?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Vaccination uptake in the Federation of Bosnia and Herzegovina (FBiH), in Bosnia and Herzegovina, is suboptimal. This study aimed to (1) assess vaccination coverage, timeliness and drop-out for children born in 2015 and 2016 and compare these with official administrative coverage estimates, (2) identify associations between characteristics of children/caregivers and vaccination uptake. This was a cross-sectional study based on patient files for children 12–23 months (n = 1800) and 24–35 months (n = 1800). Methods were adapted from the World Health Organization cluster survey methodology. A two-stage stratified sampling procedure was conducted in urban and rural strata. A structured paper-based form was completed by a pediatrician/nurse from randomly selected primary care centers and patient files. Estimates were based on weighted analysis with a 95% confidence interval to account for the survey sampling design. Vaccination coverage was consistent with administrative coverage levels for BCG, DTP and MMR, and lower for HepB; all considerably lower than regional targets. Children in urban areas had lower vaccination uptake. An assumption that anti-vaccination sentiment prevails among caregivers was not confirmed; only 2% of children were not vaccinated at all, instead challenges related to delays and drop-out. An assumption of caregiver concerns for the MMR vaccine was confirmed with low uptake and delays. The FBiH has experienced vaccination schedule changes due to supply issues; findings confirmed that sustainability in supply and schedule is high priority. These data are new and provide important information for developing strategies to increase uptake.
Introduction
Vaccination saves lives and prevents suffering for millions of people every year,Citation1 yet vaccination uptake at national and sub-national levels across the World Health Organization (WHO) European Region remains insufficient to stop the spread of measles and other potentially life-threatening vaccine-preventable diseases.Citation2 The Federation of Bosnia and Herzegovina (FBiH) is one of the two entities comprising Bosnia and Herzegovina. Vaccination uptake in the FBiH has been declining in recent years. According to administrative official data, coverage for three doses of diphtheria-tetanus-pertussis (DTP) and polio vaccine in children under 1 year declined from 86.2% to 72.3% during 2014–2018; while uptake of the first dose of measles-mumps-rubella vaccine (MMR) declined from 89.1% to 68.4%,Citation3 far from the 95% target set for both vaccines in the European Vaccine Action Plan.Citation4
A measles outbreak in the FBiH in 2014–2015 reached 5084 cases,Citation5 and a current measles outbreak has seen 1332 cases during 2019, with two related deaths of children under 1 year. In its most recent report, the European Regional Certification Commission for Poliomyelitis Eradication concluded that the FBiH, due to its low population immunity, remains at high risk of sustained polio outbreak following importation.Citation6
There are no evidence-based data on the reasons for the suboptimal vaccination uptake in the FBiH to inform the development of tailored public health interventions. Some frequent assumptions amongst key stakeholders include caregiver and health worker vaccine hesitancy, misinformation, lack of trust, health worker shortage, vaccine shortages, and immunization schedule changes. Moreover, a comprehensive analysis has not been done to describe vaccinated and unvaccinated population groups in terms of characteristics such as birth order, residence, education, age or community affiliations. Lastly, there are concerns as regards the denominators used to calculate administrative coverage due to inaccurate projections and thus the accuracy of assessment. Administrative coverage is assessed by routine reporting, starting from a health-care facility that performs mandatory immunization, which is compiled by cantonal public health institutes and passed to the Institute for Public Health of FBiH. The number of doses administered to the target population is divided by the total estimated number of people in the target population.
Given these challenges, the Institute for Public Health of the FBiH decided to initiate a WHO Tailoring Immunization Programmes (TIP) project to understand barriers and drivers to childhood vaccination, to inform a strategy to increase vaccination uptake.Citation7–9 The TIP approach applies a people-centered approach and draws on the COM-B (capability, opportunity, motivation-behavior) system as a framework for changing behavior.Citation10 Three TIP formative research studies were conducted in the FBiH from 2017 to 2019: two qualitative interview studies with health workers and caregivers,Citation11 to identify barriers and drivers to positive vaccination behaviors, and the patient file study presented in this paper. The aims of this study were to:
assess the vaccination coverage, timeliness and drop-out for children born in 2015 and 2016; and compare these with official administrative coverage estimates;
identify associations between the characteristics of children/caregivers and vaccination uptake.
