ABSTRACT
China has achieved high vaccination coverage under the Expanded Program on Immunization (EPI) in children 1–2 years of age. However, a knowledge gap exists regarding vaccination coverage and timeliness for children >2 years of age. As such, this study aimed to estimate coverage and timeliness for all EPI and selected non-EPI vaccines within a rural area of China. Immunization data for 5091 children, born between September 2003 and November 2015, were collected from vaccination cards obtained during sero-surveillance follow-up visits and/or from the Hunan immunization information system. For each dose of both EPI and non-EPI vaccines, vaccination coverage and timeliness were calculated, and temporal variations were examined across birth cohorts. We found coverage for EPI vaccines scheduled for <12 months was 97.1%-99.4%. However, for EPI vaccines scheduled at 6 years coverage was 44.4%-51.7%. The timeliness for EPI vaccines was generally poor, especially for EPI vaccines introduced after 2008 or scheduled for administration at ≥12 months, with a maximum of 35.4% of children vaccinated according to schedule. Despite this, we found increasing trends in vaccination coverage and improvements in timeliness for EPI vaccines. However, for non-EPI vaccines, we found only moderate increases, and in some cases decreases, in vaccination coverage. This study demonstrates the success and improvement of the Chinese immunization program, but also highlights some challenges to be addressed. We recommend that future changes in vaccine practice and policy should primarily focus on improving coverage and timeliness of vaccines introduced after 2008, and/or scheduled for administration ≥12 months.
Introduction
In recent history, China has achieved many milestones in terms of infectious disease control and prevention, including polio eradication, hepatitis B control, and maternal and neonatal tetanus elimination.1,Citation2 Currently, incidence rates of many vaccine-preventable diseases are comparable to those in high-income countries. The important role of the national Expanded Program on Immunization (EPI) to these successes is undeniable.Citation1–3 Established in 1978, the EPI provides free vaccinations and associated immunization services to the Chinese population, and offers a group of vaccine products referred to as the “EPI vaccines”. Initially, only five vaccines against five diseases were included in the EPI, but by 2008 the programme had expanded to include 11 vaccines against 12 diseases.Citation4 Vaccine products not provided by the EPI are referred to as “non-EPI vaccines”. They are available from relevant vaccination services and associated expenses are covered solely by out-of-pocket payments.
Vaccination coverage is considered as a key performance indicator of the EPI. Achieving high coverage for routine immunizations is an important step toward the elimination, and subsequent eradication, of vaccine preventable diseases. In China, the vaccination coverage rates of EPI vaccines are assessed through periodic surveys, such as the EPI Cluster Survey.Citation5,Citation6 The 2013 EPI Cluster Survey indicated that national level immunization coverage rate of EPI vaccines scheduled before 24 months were all above 95%. However, township coverage rates varied substantially by vaccine, dose within a vaccine series, and geographic location.Citation5,Citation6 Unfortunately, the EPI Cluster Survey lacks vaccination coverage data for EPI vaccines scheduled beyond 24 months, does not collect data regarding non-EPI vaccines,Citation7 and does not investigate temporal variations in vaccine uptake rates, which is a crucial quality measure of public health services.Citation8,Citation9 Further, the survey does not investigate the proportion of children vaccinated according to schedule (vaccine timeliness), a factor important to understand population-level disease susceptibility.Citation8,Citation9
To address these knowledge gaps, we investigated both EPI and non-EPI vaccines scheduled at 0–6 years of age. We aimed to estimate coverage rates, timeliness of administration, and temporal variations for EPI and non-EPI vaccines in the rural Anhua county, Hunan province of China. Here we present our findings and propose recommendations to further strengthen the national EPI.
Methods
Study site and study population
Anhua is a rural county in southern China, with 23 townships and a population of approximately 1 million.Citation10 This study is based on data collected from a previously established prospective, population-based cohort study. This previous sero-survey investigated sero-epidemiological characteristics of pediatric enterovirus A71 infections in three townships of Anhua county. All children who participated in the sero-survey were included in the current study. The current study population, a total of 5254 children, consisted of:
4188 children aged between 1–9 years recruited between September and November 2013, who received annual follow-up visits (between August and November) until September 2016; 1066 new-borns delivered in six local hospitals recruited between September 2013 and November 2015, who were followed up at the age of 2, 4, 6, 12, 24 and 36 months.Citation11
Data sources
In China, the National Vaccination Certificate System was implemented in 2005. Vaccination cards serve as the official and legal documents required to enroll children in school and to show that their vaccination schedules are complete. The Hunan Provincial Immunization Information System (HNIIS), on the other hand, is a confidential, digitized information system implemented in 2014. HNIIS holds demographic and vaccination data for children aged under seven years of age and living in Hunan province. Vaccine records from 2003 to 2013 have been retrospectively added to the HNIIS. Due to the comprehensive access to HNIIS for all vaccination clinics, the HNIIS database avoids data loss due to missing physical copies of vaccination records, lost or not updated by parents.
