2013 in the young and aged cotton rats. Animals immunized once with FluLaval or FluMist were infected i.n. with A/California (A/Cal) three weeks later and sacrificed 1 day later for analysis of influenza load in the lung by qPCR. Control animals were infected with A/Cal and re-infected 3 weeks later (secondary infection, °2) or mock immunized and infected with A/Cal (primary infection, °1). Results represent GMT±SE for 5 animals per group. *p< .05 when compared to influenza M mRNA level in the same age animals with primary infection. No significant differences were found between influenza M mRNA levels in animals of different ages treated the same way
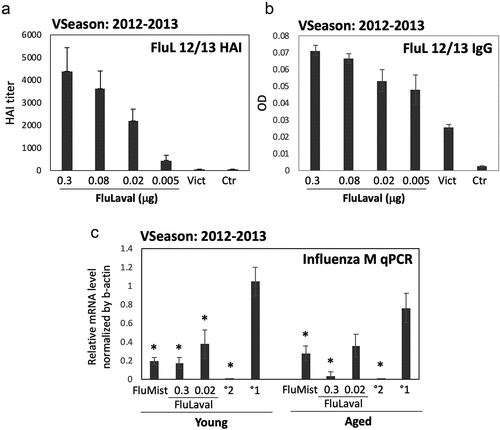
FluLaval immunization was accompanied by a strong induction of binding IgG and HAI against the vaccine formulation (). Vaccine antigens can be used as hemagglutinating antigens for the HAI test.Citation48 Split vaccines represent a good source of functional HA for HAI assay and allow for assessment of HAI response to several vaccine strains simultaneously.Citation49 The response to FluLaval was dose-dependent, with 0.3 μg and 0.02 μg of FluLaval inducing the strongest and intermediate HAI titer, respectively. Serum of animals infected intranasally with A/Victoria demonstrated the presence of IgG reactive with FluLaval 2012–2013 (), but no HAI response against FluLaval 2012–2013 was found (). The latter may reflect diversity of binding and HAI antibody response generated by natural influenza infection and/or stricter requirement for preservation of antigens for HAI vs. binding IgG assays.Citation48
To determine whether there are any age-related differences in antiviral efficacy, young and aged cotton rats were immunized with FluLaval 2012–2013 at 0.3 μg or 0.02 μg once and challenged with influenza A/California (H1N1 influenza strain whose antigens were included in the 2012–2013 vaccine formulation) three weeks later (). Controls included animals infected and then challenged with A/California three weeks after the original infection (secondary infection control), and mock-immunized animals that were challenged with A/California once (primary infection controls). Animals immunized intranasally with FluMist 2012–2013 once and challenged with A/California three weeks later were also included in the study. Viral presence in the lung was evaluated by RT-PCR analysis of influenza M mRNA expression. FluMist significantly reduced influenza gene expression in the lungs of both young and aged animals (). The higher FluLaval dose also significantly inhibited influenza mRNA load in the lungs of both young and aged animals. The lower vaccine dose reduced influenza mRNA level, but the inhibition was less and the difference was significant only for the young animals. Overall, these studies demonstrated that the FluLaval doses of 0.3 and 0.02 μg per 100 g animal were appropriate for the comparative immunogenicity and efficacy studies in the young and aged cotton rats.
Efficacy of FluLaval from another influenza season (2016–2017) was evaluated next with regard to protection of both the lower and upper respiratory tracts against influenza challenge (). The same influenza A H1N1 strain (A/California) was included in both 2012–2013 and 2016–2017 formulations that allowed for a more direct comparison between the two FluLaval vaccines in the cotton rat model. Animals were immunized with FluLaval 2016–2017 once or twice as described above, challenged with influenza A/California 3 weeks after the last immunization and sacrificed for analysis of viral load in the lungs and nose on day 1 post-infection.
Figure 2. Antiviral efficacy of FluLaval 2016–2017 in the young and aged cotton rats when given as a two-times (a) vs. one-time (b) immunization. (a) Two-times vaccination. Young and aged cotton rats were immunized with the indicated doses of FluLaval (FluL) 2016–2017 and boosted 3 weeks later. After another 3 weeks animals were infected i.n. with influenza A/California (A/Cal) at 107 TCID50 per 100 g animal and sacrificed one day later for analysis of viral load in the lung and nose. Results of viral titration by TCID50 assay (VT) are shown. Control animals were infected i.n. with A/Cal and re-infected 6 weeks later (°2) or mock immunized and infected with A/Cal (°1). (b) One-time vaccination. Young and aged cotton rats were immunized with FluLaval once, infected i.n. with A/Cal 3 weeks later, and sacrificed 1 day after infection. Control animals were mock-immunized with PBS and infected i.n. with A/Cal once (°1) or twice with an interval of 3 weeks (°2) and sacrificed one day after challenge. Unimmunized and uninfected animals were included as additional controls (“-“). Results represent GMT±SE for 5–6 animals per group. *p < .05 when compared to primary infection of the same age animals, #p < .05 when compared to the same treatment, opposite age
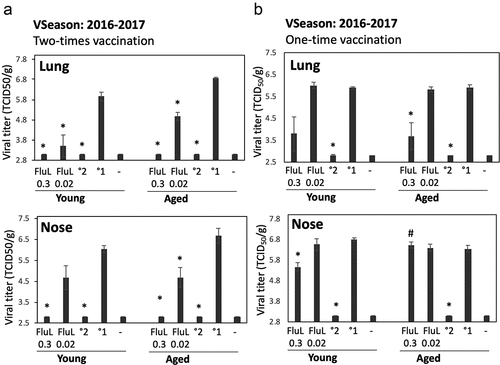
The two-times immunization with FluLaval 2016–2017 was highly efficacious in the lungs of both young and aged animals as determined by viral titration (). Both high and low doses of vaccine inhibited influenza replication in both age groups, with protection being stronger for the higher vaccine dose. In the nose, the higher dose of FluLaval reduced viral load to undetectable levels in both young and aged animals. The lower vaccine dose was less protective, but viral replication was reduced in both the aged and young animals to a comparable extent.