Methods
The childhood schedule for the FBiH is presented in . Throughout the paper, we refer to vaccines by their abbreviation with a number to indicate the dose (e.g. DTP3 is the third dose of diptheria-tetanus-pertussis-containing vaccine).
Table 1. Childhood immunization schedule in FBiH in 2016
Study design and target population
This was a cross-sectional study, based on Primary Health Care (PHC) facility patient files of children, which included data about their caregivers.
We included two cohorts of children, born in 2015 (aged 24–35 months at the time of data analysis) and 2016 (aged 12–23 months at the time of data analysis) because of the change in pertussis vaccine that occurred at this time (see footnote). Children born in 2015 temporarily received a vaccine containing whole-cell pertussis (not acellular) which has been the issue of dispute in the past;Citation12 thus, to minimize bias related to this change in the schedule and the fact that it also affected HepB3 vaccine coverage, which is given simultaneously, we only reviewed MMR uptake for these children, enabling us to review MMR1 uptake beyond 12 months of age. Children born in 2016 mainly received acellular pertussis-containing vaccine; we, therefore, included all vaccines except MMR1 in studying this cohort. The inclusion of this cohort enabled us to review uptake in vaccinations that are scheduled in the first 12 months of life.
Operational definitions
Fully vaccinated
Child aged 12–23 months who received BCG, DTP3, HepB3
Child aged 24–35 months who received MMR1
Unvaccinated
Child aged 12–23 months who had not received any of BCG, DTP or HepB. To account for the fact that many children receive their first doses of two vaccinations (BCG and HepB1) in birth clinics, we also investigated children who were unvaccinated except for those first doses.
Child aged 24–35 months who had not received MMR1.
Timeliness
Child who had received vaccines on time according to the childhood schedule for the FBiH
Drop-out
Child who had received the first dose, but not the third dose of DTP or HepB:
DTP drop-out: (DTP1 – DTP3/DTP1) * 100
HepB drop-out: (HepB1 – HepB3/HepB1) * 100
Sample size and sampling technique
The sampling frame and procedures were adapted from the WHO Expanded Programme on Immunization (EPI) cluster survey.Citation13
The sample size was calculated to allow us to estimate the FBiH coverage with a confidence level no wider than 8%, when coverage is between 50% and 70%, and with a design effect of 5.67. Estimated total target participants: 2 (strata – urban/rural) x 159 (effective sample size) x 5.67 (design effect) = 1803. The final sample size was rounded down to 1800 participants per age group (900 for each urban and rural strata). The number of patient files to check per cluster was 15, based on the number of households a data collection team can visit in a day as well as the total number of target respondents expected in an average size cluster, assuming that all eligible respondents in those households visited are interviewed. Since the sample size selection was done in the PHC facility (not in the community), we defined a cluster as a unit of 60 (4x15) patient files.
To categorize settlements as rural/urban, a 2013 census list of FBiH municipalities by settlement type (rural/urban) was used. Municipalities with at least 65% urban households were classified as urban.Citation14
Seventy-nine PHCs in the FBiH provide childhood vaccination. The sizes of the birth cohorts were 19,358 in 2015 and 19,655 in 2016.Citation15,Citation16
A two-stage stratified sampling procedure was conducted in each stratum (urban/rural). A sampling frame was created by listing all the PHCs alongside the number of children in the birth cohort from each PHC and then further segmenting the PHCs into clusters (primary sampling units) comprising 60 patient files. PHCs with fewer than 60 patient files were combined with neighboring PHCs to create a single cluster. Next, we randomly selected 15 clusters in each stratum using Microsoft Excel with the formula for randomization. Since the sampling units had been split into sub-units (clusters), the weight template was adapted. We computed a new measure of size by dividing the sum of patient files in a PHC (or collection of PHCs) by the resultant number of units created. All selected PHCs agreed to participate and received a small fee for their participation.
Within the clusters selected in the first stage of the sampling procedure, we randomly selected patient files for children born in 2015 and 2016, using Microsoft Excel with the formula for randomization. Patient files from a total of 3600 (1800 children per age group) were reviewed during January 2018.