Data collection and management
Information regarding the vaccination history of recruited children was collected from vaccination cards. If vaccination cards were missing or incomplete, vaccination data were collected solely from the HNIIS. Data collected from vaccination cards were linked to HNIIS records for verification. Demographic and socio-economic information of the study population was generated from data collected at the baseline visit of the previously established sero-survey.
For the sero-survey cohort of children aged 1–9 years who participated in the final follow-up visit during September 2016, vaccination cards were photographed by trained investigators. For those who did not receive a final follow-up visit, vaccination cards were collected by village doctors door-to-door and were subsequently photographed by trained investigators.
For the sero-survey cohort of new-borns, data from vaccination cards were collected during the final three follow-up visits conducted in September 2016, March 2018 and August 2018.
Data from photographed vaccination cards, including children’s demographic information (name, date of birth, and sex) and vaccination histories (dates and doses administered), were manually double-entered by a contracted data company using Epidata 3.1. The study population was identified in HNIIS records by matching on name, sex, and date of birth. When multiple matches were identified, we further matched using the names of the parents.
Outcome measures
We considered all EPI vaccines and four non-EPI vaccines. EPI vaccines included Bacillus Calmette-Guérin vaccine (BCG); Hepatitis B vaccine (HepB); Poliovirus vaccine (PV); Diphtheria, tetanus and pertussis vaccine (DTP); Measles containing vaccine (MCV); Hepatitis A vaccine (HepA); Japanese encephalitis vaccine (JEV); Meningococcal polysaccharide vaccine type A (MenA); Meningococcal polysaccharide vaccine type A and C (MenAC); and Diphtheria and tetanus vaccine (DT).
Non-EPI vaccines include varicella vaccine (VarV); Haemophilus influenzae type B vaccine (Hib); oral rotavirus vaccine (ORV); and the 7-valent pneumococcal vaccine (PCV7). The human papillomavirus (HPV) vaccine and 13-valent pneumococcal (PCV13) vaccine were not included in this study due to their very recent introduction.
The vaccination schedule of EPI vaccines and selected non-EPI vaccines are summarized in Supplementary Table 1.
Vaccination coverage is defined as the percentage of children who received the recommended dose(s), regardless of their age at vaccination. Vaccination timeliness is defined as the proportion of children who were vaccinated within one month of the recommended age for each vaccine. For example, children who received the third dose of HepB (recommended at 6 months) between six and seven months of age were considered to have been vaccinated on time. Special definitions were applied for the BCG and the first dose of HepB vaccine, where timeliness was considered to be within 24 hours after birth.
Data analysis
When both vaccination cards and HNIIS data were available, the analysis relied on data from the vaccination cards. When both vaccination cards and HNIIS data were unavailable, children were excluded. Children were also excluded when they fell outside of the study age range or when they did not have access to the vaccines under consideration in this study. EPI vaccines are accessible as soon as they are introduced. Non-EPI vaccines are only accessible if they are available in the local market, and therefore access varies across China.
To estimate the vaccination coverage of EPI vaccines, we categorized the vaccines into two groups based on the recommended vaccination age: <12 months and ≥12 months. As the schedule for MenA includes two doses administered between 6–18 months, at an interval of 3 months, it was not included in either group. We also categorized EPI vaccines into two further groups: traditional EPI vaccines introduced before 2008; and new EPI vaccines introduced after 2008.
The study population was stratified into seven birth cohorts to capture temporal variations. The 2004–2007 cohort includes those born between 20 September 2003 and 19 September 2007; the 2008 cohort includes those born between 20 September 2007 and 19 September 2008. The 2009, 2010, 2011 and 2012 cohorts were defined similarly and the 2014–2015 cohort included those children born between 20 September 2013 and 19 November 2015.
All analyses were performed using R statistical software (version 3.6.1).Citation12
Ethics
This study was approved by the Ethical Review Board of Fudan University. Informed consent was obtained verbally from the children’s parents/guardians before participation. All data were anonymized by removing all individual identifiers from the data when exported from HNIIS and individual records were assigned an “ID number” as a unique identifier. All data were stored confidentially.