The one-time immunization with FluLaval was less efficacious than the two-times immunization (compare ). The degree of lung protection afforded by the one-time immunization, however, was comparable between the young and aged animals, with the lower vaccine dose providing no protection, and the higher vaccine dose inducing moderate protection of the lungs in animals of both age categories (). Age-related differences became visible with regards to nasal protection. A moderate, but significant reduction of nasal viral load was seen in young animals vaccinated once with a high vaccine dose, but not in the aged cotton rats. The lower vaccine dose did not protect the noses of either young or aged cotton rats.
Overall, the two doses of FluLaval tested had significant antiviral efficacy in both young and aged cotton rats when administered as a two-times immunization, with the higher vaccine dose reducing viral load to undetectable in both lungs and noses of infected animals. The one-time immunization was less efficacious in both young and aged cotton rats, with some age-related differences apparent with regards to nasal protection.
Antibody response to FluLaval in the young and aged cotton rats
Side-by-side comparison of antibody response to FluLaval 2012–2013 and 2016–2017 formulations in the cotton rat model was conducted using 0.3 μg and 0.02 μg vaccine doses (). Young and aged cotton rats were immunized with FluLaval 2012–2013 () or 2016–2017 () twice and blood was collected for serum antibody analysis three weeks after the first and the second immunization. Control animals were infected with influenza A/California or left uninfected/unvaccinated. Additional animals in the 2012–2013 vaccine study were also inoculated twice intranasally with FluMist 2012–2013. FluMist induced a minimal, but significant increase in HAI titer in the young cotton rats after the first immunization and in the aged animals after the second immunization ().
Figure 3. Immunogenicity of seasonal influenza vaccines in the young and aged cotton rats. (a) Immunogenicity of FluLaval 2012–2013 and FluMist 2012–2013 in the young and aged cotton rats. Animals were immunized with the indicated doses of FluLaval 2012–2013 i.m. or with FluMist 2012–2013 i.n. during the corresponding vaccine season and boosted 3 weeks later. Control animals were infected i.n. with influenza A/California (Cal). Serum samples were collected prior to boost and three weeks after the boost for analysis of HAI titers against A/Cal. Results represent GMT±SE for 11–15 animals per group. Negative control animals (Ctr) were unimmunized and uninfected. (b,c) Immunogenicity of FluLaval (FluL) 2016–2017 in the young and aged cotton rats. Animals were immunized as described above but using the FluLaval 2016–2017 formulation during the corresponding vaccine season. Control animals were infected with influenza A/California or left unimmunized and uninfected (Ctr). Blood samples were collected for analysis of serum HAI titers against A/Cal (b) or FluLaval 2016–2017 (c). Results represent GMT±SE for 9–12 animals per group (except for Ctr group, where 5 samples were included). *p< .05 when compared to titers in the same age animals with primary infection, same day; & p< .05 when compared to d21 samples collected after the same dose vaccination for the same age animals; # p< .05 when compared to young animals, same vaccine, dose, and day of collection; ⊓ (connector) p< .05 when compared to the lower vaccine dose in the same age animals, same day of blood collection
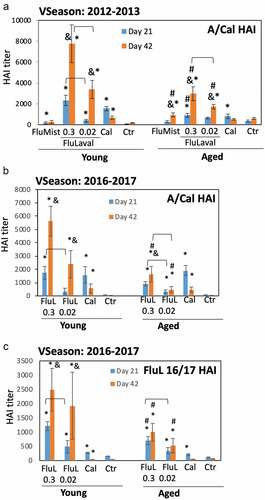
Immunization with the higher dose of FluLaval induced a significant increase in HAI titers against A/California in both young and aged animals after the first vaccine dose, with the response further increasing after the boosting (). Higher HAI titers were induced in the animals by the higher vs. lower vaccine dose after either the initial or the booster immunization. The HAI titers induced by FluLaval immunization in the aged animals were significantly lower than those in the young cotton rats. Infection with A/California significantly increased HAI levels three weeks after challenge in both age groups, although titers decreased by week 6. The magnitude of A/California HAI response to FluLaval 2016–2017 vaccination was slightly lower than to FluLaval 2012–2013 for both age categories (compare ).
Immunogenicity of FluLaval 2016–2017 in young and aged cotton rats was also evaluated in regards to HAI titers against the vaccine formulation (). Both young and aged animals showed significant rise in FluLaval HAI titers three weeks after the first immunization (). The level of FluLaval HAI response in the aged animals was lower for the higher vaccine dose compared to the young animals. Administration of a second vaccine dose significantly boosted FluLaval HAI titers in the young, but not in the aged cotton rats. Day 42 FluLaval HAI titers were lower for both vaccine doses in the aged compared to the young animals. I.n. A/California infection caused a significant increase in serum FluLaval HAI titers three weeks after immunization which declined by week 6. HAI titers remained significantly elevated in the young, but not aged animals at 6 weeks post-infection. Overall, these results demonstrate that immunogenicity of both FluLaval 2012–2013 and 2016–2017 formulations in regards to antibody induction was significantly lower in the aged cotton rats compared to the young animals.
Seroconversion among young and aged cotton rats
The percentage of animals that showed fourfold or higher increase in HAI titer against FluLaval 2016–2017 or A/California was calculated and the data are presented in as seroconversion. Seroconversion was defined as fourfold rise in HAI titers. As all animals were naïve at the beginning of the study, seroconversion was evaluated against seronegative baseline, similar to the methodology used for assessment of seroconversion induced by influenza vaccines in seronegative children.Citation50 In the FluLaval HAI assay, 100% of young animals seroconverted after the first dose of 0.3 μg FluLaval, and the number remained at 100% after the second dose (). For the lower vaccine dose (0.02 μg FluLaval), 50% of young animals seroconverted after the first vaccine dose and the number rose to 100% after the second dose. The number of seroconverted animals was lower in the aged group. Sixty-seven (67) percent of aged animals seroconverted after the first dose of 0.3 μg FluLaval, and almost all animals (92%, or 11/12) seroconverted after the second dose. The percentage of seroconverted aged animals in the group immunized with 0.02 μg FluLaval were 42% and 75%, respectively. No animals seroconverted in the FluLaval HAI assay after A/California infection in either age category.