Normalized and post-stratified weights were computed. The weights were normalized to the total number of selected units; hence, each weight was multiplied by the sum of samples divided by the sum of weighted number of children (sum of individual weights multiplied by the sample).
The normalized weights within a stratum were multiplied by a factor that limits the sum of these weights to the proportionate contribution of each strata in the sampling frame (Federation). All estimates presented in the paper are based on weighted analysis to account for the survey sampling design.
Data collection
A structured paper-based form was developed, pre-tested and completed by a pediatrician/nurse from the selected PHCs. A sub-sample was overseen by trained supervisors (MS, EP). Where data were missing the pediatrician/nurse telephoned caregivers. With no official classification of ethnicity, categorization of Roma/other was based on the knowledge of the pediatrician/nurse.
Characteristics recorded in the form were:
Child: gender, birth order, residence (urban/rural), community affiliation (Roma/other)
Caregiver: education (none/low/medium/high), age, number of children
Data were entered into MS Access 2013.
Data analysis
Descriptive statistics were generated to characterize the sample and weighted analyses to calculate coverage rates with a 95% confidence interval (CI) for each vaccine/dose and for the measurements of a fully vaccinated child. Bivariate and multivariate analyses were done to explore the association between each of the possible explanatory variables and the outcome variable of fully vaccinated. Chi-square tests were used to compare levels of uptake, and cox proportional hazard regression for comparing timeliness. Analyses were conducted using the R package srvyr.
Ethical approval
Ethical approval was secured from the Ethics Committee of the Institute for Public Health of the FBiH. Only authorized supervisors and pediatricians/nurses who already had access to patient files reviewed these. Data were recorded anonymously, no names were kept in the study database.
Results
Child and caregiver characteristics
The characteristics of the 1800 children and their caregivers in both cohorts are presented in . Both weighted (by population size) and non-weighted data are presented because the urban and rural classification did not represent an equal proportion of the population once weighted. For both age groups just over half of the sample was boys; and the majority was a first or second born child. Most mothers were aged 24–35 years and most fathers were 28–37 years old. In terms of education, medium level (high school) was dominant among mothers and fathers. Twenty children aged 12–23 months and 16 children aged 24–35 months were identified as Roma.
Table 2. Characteristics of children and their caregivers by birth cohort 2016 and 2015 (unweighted and weighted), FBiH January 2018
Vaccination coverage
Vaccination coverage data by residence among children born in 2015 and 2016 are presented in .
Table 3. Vaccination coverage by residence among children born in 2016 and 2015, FBiH January 2018
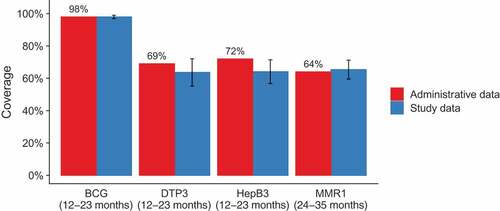
Coverage was lower for all vaccines for urban residences compared to rural. The differences were most pronounced for the three doses of DTP-containing vaccine, HepB3 and MMR1. The vaccines administered at birth, BCG and HepB1 had the highest coverage (BCG: 98%, 95% CI 97–99%; HepB1: 98%, 95% CI 96–99%). Notably, HepB coverage decreased with each dose, leading to coverage for the third dose of 64% (95% CI 57–71%). The same pattern was evident for DTP-containing vaccine with coverage decreasing from 86% (95% CI 81–90%) for first dose to 64% (95% CI 55–72%) at third dose. Among children aged 24–35 months, MMR1 coverage was 65% (95% CI 60–71%).
Just 2% (95% CI 1–3%) had received no vaccinations at all (). To account for the fact that many children routinely receive their first dose of vaccinations at birth, we also looked at children who were unvaccinated except for BCG and HepB1. A total of 8% (95% CI 4–11%) was unvaccinated using this definition.