Results
A total of 5,254 children were enrolled in the sero-survey, of whom 5,091 (96.9%) had vaccination history data from vaccination cards and/or the HNIIS. A larger proportion of participants were excluded from the 2004–2007 cohort (7.7% excluded), in comparison to other birth cohorts (maximum of 4.3% excluded) ().
Table 1. Comparison of demographic and socioeconomic characteristics, using Chi-square tests, between the children included and children excluded from this study, Anhua county, Hunan province, southern China
Vaccination coverage
For the majority of EPI vaccinations, coverage exceeded 85%. Only two vaccines, the DT and the 2nd dose of MenAC fell below this level, achieving 51.7% and 44.4% coverage respectively ().
Table 2. Vaccination coverage and timeliness for EPI vaccines, by dose. Vaccines scheduled at <12 months are shaded in gray
Coverage seems to be associated with the scheduled age of vaccination, with total coverage falling as scheduled age of vaccination increases. Coverage for vaccines scheduled at <12 months, 12–23 months, 3–5 years and 6 years were 97.1%-99.4%, 92.1%-96.2%, 86.7%-90.7%, and 44.4%-51.7%, respectively ().
Vaccination coverage for all non-EPI vaccines was below 50%. The highest vaccination coverage was for the first dose of Hib (46.0%), followed by the first dose of VarV (32.0%) and the first dose of ORV (12.9%). The vaccination coverage of PCV7 was the lowest among all, achieving only 0.2% for the first dose and 0.05% for the second dose.
Vaccines requiring multiple doses generally have lower coverage for the later doses. This was seen in the coverage for the Hib series, with the third dose (Hib-3) coverage at 20.0%, compared to 46.0% and 29.1% for Hib-1 and Hib-2 respectively. A similar trend was also seen in the ORV vaccine series, with coverage decreasing from 12.9%, to 1.0% to 0.1% from doses ORV1, ORV2, and ORV3, respectively ().
Table 3. Vaccination coverage and timeliness for non-EPI vaccines, by dose, based on the study population (rural children) in Anhua county, Hunan province, southern China
Vaccination timeliness
Among EPI vaccines, the proportion of children receiving vaccinations on time was smaller than the overall corresponding vaccination coverage. However, the difference in magnitude between timeliness and coverage varied considerably between vaccines.
The proportion of children vaccinated on time among children vaccinate was highest for HepB-1 (83.8%) and BCG (81.7%), vaccines both recommended for administration at birth. The proportion of children vaccinated on time among children vaccinate was lowest for HepA (11.9%) and MenA-2 (13.6%), vaccines recommended for administration at 18 months and between 6–18 months respectively ().
Taking both recommended vaccine schedules and time of vaccine introduction to the EPI into consideration, traditional EPI vaccines scheduled for <12 months were administered on time more frequently (range: 48.1%-83.2%) than all other vaccines (range: 9.6%-35.4%) ().
Temporal variations of vaccination coverage
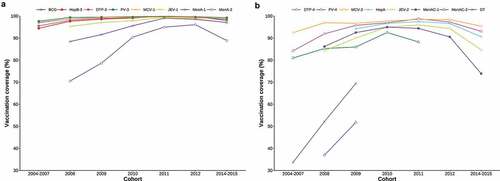
In general, the vaccination coverage of EPI vaccines gradually increased through time across all birth cohorts, with a small decrease of coverage amongst the 2014–2015 cohort. Among EPI vaccines scheduled for administration <12 months, vaccination coverage was consistently higher than 95% (). Among all but two of the EPI vaccines scheduled for administration ≥12 months, vaccination coverage was consistently above 80% and reached 90% vaccine coverage in the 2010 birth cohort. The two EPI vaccines that did not achieve this level of coverage were the DT and MenAC-2 vaccines, scheduled at 6 years of age. Although coverage was considerably lower than other EPI vaccines and far below national targets of 90% coverage, the vaccination coverage for both DT and MenAC-2 significantly increased across the birth cohorts from 33.6% to 68.9%, and from 36.8% to 51.4%, respectively ().