Table 1. Seroconversion of young and aged animals by FluLaval 2016–2017. Seroconversion was defined as an increase in HAI GMT of fourfold or higher over the lower limit of assay (HAI = 40). Percentage of seroconverted animals (if greater than 0) is given in parenthesis
In the A/California HAI assay, 70% and 100% of young animals seroconverted after the first and the second dose of 0.3 μg FluLaval, respectively, and 10% and 100% young animals seroconverted after the first and the second dose of 0.02 μg FluLaval, respectively (). Among the aged animals, 42% and 100% of animals seroconverted after the first and second dose of 0.3 μg FluLaval, respectively, and 0% and 92% of aged animals seroconverted after the first and second dose of 0.02 μg FluLaval, respectively. Eighty to 90% of young animals and 91–100% of aged animals showed seroconversion 3 and 6 weeks after A/California infection, respectively. Overall, these results showed that in the aged animals seroconversion rates were lower than in the young rats for any vaccine dose tested. The effect of age was more pronounced for the FluLaval HAI compared to A/California HAI assay. There was no age dependent difference in seroconversion in response to A/California infection.
Effect of FluLaval immunization on pulmonary pathology in young and aged cotton rats
Influenza infection in cotton rats induces a strong pulmonary inflammatory response characterized by increased peribronchiolitis, perivasculitis, interstitial inflammation, and alveolitis. In naïve animals, lung pathology is stronger on day 4 post-infection compared to day 1, while in animals pre-exposed to influenza lung pathology is already strong after one day of infection and remains elevated on day 4 ().
Figure 4. Lung histopathology in the young and aged animals immunized with FluLaval 2016–2017 twice (a) or once (b). Animals were immunized or infected as described in the legend to and sacrificed 1 or 4 days after infection for analysis of pulmonary histopathology. (a) Pulmonary histopathology in influenza-infected animals after two-times immunization with FluLaval or 6 weeks after the initial infection with A/Cal. (b) Pulmonary histopathology in influenza-infected animals after one-time immunization with FluLaval or 3 weeks after the initial infection with A/Cal. Results represent GMT±SE for 5–6 animals per group. PB: peribronchiolitis, PV: perivasculitis, IP: interstitial pneumonitis, A: alveolitis. * p < .05: a decrease compared to primary infection of the same age animals; x p < .05: a decrease compared to primary infection of the same age animals; Δ p < .05: an increase compared to primary infection of the same age animals; @ p < .05: an increase compared to primary infection of the same age animals
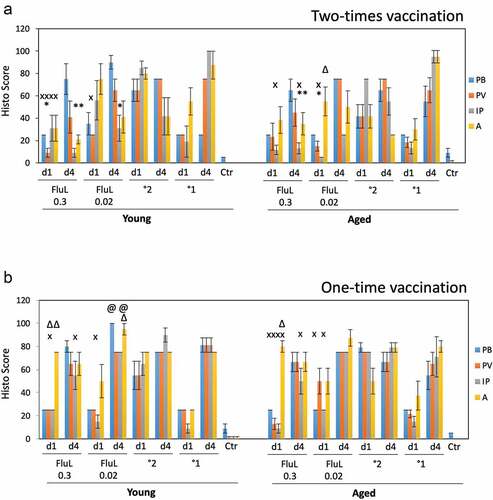
Pulmonary histopathology was evaluated in young and aged animals immunized with FluLaval 2016–2017 twice or once and infected i.n. with A/California (). In the two-times immunization study (), 0.3 μg FluLaval reduced multiple parameters of pulmonary inflammation compared to either primary or secondary influenza infection for both young and aged cotton rats. The lower vaccine dose also reduced some parameters of inflammation, but the effect was less than what was seen for the higher vaccine dose. Alveolitis in the aged animals immunized with 0.02 μg FluLaval was increased on day 1 post-infection (alveolitis score of 55) compared to animals with primary influenza infection (alveolitis score of 30).
The effect of the one-time immunization with FluLaval on pulmonary pathology () was different from the effect of the two-time immunization. No parameters of inflammation were decreased in comparison to primary infection. In fact, multiple parameters of inflammation were increased in the vaccinated animals that received only one vaccine dose. The increases were evident in comparison to both primary and secondary infection and were present primarily among young animals. Overall, these results suggest that one-time immunization of naïve cotton rats with TIV may result in an increased pulmonary inflammation upon virus encounter, with the effect being more pronounced in the younger compared to older animals.
HMGB as the serum marker of immunization efficacy
In our previous work we showed that influenza infection in cotton rats is accompanied by an increase in serum HMGB1,Citation45 a protein previously shown to be a marker of systemic inflammation. To evaluate whether the circulating HMGB1 can serve as a marker of influenza vaccine efficacy, HMGB1 ELISA was run on serum samples of aged and young animals immunized twice with FluLaval 2016–2017 at two different doses and challenged with A/California (). Serum samples from young animals immunized twice with FluLaval 2012–2013 and infected with A/California were included in the same analysis.
Figure 5. Serum HMGB1 levels in cotton rats immunized with FluLaval and challenged with A/California. Young and aged animals were immunized with the indicated doses of FluLaval 2016–2017 (FluL) twice with an interval of 3 weeks and infected with influenza A/Cal 3 weeks after the second immunization. Control animals were infected with A/Cal and re-infected 6 weeks later (secondary infection, °2) or mock immunized and infected with A/Cal (primary infection, °1). Serum from a group of young animals immunized in a similar manner with FluLaval 2012–2013 (FluL 12/13) and challenged with A/Cal was included in the analysis. Serum was obtained on day 4 post-challenge. Results represent GMT±SE for 5–6 animals per group. *p < .05 when compared to HMGB1 levels in serum of the same-age animals with primary infection
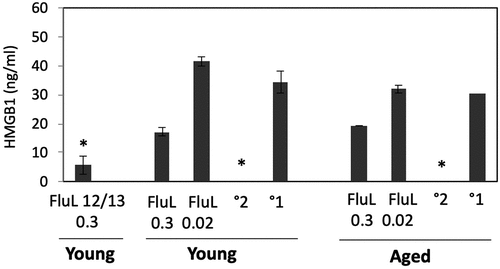
HMGB1 level was significantly reduced in both young and aged animals with secondary influenza infection (). HMGB1 was also reduced in both young and aged animals vaccinated with the higher dose of FluLaval 2016–2017, albeit the difference did not reach statistical significance for either age. Immunization with FluLaval 2012–2013 in the same dose, however, caused significant reduction in serum HMGB1 level in young cotton rats.