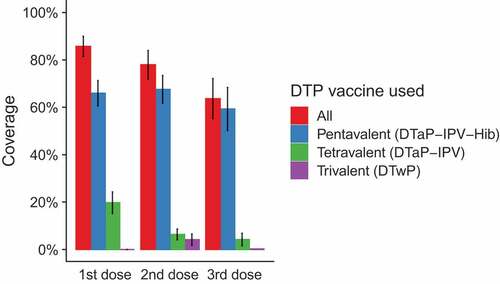
For DTP, pentavalent vaccine was the most commonly administered (coverage with 1st dose 66%, 2nd dose 68%, 3rd dose 59%), and trivalent vaccine was the least frequently administered (coverage with 1st dose 0%, 2nd dose 4%. 3rd dose 0%). The distribution of vaccines used for the first, second and third dose is presented in .
Patient file data versus official administrative coverage estimates
Vaccination coverage data from the patient files were compared with the data reported in the official annual report (). Coverage levels estimated in our study were consistent with administrative levels for three of the four vaccines (p-values for null hypothesis of no difference: BCG 0.93, DTP3 0.21, MMR1 0.63), but to a lesser degree for HepB3 coverage, which was estimated to be lower than the administrative level (64% vs. 72%, p = .04).
Timeliness
To assess timeliness, the age of children at the time of vaccination was assessed for HepB, DTP, and MMR1.
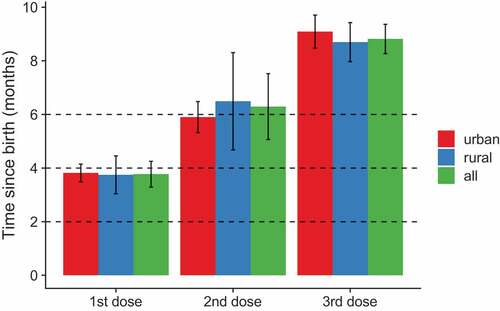
The recommended timing for the three doses of HepB was at birth, 1 and 6 months (or at least 6 months after the first dose) and for DTP at 2, 4 and 6 months (). For children who received all three doses of each vaccine, the timing of vaccination by residence for each dose is presented in . Mean age for all three doses was older than the recommended age for both vaccines, with completion of each vaccine at 8–9 months instead of the recommended 6 months. Vaccination occurred later in urban than rural settings for MMR1, and a slightly significant difference was found for HepB1 (, ). Mean age for DTP1 was 3.8 months.
Table 4. Time of vaccination in months by residence among children born in 2016 and 2015, FBiH January 2018
Drop out
The drop-out rate for HepB from first to third dose was 34% (95% CI 27–41%). For DTP the rate was 26% (95% CI 19–33%).
Characteristics associated with full vaccination coverage
presents the association between child and caregiver characteristics in relation to full and no vaccination coverage among children born in 2016, aged 12–23 months (excluding MMR). Just over half of the children (59%, 95% CI 51–68%) were fully vaccinated. The odds of being fully vaccinated were three times (95% CI 1.6–5.4) higher for a child living in a rural area compared to an urban area. Conversely, a child of Roma community affiliation was less likely to be fully vaccinated than a non-Roma child (although this is based on a small sample). None of the other child or caregiver characteristics were strongly associated with full vaccination coverage.
Table 5. Association between child and caregiver characteristics in relation to BCG, DTP3, HepB3 (full vaccination) coverage among children born in 2016 and aged 12–23 months, FBiH January 2018
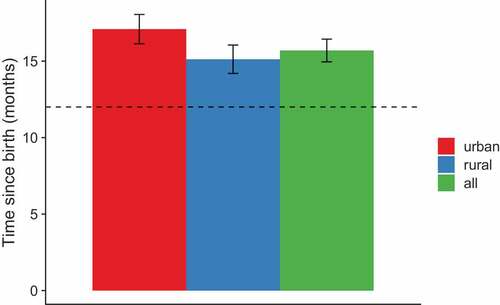
presents the association between child and caregiver characteristics in relation to full vaccination coverage for MMR1 among children born in 2015, aged 24–35 months. Two-thirds of the children were fully vaccinated (65%, 95% CI 60–71%). The odds of being vaccinated with MMR1 were 4.7 times (95% CI 2.6–8.2) higher for a child living in a rural area compared to an urban area; and 1.4 times higher (95% CI 1.1–1.8) if the mother is older compared to younger (>35 years to 30–35 years). None of the other child or caregiver characteristics were strongly associated with MMR1 vaccination coverage.
Table 6. Association between child and caregiver characteristics in relation to MMR1 (full vaccination) coverage, among children born in 2015 and aged 24–35 months, FBiH January 2018
Discussion
This study assessed childhood vaccination coverage, timeliness and drop-out in the FBiH and described the characteristics of fully vaccinated children and their caregivers. These data provide detailed insight, and alongside two qualitative TIP studies conducted in parallel,Citation11 are important information for developing evidence-informed tailored interventions to improve vaccination coverage in the FBiH.Citation7–9
The study confirmed that vaccination coverage in the FBiH in 2016 was considerably lower than regional targets and coverage in other central European countries;Citation17,Citation18 and that there are issues related to delay and drop-out for a considerable proportion of children. This increases the risk of outbreaks of vaccine-preventable diseases with personal and financial implications for individuals and health systems. Our findings also confirmed the accuracy of the administrative data.Citation3
Prior to conducting this study, there were a number of assumptions in the FBiH about factors related to vaccination of which some were confirmed, and others challenged by the study findings. There was an assumption that anti-vaccination sentiment prevails among caregivers in the FBiH. This notion is challenged by the high coverage for birth doses and vaccines given at 1 and 2 months of age. Indeed, only 2% of the children in this study were completely unvaccinated. Instead, the study identified challenges related to the completion of the vaccination schedule and timeliness. For all assessed vaccines, the study confirmed considerable delays and drop-out. Insight from our qualitative studies with caregivers (unpublished data) and health workers;Citation11 and a study in neighboring Serbia,Citation19 offer potential explanations for suboptimal vaccination for later doses (delay or drop-out), including changes in immunization schedule, lack of knowledge and/or information about the importance of completing the schedule, lack of encouragement from health workers to return for vaccination; inefficient or a lack of reminders, poorer doctor-patient relationship in urban comparing to rural setting and overloaded pediatricians in urban settings, and competing priorities for caregivers in urban settings.
Another assumption was that caregivers have particular concerns about the MMR vaccine. Indeed, this was evident from our interview studies with caregivers (unpublished) and health workers.Citation11 Anti-MMR vaccine information is increasingly circulating in the Balkan countries (sharing similar languages),Citation20 and MMR vaccination rates in these countries have declined more significantly than other vaccines.Citation18 It is known that some parents delay MMR vaccination due to misperceived risks of potential side effects affecting walking and speaking abilities.Citation21,Citation22 This current study confirmed that one-third of eligible children had not received MMR vaccination, higher than for any other vaccines. Specifically, MMR1 vaccine compared to DTP1 vaccine was less accepted (65% versus 86%) and more delayed (4 months versus 2 months).
Another explanation to the general pattern of low coverage at 12 months may be that at the time there were specific concerns to the DTP vaccine. As mentioned, whole-cell pertussis had been temporarily reintroduced. Even if this did not affect the cohort studied to great degree in terms of the vaccines which were administered to them, the debate related to whole-cell pertussis may have affected both health workers and caregivers. This means that the delays related to DTP vaccine and low uptake of DTP3 may be related to vaccine safety concerns as well.
The study confirmed a considerable delay for vaccines not provided at birth or during initial months. In 2017 (the cohort following our study), due to cost and supply issues, the immunization schedule in the FBiH was changed with DTP3 being postponed from 6 to 10 months. Given the findings of this study, such rescheduling may postpone DTP vaccination even further into the child’s second year. In 2019, thanks to a multi-year tender resulting in a decreased vaccine price and sustainable supply, DTP doses were changed back to 2–4–6 months (including a booster dose in the second year and a pre-school booster dose). The findings of this study indicate that in order to achieve early and durable protection against pertussis disease, the priming schedule with three DTP doses at 2–4–6 months or with a shorter gap between second and third doses, is better optimized compared to a schedule with two doses at 2–4 months and a booster at 10 months of age.