Discussion
The goal of this work was to evaluate the effect of advanced age on immune responses to influenza vaccination and vaccine effectiveness, while expanding on our earlier observation that cotton rats can be used for human influenza vaccines evaluation and that trivalent inactivated vaccines are efficacious in the model.Citation51,Citation52 Another important goal was to reconcile seemingly contradictory findings of reduced antibody response to and potent effectiveness of TIVs in human elderly over 65 years of age. Included in this was the evaluation of cotton rat responses to different doses of TIV, as increasing vaccine dose was shown to improve immunogenicity of TIV in human elderlyCitation10,Citation11 and reduce incidence of respiratory illness-related hospital admissions.Citation53 In this work, it was possible to test influenza vaccine efficacy against challenge with an unadapted seasonal influenza virus (H1N1 A/California) whose antigens were included in both 2012–2013 and 2016–2017 vaccine preparations, as no adaptation of influenza viruses to cotton rats is required for infection of these animals and replication of viruses in the respiratory tract.Citation36,Citation37,Citation39 Vaccines were purchased from a commercial distributor, while virus was obtained from the CDC and propagated in-house. The expedience of the process allowed for testing of current seasonal influenza vaccine against challenge with a predicted circulating influenza strain. Results of this work demonstrated that while antibody response to vaccination was reduced in old cotton rats, TIV given in a prime-boost regime exhibited comparable ability to protect the respiratory tract against influenza replication in both age categories.
Observational studies run by the U.S. Flu VE and European I-MOVE networks compare odds of influenza vaccination in outpatients with acute respiratory infections (ARI) who test positive for laboratory-confirmed influenza to the odds of influenza vaccination in outpatients with ARI who test negative for influenza. While highly informative, these studies assess vaccine efficacy only in people who already display signs of ARI and seek outpatient help. The randomized placebo-controlled clinical trials (RCTs) may provide a better estimate of vaccine efficacy with the proper selection of endpoints. RCTs of influenza vaccines in the elderly, however, are scarce due to the ethical considerations of the recommendation to vaccinate all elderly against influenza. Nevertheless, the meta-analysis of combined results of available RCTs and observational studies in the elderly also determined comparable efficacy of parenteral inactivated vaccines against laboratory-confirmed cases in healthy adults (60%) and the elderly (58%).Citation53 The effectiveness of vaccines at preventing clinically confirmed cases was lower for both age categories, with 24% reported for the elderly and 19–22% for adults.Citation53 The only large RCT of purified split virion influenza vaccine conducted exclusively in the elderly took place during the winter of 1991–1992 in the Netherlands and involved 1,838 individuals ≥60 years old.Citation54 That study showed 58% risk reduction against clinical serologically confirmed influenza in the elderly, in line with the 60% estimate for younger adults.Citation53 Overall, it appears that influenza vaccines may show comparable effectiveness among adults and elderly, but these results have been difficult to reconcile until now with multiple reports of reduced antibody response to influenza vaccines in individuals ≥65 years old.
Our results, obtained in the cotton rat model, show that the antibody response to FluLaval (as indicated by HAI titers) is significantly reduced in the aged animals compared to the young ones. The effect was seen for FluLaval formulations from two different seasons: 2012–2013 and 2016–2017 and therefore must represent a general phenomenon. Both the GMTs and seroconversion rates were reduced in the aged rats. The GMTs of HAI against A/California were significantly lower in the aged animals vaccinated with either vaccine dose. In the HAI assay against FluLaval 2016–2017, aged animals mounted significantly lower HAI GMTs in response to the 0.3 μg dose, and were not able to boost antibody response as efficiently as young animals after the second immunization. Seroconversion rates of aged rats in the FluL 2016–2017 HAI assay were lower than that in young animals. The effect of age was less evident in A/California HAI assay, suggesting that the choice of assay may influence the results of seroconversion analysis. Since all animals were naïve at the beginning of the study, seroconversion was evaluated against a negative baseline level (assay limit of detection), so results should be interpreted with caution. Nevertheless, significant increase in antibody response to vaccination was seen for the higher compared to the lower vaccine dose after the first immunization, present in both aged and young animals. Even though the difference between the higher and lower vaccine doses tested here (16-fold) exceeds the fourfold difference between the high-dose and standard TIV vaccine, these results experimentally confirm the observation that immunogenicity of TIV in the aged population can be boosted with the use of a higher vaccine dose.Citation10,Citation11
Interestingly, we have detected differences in reactivity of sera induced by inactivated influenza vaccines and seasonal strains of influenza included in vaccine formulations. Thus, serum of animals infected with influenza H3N2 A/Victoria did not show HAI response against FluLaval 2012–2013 () and A/California HAI response to FluLaval 2016–2017 formulation was lower than the response to FluLaval 2012–2013 formulation (). These differences could be due to the changes in vaccine viruses acquired during virus propagation in eggs. Large-scale propagation of viruses in embryonated eggs were shown to lead occasionally to influenza mutations that cause antigenic mismatch.Citation55,Citation56 Both 2012–2013 and 2016–2017 vaccines included viruses that had egg-adapted mutationsCitation56,Citation57 and could explain some of the differences observed in regards to HAI assays performed using A/California and FluLaval as antigens. It also shows that the cotton rat model could be valuable for assessing antigenic match between seasonal influenza vaccines and circulating strains of virus, similar to the way ferrets are used for the task.Citation31–33 Recent studies, however, show that ferrets that have been influenza-primed may be better suited for serological assessment of human influenza vaccine efficacy than naïve ferrets.Citation32,Citation33 Similarly, influenza-primed cotton rats may provide a better model for serologic assessment of vaccine match than naïve animals, but further studies on the subject are needed.