In terms of caregiver/child characteristics, the study clearly showed that children living in urban areas have lower vaccination coverage, evident for all vaccinations except BCG. Differences in the timeliness of vaccination by residence only applied for MMR1 where the delay was more frequent for urban areas. International literature reports a positive impact of maternal education on vaccination coverage,Citation23 although conversely a popular assumption, especially with MMR, is that highly educated parents are those most likely to reject vaccination.Citation24 Neither were supported by the findings. We found no association with education, and with complete vaccination coverage in urban areas lower than 50%.
Earlier data from the FBiH and wider Europe suggest low vaccination coverage among Roma,Citation25–27 and this was confirmed in this study, although this finding should be viewed with caution given the small sample size. Given this finding, additional explanations for suboptimal vaccination based on evidence from other countries could again be speculated and further explored for verification.
Finally, the use of digital technology for the vaccination reporting would ensure accurate coverage estimates, and should be considered for the future activities.
Strengths and limitations
The study method was unconventional, although based on an accepted WHO methodology.Citation13 Probability samples and weighted statistical analyses were used at all stages of sampling, thus reducing the potential for selection bias, and allowing the calculation of meaningful confidence intervals and confidence bounds.Citation13
Regular surveys on national vaccination coverage provide important information for public health officials and decision makers, and public health practitioners and researchers are encouraged to conduct them. Typically, evidence is usually collected from home-based records (household surveys), as well as from a vaccination history as recalled by the individual or the child’s caregivers, which can be very expensive and time-consuming. Our approach, using vaccination documentation at the child’s health-care facility, was sufficient to confirm low coverage and provided additional valuable data (which are not included in the administrative data), about timeliness, and the characteristics of children and their caregivers. This method could be adapted and used as a quick and less expensive approach to cluster vaccination coverage survey, if there is documented evidence of vaccination in health facilities. Feedback from participating pediatricians and nurses was that the work was not a significant burden, and they all provided the data within 1 week.
However, this method has several limitations: there might be undocumented vaccination as children may have been vaccinated at multiple health facilities, or in private clinics that were not recorded in the patient file. Although we tried to assess data on evidence for vaccination elsewhere in the files. We found that 1% of children in both age groups had documented vaccination elsewhere. Also, data on caregivers’ characteristics, for example, education and community affiliation were inconsistently registered in files and required additional data collection via phone by the pediatrician/nurse.
Due to vaccine supply issues, coverage was assessed for two cohorts. Broader insights into vaccination coverage and delayed vaccination would have allowed an assessment for a broader age group, for example, up to 5 years; this was not possible within the limitations of this project.
To minimize bias, categories were developed for each question and data collection was supervised by appointed supervisors. As part of quality control, analyses also included internal consistency checks. As there is no official categorization of specific population groups, the categorization of “Roma” vs “other” had to be done based on the knowledge of the person completing the data collection form. At the same time, the Roma part of the sample was small. Findings related to this population group should be interpreted with care and inspire more research.
The findings of this study indicate that comprehensive action, investment and prioritization from decision-makers are immediately necessary for the FBiH to ensure the protection of the population against vaccine-preventable diseases. It is important to ensure a stable supply of mandatory vaccines to avoid vaccine shortages and consequential adjustment of the immunization calendar; to educate health workers and caregivers to improve knowledge related to safety concerns; to improve urban vaccination settings in order to encourage urban caregivers to bring children for vaccination; to support Roma families to bring children for vaccination and finally, and to digitalize the vaccination reporting system to ensure accurate coverage estimates.
Conclusions
The study confirmed the administrative coverage estimates and that vaccination coverage for children born in 2015 (MMR1) and 2016 (BCG, HepB, DTP) is significantly below the target immunization rates and is associated with considerable delays and drop-out. Very close to no children were not vaccinated at all. The later doses have lower coverage than doses given earlier. This is associated with high drop-out rates and does not confirm a general anti-vaccination sentiment. The study indicated that suboptimal vaccination coverage is associated with: child living in urban area (low coverage and delayed vaccination). Assessment of vaccination coverage of children of caregivers of Roma origin indicated low coverage and needs further exploration.
Author contributions
SM co-conceived the work, co-led on the design and development of the study protocol, led on coordination and recruitment of PHC facilities, co-led the data cleaning and interpretation of the study findings, co-led on drafting and revising the manuscript.
KBH co-conceived the work, co-led the design and development of the study protocol, co-led on the interpretation of the study findings, co-led on drafting and revising the manuscript.
CJ co-conceived the work, contributed to the design and development of the study protocol, the interpretation of the study findings, drafting and revising the manuscript.
AK co-led on the interpretation of the study findings, contributed to drafting and revising the manuscript.
EP and MS trained health workers and supervised the process of data collection, contributed to drafting and revising the manuscript.
SF co-led the design and development of the study protocol, conducted data analysis, co-led on the interpretation of the study findings, co-led on drafting and revising the manuscript.
All authors approved the submitted manuscript and are accountable for all aspects of this work.
The authors alone are responsible for the views expressed in this manuscript and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Tip advisory group
Goran Čerkez, Federal Ministry of Health, Federation of Bosnia and Herzegovina, Bosnia and Herzegovina
Zlatan Peršić, Federal Ministry of Health, Federation of Bosnia and Herzegovina, Bosnia and Herzegovina
Davor Pehar, Institute for Public Health of the Federation of Bosnia and Herzegovina, Bosnia and Herzegovina
Mirsada Mulaomerović, Institute for Public Health of the Federation of Bosnia and Herzegovina, Bosnia and Herzegovina
Victor Olsavszky, WHO Country Office in Bosnia and Herzegovina
Fatima Čengić, UNICEF Bosnia and Herzegovina
Amra Junuzović, Health Centre of Sarajevo Canton, Bosnia and Herzegovina
Jelena Kalinić, Society for Science Advocacy “Science and the World“, Bosnia and Herzegovina
Acknowledgments
The TIP team would like to thank Carolina Danovaro (WHO) and John Wagai (Independent Consultant) for reviewing the study protocol and report. We would like also to thank all the primary health-care facilities and health workers who took part in the study. We are grateful to Mirza Palo and Victor Olsavszky at the WHO, and the TIP Advisory Group (listed below) who have advised on all steps of the TIP process. Thanks also to Azemina Bešić for providing administrative support.
Additional information
Funding
References
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–46. doi:10.2471/BLT.07.040089.
- World Health Organization Regional Office for Europe. WHO Epi Brief: A report on the epidemiology of selected vaccine-preventable diseases in the European Region. Copenhagen: World Health Organization; 2019 [accessed 2019 November 26]. http://www.euro.who.int/__data/assets/pdf_file/0017/410714/EpiBrief_2_2019_EN.pdf
- Institute for Public Health FBiH. Epidemiological surveillance of communicable diseases in the Federation of Bosnia and Herzegovina. Sarajevo; 2019.
- World Health Organization Regional Office for Europe. European Vaccine Action Plan 2015-2020. Copenhagen (Denmark): World Health Organization; 2014 [accessed 2019 May 30]. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020-2014
- Musa S, Topalović B, Ćatić S, Smajlagić Z. Assessment of vaccine effectiveness during measles outbreak in the Federation of Bosnia and Herzegovina, 2014-2015. Cent Eur J Public Health. 2018;26:79–82. doi:10.21101/cejph.a4754.
- World Health Organization Regional Office for Europe. 32nd meeting of the European regional certification commission for poliomyelitis eradication. Copenhagen (Denmark): World Health Organization; 2018 [accessed 2019 Nov 27]. http://www.euro.who.int/en/health-topics/communicable-diseases/poliomyelitis/publications/2018/32nd-meeting-of-the-european-regional-commission-for-certification-of-poliomyelitis-eradication-rcc-report-2018
- World Health Organization Regional Office for Europe. Tailoring immunization programmes to reach underserved groups – the TIP approach. Copenhagen (Denmark): World Health Organization; 2019 [accessed 2019 Nov 26]. http://www.euro.who.int/tip
- Dube E, Leask J, Wolff B, Hickler B, Balaban V, Hosein E, Habersaat K. The WHO Tailoring Immunization Programmes (TIP) approach: review of implementation to date. Vaccine. 2017;36:1509–15. doi:10.1016/j.vaccine.2017.12.012.