Efficacy of vaccination against influenza replication in the lungs and noses of aged and young cotton rats was evaluated as a part of this work. Our data showed that FluMist 2012–2013 significantly reduced influenza load in the lungs of both young and aged animals, potentially reflecting comparable antiviral efficacy reported for FluMist in the young and elderly humans.Citation53,Citation58 While FluMist studies in aged animals could not be continued as the vaccine became unavailable at the time, the studies with FluLaval could be carried over two different seasons (2012–2013 and 2016–2017). These studies compared two vaccine doses, one causing high and the other intermediate levels of antibody response in cotton rats. Administered twice, both FluLaval vaccine doses significantly inhibited influenza replication in the lungs of young and aged animals, without differences between the ages. The effect was vaccine dose-dependent. Inhibition to undetectable level was induced in the lungs of young animals vaccinated with 0.3 μg FluLaval, consistent with our previous report for FluLaval 2006–2007.Citation39 Both doses of FluLaval 2016–2017 tested here (0.3 and 0.02 μg per 100 g weight) suppressed influenza replication in the nose when vaccine was given two times. One-time immunization with FluLaval showed lower efficacy for either vaccine dose in both age groups. The dose-dependent effect was still seen for the lung protection, with the higher vaccine dose being more efficacious. The biggest difference in regards to age was noted in nasal protection in infected animals vaccinated with FluLaval once. The higher vaccine dose was still capable of moderately, yet significantly, reducing nasal viral load in the nose of young, but not aged animals. Immunity of the upper respiratory tract of aged animals with respect to influenza vaccination needs to be explored in more detail in the future. Boosting of immune responses can be more important for the aged in order to achieve sufficient nasal protection against influenza infection. Although the situation of one-time immunization of naïve aged animals may not be a realistic scenario for human elderly with multiple influenza exposures, this effect can have important implications for protection of the upper respiratory tract of the elderly during the seasons of poor match between the vaccine and circulating strains.
The effect of vaccination on pulmonary pathology was different from the effect on viral replication. While the two-time immunization with 0.3 μg FluLaval conferred strong immunity in the lung of young and aged animals, pulmonary pathology was not completely eliminated. There was a significant reduction in multiple parameters of inflammation compared to primary and secondary infection, although the effect was less obvious for the lower vaccine dose. One-time immunization with FluLaval had a smaller pathology-reducing ability. In fact, several parameters of inflammation were increased in vaccinated animals, particularly in the young cotton rats. Increases in lung pathology were seen in vaccinated animals in comparison to both primary and secondary influenza infection, suggesting that pathology may be increased when intermediate level of immunity against influenza is induced by a vaccine in naïve individuals, mostly children. This finding can be particularly important in light of existing concern over the potential harm of seasonal influenza vaccines in otherwise healthy children.Citation53 The interesting parallel with our model could be the relative difference in potential harm of seasonal influenza vaccines in children versus older adults, and the fact that children may be influenza-naïve at the time of the first seasonal influenza vaccine administration, like young animals in the one-time immunization studies.
The question still remains as to what should be used as the marker(s) of influenza vaccine effectiveness in animal models that can translate to a successful outcome in humans. Relevance of serologic correlates of protection obtained from naïve animal models has been questioned by recent studies in naïve vs influenza-primed ferrets.Citation32,Citation33 The matter is further complicated by the fact that antigenic changes acquired by vaccine strains during manufacturing discussed above may influence HAI readouts. The age of vaccinated animals may present another complication. While it may appear that a titer of ~1,000 or higher correlates with a certain degree of nasal protection in young and aged cotton rats (), the aged animals vaccinated twice with the low dose of FluLaval do not achieve that level of HAI response even after boosting, yet significant nasal and lung protection is evident. Some alternative markers of disease (e.g., HMGB1) may be better reflective of disease severity in the influenza model. Immunization of cotton rats with FluLaval 2012–2013 appeared to be more effective at inducing HAI titers and reducing serum HMGB1 levels than immunization with FluLaval 2016–2017. Interestingly, effectiveness of FluLaval 2012–2013 formulation against influenza A or B viruses in humans surpassed that of FluLaval 2016–2017 formulation, according to the CDC 2016–2017 report.Citation3 The stronger inhibition of HMGB1 level and increased serum HAI response induced by 2012–2013 compared to 2016–2017 FluLaval immunization in cotton rats may be reflective of that difference in VE and may suggest that HMGB1 could become a relevant marker of influenza vaccine immunogenicity and effectiveness. The correlation between serum HMGB1 with protection from viral replication might indicate a reduction in the general symptoms of flu infection, as well as highlight a difference between lung damage in influenza-infected humans and lung inflammation as measured by cellular infiltration in the animal models. Further studies on the subject are needed.
The choice of the VE markers in animal models is further complicated by the fact that it is still not clear to what extent well-matched influenza vaccines protect humans against respiratory illness. The clinical overlap between ARI and influenza-like illness (ILI) has been reported.Citation59 The consensus at the moment is that parenteral influenza vaccines are only moderately effective against laboratory confirmed influenza, and that individuals vaccinated against influenza still present with symptoms of ARI (the premise that forms the basis of observational studies conducted by U.S. Flu VE and European I-MOVE networks). This is true for both elderly and younger individuals. If this is the case, and if an animal model is to accurately reproduce moderate effectiveness of influenza vaccines in humans, one should not expect a complete inhibition of viral replication or eradication of disease by a standard vaccine dose in either young or aged model animals. In fact, this was exactly what we saw in this work when the prime-boost immunization model was used. The “standard” dose of FluLaval (corresponding to the 0.02 μg dose) given twice to naïve animals reduced influenza load in the respiratory tract, but did not eliminate it, reflecting moderate antiviral efficacy. The ability of the “standard” dose to reduce pulmonary pathology or serum HMGB1 in cotton rats was limited, and a 16-fold higher vaccine dose was required to reduce these markers. In general, it is believed that efficacy of human seasonal influenza vaccines should be further improved. For pre-pandemic H5N1 vaccines, the current approach is to include vaccine adjuvants (e.g., oil-in-water emulsion) to improve vaccine performance,Citation60,Citation61 in spite of the reports of increased adverse effects. The trade-off between improved protection and relative increase in adverse effects may be justified for pre-pandemic vaccines,Citation60 but is still debatable for seasonal influenza vaccines. Altogether, this indicates that further work is needed to improve seasonal influenza vaccines for different age groups and to define appropriate correlates of immunity and protection. It appears that inhibition of viral replication in the respiratory tract and reduction in pulmonary pathology/systemic markers of inflammation (including serum HMGB1) could be relevant markers of influenza vaccine efficacy in cotton rats S.hispidus.