- Bach Habersaat K, Jackson C. Understanding vaccine acceptance and demand—and ways to increase them. Bundesgesundheitsbl. 2020;63:32–39. doi:10.1007/s00103-019-03063-0.
- Michie S, Atkins L, West R. The Behaviour Change Wheel. A Guide to Designing Interventions. Bream (UK): Silverback Publishing; 2014.
- Musa S, Skrijelj V, Kulo A, Bach Habersaat K, Smjecanin M, Primorac E, Becirovic D, Jackson C. Identifying barriers and drivers to vaccination: a qualitative interview study with health workers in the Federation of Bosnia and Herzegovina. Vaccine. 2020;38:1906–14. doi:10.1016/j.vaccine.2020.01.025.
- Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveillance. 2013;18:20587. doi:10.2807/1560-7917.es2013.18.38.20587.
- World Health Organization. Vaccination coverage cluster surveys: reference manual, Version 3. [accessed 2019 May 30]. https://www.who.int/immunization/monitoring_surveillance/Vaccination_coverage_cluster_survey_with_annexes.pdf
- Bodin J. and Cassel C. Advice on master sample for Bosnia and Herzegovina (BiH), Part II; Report from a mission to BiH. Statistics Sweden, International Consulting Office; 2000.
- Institute for statistics of FBiH. Statistical yearbook. Sarajevo; 2016.
- Institute for statistics of FBiH. Statistical yearbook. Sarajevo; 2017.
- World Health Organization. Measles – european Region. Disease outbreak news – update. Copenhagen (Denmark): World Health Organization; 2019 [accessed 2019 Aug 12]. https://www.who.int/csr/don/06-may-2019-measles-euro/en/
- World Health Organization/UNICEF. Estimates of National Immunization Coverage (WUENIC). Geneva (Switzerland): World Health Organization; 2018 [accessed 2019 Nov 26]. https://www.who.int/immunization/monitoring_surveillance/data/en/
- UNICEF. Knowledge, Attitudes and Practices in Relation to Immunization of Children in Serbia. 2018 [accessed 2019 Nov 21]. https://www.unicef.org/serbia/media/9146/file/Knowledge,%20attitudes%20and%20practices.pdf
- Radovanovic Z. Anti-vaccinationists and their arguments in the Balkan countries that share the same language. Srp Arh Celok Lek. 2017;145:46. doi:10.2298/SARH161214046R.
- Allan N, Harden J. Parental decision-making in uptake of the MMR vaccination: a systematic review of qualitative literature. J. Public Health. 2015;37:678–87. doi:10.1093/pubmed/fdu075.
- Herzog R, Alvarez-Pasquin MJ, Diaz C, Del-Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2019;13:154. doi:10.1186/1471-2458-13-154.
- Forshaw J, Gerver SM, Gill M, Cooper E, Manikam L, Ward H. The global effect of maternal education on complete childhood vaccination: a systematic review and meta-analysis. BMC Infect Dis. 2017;17:801. doi:10.1186/s12879-017-2890-y.
- Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vacc Immunother. 2013;9:1755–62. doi:10.4161/hv.25085.
- UNICEF. Bosnia and Herzegovina Multiple Indicator Cluster Survey 2011-2012. 2014 [accessed 18 December 2019]. https://mics-surveys-prod.s3.amazonaws.com/MICS4/Europe%20and%20Central%20Asia/Bosnia%20and%20Herzegovina/2011-2012/Final/Bosnia%20and%20Herzegovina%202011-12%20MICS_English.pdf
- Fournet N, Mollema L, Ruijs WL, Harmsen IA, Keck F, Durand JY, Cunha MP, Wamsiedel M, Reis R, French J, et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Pub Health. 2018;18:196. doi:10.1186/s12889-018-5103-8.
- Cook B, Wayne GF, Valentine A, Lessios A, Yeh E. Revisiting the evidence on health and health care disparities among the Roma: a systematic review 2003–2012. Int J Public Health. 2013;58:885–911. doi:10.1007/s00038-013-0518-6.