Several limitations have to be taken into account when interpreting results of this work. First, cotton rats were influenza naïve at the time of the first immunization, which complicates evaluation of both susceptibility and seroconversion. As mentioned above, this situation could be appropriate for influenza-naïve children, but not for adults and elderly who have been infected multiple times. Prime-boost immunization of naïve animals would, to some extent, reflect boosting of preexistent immunity, although vaccination of previously infected animals would represent a better experimental model. Second, only female animals were used for these studies. Choice of females was guided by the fact that all aged animals were retired female breeders and young female animals had to be used as appropriate controls. Additionally, delayed dynamics of respiratory infection and disease in immunosenescent animals needs to be taken into account,Citation34,Citation41-44,Citation62-64 and more numerous time points may need to be included for a more comprehensive analysis of responses with regards to aging. Finally, the estimation of antiviral efficacy in cotton rats was based on quantification of viral load/gene expression in the lung and nose homogenates, not in swabs or washes collected from nasopharyngeal and throat surfaces, as in humans. The latter technique was not used in cotton rats because it is more prone to variation. Nevertheless, potential differences with human samples collected by standard methods need to be considered. In spite of these factors, however, this work is important, as it was able to reproduce some of the seemingly contradictory findings of reduced antibody response induction and yet effective antiviral defense in human elderly. Results presented here may suggest the existence of compensatory mechanisms of defense (e.g., more efficient cellular immune response) that develop with advanced age. These results also suggest that altered and/or incomplete immunity to influenza may be detrimental at any age, resulting in insufficient protection of the respiratory tract in both young and aged, and, perhaps, enhanced pathology in the young.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Charles Smith, Fredy Rivera, Martha Malache, and Ana Rivera for help with the animals.
Additional information
Funding
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi:10.1001/jama.289.2.179. PMID: 12517228
- Schanzer DL, Tam TW, Langley JM, Winchester BT. Influenza-attributable deaths, Canada 1990-1999. Epidemiol Infect. 2007;135(7):1109–16. doi:10.1017/S0950268807007923. PMID: 17306052 PMCID: PMC2870678
- CDC 2016-2017 report. [accessed 2019 Aug 8]. https://www.cdc.gov/flu/professionals/vaccination/ effectiveness-year/2016-2017.html
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427–36. doi:10.1093/cid/cis211. PMID: 22495079 PMCID: PMC3334364
- Langmuir AD, Henderson DA, Serfling RE. The epidemiological basis for the control of influenza. Am J Public Health Nations Health. 1964;54(4):563–71. doi:10.2105/ajph.54.4.563. PMID: 14136320 PMCID: PMC1254817.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 Influenza Season. MMWR Recomm Rep. 2018;67(3):1–20. doi:10.15585/mmwr.rr6703a1. PMID: 30141464 PMCID: PMC6107316
- NVIC (National Vaccine Information Center). What is the history of influenza vaccine use in America? 2019 [accessed 2019 Aug 8]. https://www.nvic.org/vaccines-and-diseases/influenza/vaccine-history.aspx
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. doi:10.1016/j.vaccine.2005.08.105. PMID: 16213065
- Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121(8):3109–19. doi:10.1172/JCI57834. PMID: 21785218 PMCID: PMC3148747
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172–80. doi:10.1086/599790. PMID: 19508159
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–45. doi:10.1056/NEJMoa1315727. PMID: 25119609
- Frey SE, Reyes MR, Reynales H, Bermal NN, Nicolay U, Narasimhan V, Forleo-Neto E, Arora AK. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine. 2014;32(39):5027–34. doi:10.1016/j.vaccine.2014.07.013. PMID: 25045825
- McElhaney JE, Coler RN, Baldwin SL. Immunologic correlates of protection and potential role for adjuvants to improve influenza vaccines in older adults. Expert Rev Vaccines. 2013 Jul;12(7):759–66. doi:10.1586/14760584.2013.811193.
- Trucchi C, Paganino C, Orsi A, De Florentiis D, Ansaldi F. Influenza vaccination in the elderly: why are the overall benefits still hotly debated? J Prev Med Hyg. 2015;56(1):E37–E43. PMID: 26789831 PMCID: PMC4718343
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, Petrie JG, Lofthus G, Meece JK, Williams JV, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55(7):951–59. doi:10.1093/cid/cis574. PMID: 22843783 PMCID: PMC3657521
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM, et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529–40. doi:10.1093/infdis/jiu647. PMID: 25406334 PMCID: PMC4407759
- Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP, et al. Influenza vaccine effectiveness against 2009 Pandemic Influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis. 2016;213(10):1546–56. doi:10.1093/infdis/jiv577. PMID: 26743842 PMCID: PMC4837903
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. doi:10.1016/S1473-3099(16)00129-8. PMID: 27061888
- Russell K, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018;36(10):1272–78. doi:10.1016/j.vaccine.2018.01.045. PMID: 29402578 PMCID: PMC5812289
- Kissling E, Valenciano M, Falcao J, Larrauri A, Widgren K, Pitigoi D, Oroszi B, Nunes B, Savulescu C, Mazick A, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008-9. Euro Surveill. 2009;14(44):pii: 19388. Erratum in: Euro Surveill. 2009;14(45)pii: 19399. PMID: 19941774
- Mbawuike IN, Wyde PR, Anderson PM. Enhancement of the protective efficacy of inactivated influenza A virus vaccine in aged mice by IL-2 liposomes. Vaccine. 1990 Aug;8(4):347–52. doi:10.1016/0264-410X(90)90093-2. PMID: 1697721.
- McDonald JU, Zhong Z, Groves HT, Tregoning JS. Inflammatory responses to influenza vaccination at the extremes of age. Immunology. 2017 Aug;151(4):451–63. doi:10.1111/imm.12742. PMID: 28375554 PMCID: PMC5506419.
- Baldwin SL, Hsu FC, Van Hoeven N, Gage E, Granger B, Guderian JA, Larsen SE, Lorenzo EC, Haynes L, Reed SG, et al. Improved immune responses in young and aged mice with adjuvanted vaccines against H1N1 influenza infection. Front Immunol. 2018 Feb 19;9:295. doi:10.3389/fimmu.2018.00295. PMID: 29515589 PMCID: PMC5826078.
- Vassilieva EV, Taylor DW, Compans RW. Combination of STING pathway agonist with saponin is an effective adjuvant in immunosenescent mice. Front Immunol. 2019 Dec 23;10:3006. doi:10.3389/fimmu.2019.03006. eCollection 2019.
- Ramirez A, Co M, Mathew A. CpG improves influenza vaccine efficacy in young adult but not aged mice. PLoS One. 2016 Mar 2;11(3):e0150425. doi:10.1371/journal.pone.0150425. PMID: 26934728 PMCID: PMC4774967.
- Gagneux P, Cheriyan M, Hurtado-Ziola N, van der Linden EC, Anderson D, McClure H, Varki A, Varki NM. Human-specific regulation of alpha 2-6-linked sialic acids. J Biol Chem. 2003;278(48):48245–50. doi:10.1074/jbc.M309813200. PMID: 14500706.
- Ibricevic A, Pekosz A, Walter MJ, Newby C, Battaile JT, Brown EG, Holtzman MJ, Brody SL. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80(15):7469–80. doi:10.1128/JVI.02677-05. PMID: 16840327 PMCID: PMC1563738.
- Xu Q, Wang W, Cheng X, Zengel J, Jin H. Influenza H1N1 A/Solomon Island/3/06 virus receptor binding specificity correlates with virus pathogenicity, antigenicity, and immunogenicity in ferrets. J Virol. 2010 May;84(10):4936–45. doi:10.1128/JVI.02489-09. PMID: 20200248 PMCID: PMC2863823.
- Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Dis Model Mech. 2011 Sep;4(5):575–79. doi:10.1242/dmm.007823. Epub 2011 Aug 2. PMID: 21810904 PMCID: PMC3180220.
- Enkirch T, von Messling V. Ferret models of viral pathogenesis. Virology. 2015 May;479-480:259–70. doi:10.1016/j.virol.2015.03.017. Epub 2015 Mar 26. PMID: 25816764.
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000 May;61(1):94–99. doi:10.1002/(SICI)1096-9071(200005)61:1<94::AID-JMV15>3.0.CO;2-C. PMID: 10745239.
- Cobey S, Gouma S, Parkhouse K, Chambers BS, Ertl HC, Schmader KE, Halpin RA, Lin X, Stockwell TB, Das SR, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012-2013. Clin Infect Dis. 2018 Jul 18;67(3):327–33. doi:10.1093/cid/ciy097. PMID: 29471464 PMCID: PMC6051447.
- Kosikova M, Li L, Radvak P, Ye Z, Wan XF, Xie H. Imprinting of repeated influenza A/H3 exposures on antibody quantity and antibody quality: implications for seasonal vaccine strain selection and vaccine performance. Clin Infect Dis. 2018 Oct 30;67(10):1523–32. doi:10.1093/cid/ciy327. PMID: 29672713 PMCID: PMC6206119.
- Bissel SJ, Carter CE, Wang G, Johnson SK, Lashua LP, Kelvin AA, Wiley CA, Ghedin E, Ross TM. Age-related pathology associated with H1N1 A/California/07/2009 influenza virus infection. Am J Pathol. 2019 Dec;189(12):2389–99. doi:10.1016/j.ajpath.2019.08.017. Epub 2019 Oct 1.
- Ottolini MG, Blanco JCG, Eichelberger MC, Porter DD, Pletneva L, Richardson JY, Prince GA. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J Gen Virol. 2005 Oct;86(Pt 10):2823–30. doi:10.1099/vir.0.81145-0. PMID: 16186238.
- Eichelberger MC. The cotton rat as a model to study influenza pathogenesis and immunity. Viral Immunol. 2007 Summer;20(2):243–49. doi:10.1089/vim.2007.0017. PMID: 17603841
- Blanco JC, Pletneva LM, Wan H, Araya Y, Angel M, Oue RO, Sutton TC, Perez DR. Receptor characterization and susceptibility of cotton rats to avian and 2009 pandemic influenza virus strains. J Virol. 2013;87(4):2036–45. doi:10.1128/JVI.00638-12. PMID: 23192875 PMCID: PMC3571489
- Ottolini M, Blanco J, Porter D, Peterson L, Curtis S, Prince G. Combination anti-inflammatory and antiviral therapy of influenza in a cotton rat model. Pediatr Pulmonol. 2003 Oct;36(4):290–94. doi:10.1002/ppul.10320. PMID: 12950040.
- Yim K, Miles B, Zinsou R, Prince G, Boukhvalova M. Efficacy of trivalent inactivated influenza vaccines in the cotton rat Sigmodon hispidus model. Vaccine. 2012;30(7):1291–96. doi:10.1016/j.vaccine.2011.12.084. PMID: 22210139
- Braun LE, Sutter DE, Eichelberger MC, Pletneva L, Kokai-Kun JF, Blanco JC, Prince GA, Ottolini MG. Co-infection of the cotton rat (Sigmodon hispidus) with Staphylococcus aureus and influenza A virus results in synergistic disease. Microb Pathog. 2007 Nov-Dec;43(5–6):208–16. doi:10.1016/j.micpath.2007.03.005. PMID: 17689046.
- Curtis SJ, Ottolini MG, Porter DD, Prince GA. Age-dependent replication of respiratory syncytial virus in the cotton rat. Exp Biol Med (Maywood). 2002;227(9):799–802. doi:10.1177/153537020222700912. PMID: 12324660
- Boukhvalova MS, Yim KC, Kuhn KH, Hemming JP, Prince GA, Porter DD, Blanco JC. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J Infect Dis. 2007;195(4):511–18. doi:10.1086/510628. PMID: 17230410
- Guichelaar T, Hoeboer J, Widjojoatmodjo MN, Reemers SS, van Els CA, Otten R, van Remmerden Y, Boes J, Luytjes W. Impaired immune response to vaccination against infection with human respiratory syncytial virus at advanced age. J Virol. 2014;88(17):9744–50. doi:10.1128/JVI.01101-14. PMID: 24920795 PMCID: PMC4136321
- Guichelaar T, van Erp EA, Hoeboer J, Smits NAM, van Els CACM, Pieren DKJ, Luytjes W. Diversity of aging of the immune system classified in the cotton rat (Sigmodon hispidus) model of human infectious diseases. Dev Comp Immunol. 2018;82:39–48. doi:10.1016/j.dci.2017.12.026. PMID: 29305168.
- Patel MC, Shirey KA, Boukhvalova MS, Vogel SN, Blanco JCG. Serum high-mobility-group box 1 as a biomarker and a therapeutic target during respiratory virus Infections. MBio. 2018;9(2). doi:10.1128/mBio.00246-18. PMID: 29535197 PMCID: PMC5850323
- Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82(Pt12):2881–88. doi:10.1099/0022-1317-82-12-2881. PMID: 11714962
- Boukhvalova MS, Yim KC, Prince GA, Blanco JC. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription-PCR in vivo: detection of abortive viral replication. Curr Protoc Cell Biol. 2010;46(1). doi:10.1002/0471143030.cb2606s46. Chapter 26:Unit26.6;PMID: 20235102 PMCID: PMC3049332
- Olafsdottir TA, Alexandersson KF, Sveinbjornsson G, Lapini G, Palladino L, Montomoli E, Del Giudice G, Gudbjartsson DF, Jonsdottir I. Age and influenza-specific pre-vaccination antibodies strongly affect influenza vaccine responses in the icelandic population whereas disease and medication have small effects. Front Immunol. 2018;8. doi:10.3389/fimmu.2017.01872.
- Jonges M, Liu WM, van der Vries E, Jacobi R, Pronk I, Boog C, Koopmans M, Meijer A, Soethout E. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J Clin Microbiol. 2010 Mar;48(3):928–40. doi:10.1128/JCM.02045-09.
- Lee MS, Mahmood K, Adhikary L, August MJ, Cordova J, Cho I, Kemble G, Reisinger K, Walker RE, Mendelman PM. Measuring antibody responses to a live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2004 Sep;23(9):852–56. doi:10.1097/01.inf.0000137566.87691.3b. PMID: 15361726.
- Planty C, Mallett CP, Yim K, Blanco JC, Boukhvalova M, March T, van der Most R, Destexhe E. Evaluation of the potential effects of AS03-adjuvanted A(H1N1)pdm09 vaccine administration on the central nervous system of non-primed and A(H1N1)pdm09-primed cotton rats. Hum Vaccin Immunother. 2017;13(1):90–102. doi:10.1080/21645515.2016.1227518. PMID: 27629482 PMCID: PMC5287305
- Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, Mor V. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5(9):738–46. doi:10.1016/S2213-2600(17)30235-7. PMID: 28736045
- Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother. 2012;8(7):851–62. doi:10.4161/hv.19917. PMID: 22777099 PMCID: PMC3495721
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–65. doi:10.1001/jama.1994.03520210045030. PMID: 7966893.
- Raymond DD, Stewart SM, Lee J, Ferdman J, Bajic G, Do KT, Ernandes MJ, Suphaphiphat P, Settembre EC, Dormitzer PR, et al. Influenza immunization elicits antibodies specific for an egg-adapted vaccine strain. Nat Med. 2016;22(12):1465–69. doi:10.1038/nm.4223. PMID: 27820604 PMCID: PMC5485662.
- Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578–83. doi:10.1073/pnas.1712377114. PMID: 29109276 PMCID: PMC5703309.
- Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, Fonseca K, Winter A-L, Gubbay JB, Krajden M, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9(3):e92153. doi:10.1371/journal.pone.0092153.
- Rudenko LG, Arden NH, Grigorieva E, Naychin A, Rekstin A, Klimov AI, Donina S, Desheva J, Holman RC, DeGuzman A, et al. Immunogenicity and efficacy of Russian live attenuated and US inactivated influenza vaccines used alone and in combination in nursing home residents. Vaccine. 2000;19(2–3):308–18. doi:10.1016/s0264-410x(00)00153-5. PMID: 10930686
- Bellei N, Carraro E, Perosa A, Watanabe A, Arruda E, Granato C, Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S. Acute respiratory infection and influenza-like illness viral etiologies in Brazilian adults. J Med Virol. 2008;80(10):1824–27. doi:10.1002/jmv.21295. PMID: 18712837
- Manzoli L, Salanti G, De Vito C, Boccia A, Ioannidis JP, Villari P. Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis. 2009;9(8):482–92. doi:10.1016/S1473-3099(09)70153-7. PMID: 19628173
- Prieto-Lara E, Llanos-Méndez A. Safety and immunogenicity of prepandemic H5N1 influenza vaccines: a systematic review of the literature. Vaccine. 2010;28(26):4328–34. doi:10.1016/j.vaccine.2010.03.068. PMID: 20403350
- Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009 Nov 18;10(1):112. doi:10.1186/1465-9921-10-112.
- Schneider-Ohrum K, Giles BM, Weirback HK, Williams BL, DeAlmeida DR, Ross TM. Adjuvants that stimulate TLR3 or NLPR3 pathways enhance the efficiency of influenza virus-like particle vaccines in aged mice. Vaccine. 2011 Nov 8;29(48):9081–92. doi:10.1016/j.vaccine.2011.09.051.
- Paquette SG, Huang SSH, Banner D, Xu L, Leόn A, Kelvin AA, Kelvin DJ. Impaired heterologous immunity in aged ferrets during sequential influenza A H1N1 infection. Virology. 2014 Sep;464-465:177–83. doi:10.1016/j.virol.2014.07.013.