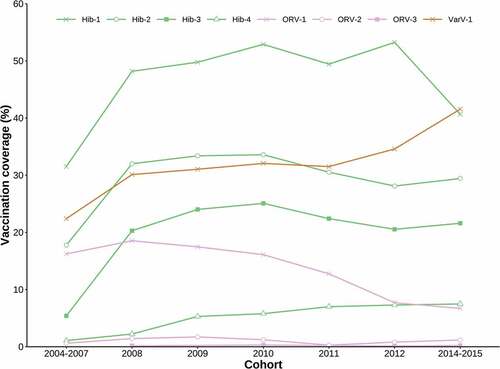
Figure 1. Vaccination coverages of EPI vaccines by birth cohort. No data was collected for a 2013 cohort in the original sero-survey study. Each line begins from the cohort in which the vaccine was first introduced to the EPI, and ends with the cohort for which the vaccine is not age-appropriate
Among non-EPI vaccines, vaccination coverage of Hib-1, although variable, remained higher than that of both VarV and ORV-1 across all cohorts. The vaccination coverage of VarV increased slowly but gradually across cohorts, while ORV-1 coverage decreased steadily over the years ().
Temporal variations of vaccination timeliness
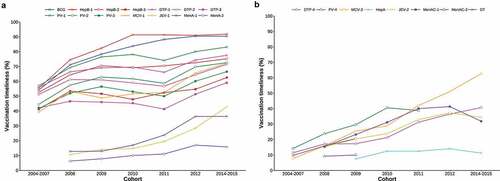
Overall, we found that the timeliness of EPI vaccine administration improved across birth cohorts. Among EPI vaccines scheduled for <12 months, vaccination timeliness was highest for HepB-1, reaching it’s maximum of 90% in the 2010–2012 cohort (). Although lower than other traditional vaccines, the proportion of children receiving the JEV-1 on time gradually increased from 9.8% in the 2008 cohort to 41.9% in the 2014–2015 cohort (). Among EPI vaccines scheduled for ≥12 months, vaccination timeliness for MCV-2 and DTP-4 increased from 7.7% to 61.5%, and 11.4% to 39.9%, respectively. For the HepA vaccine, vaccination timeliness remained at relatively low levels, but increased slowly across cohorts, from 7.5% in the 2008 cohort to 14.1% in the 2014/15 cohort ().
Figure 3. Vaccination timeliness for EPI vaccines, by birth cohort. No data was collected for a 2013 cohort in the original sero-survey study. Each line begins from the cohort in which the vaccine was first introduced to the EPI, and ends with the cohort for which the vaccine is not age-appropriate
Discussion
This study estimated vaccination coverage, timeliness, and temporal variations for both EPI and non-EPI vaccines. The study was conducted among 5,091 children born between 2004 and 2015 in the rural Anhua county, southern China. Our results found vaccination coverage for primary EPI vaccines to be satisfactory, but the administration of subsequent booster doses was insufficient to prevent disease outbreak. Further to this, we found that the EPI vaccination timeliness was generally poor, especially for relatively new vaccines introduced after 2008 and for vaccines scheduled for administration in children ≥12 months. Despite this, our results showed that over time there was generally a significant improvement in vaccination coverage and the timeliness of EPI vaccinations. Unfortunately, this was not observed in non-EPI vaccines, where only small increases or considerable decreases in vaccination coverage were seen.
For EPI vaccines, coverage for vaccines scheduled for children <12 months was as high as 97.1%-99.4%. However, for vaccines scheduled for administration in older age groups, vaccination coverage was much lower. Especially noteworthy was the poor vaccine coverage for doses scheduled for children at 6 years of age, with coverage as low as 44.4%-51.7%, levels that fall far below the national goal of 90% vaccine coverage set by the Chinese EPI. This is concerning as low uptake of booster doses may lead to reductions in long-lasting protection, and overall low vaccination coverage compromises herd immunity. The decrease in vaccination coverage with increasing recommended vaccination age has also been found in register-based vaccination coverage studies conducted in Zhejiang and Shandong.Citation13,Citation14 Possible explanations for low vaccine uptake may be related to parents’ loss of vaccination awareness as their children get older, or due to lack of emphasis on the importance of vaccination booster doses from immunization related healthcare workers.Citation15,Citation16 However, there are other opportunities in which to address missing vaccines in children. For example, in China, it is a national requirement that vaccination records are checked at the point of child enrollment to kindergarten and school. Previous studies have indicated that an effective examination mechanism of vaccination cards at this enrollment event significantly improved overall vaccination coverage rates of EPI vaccines, especially for vaccines scheduled for administration after 3 years of age.Citation17 Therefore, interventions designed based on well-organized examination of vaccination cards at school enrollment could help to further improve EPI vaccine coverage, particularly for low uptake vaccines in older children.
We found that over time, across the study birth cohorts, vaccine coverage and timeliness of EPI vaccine administration generally improved. The slightly lower vaccine coverage seen in the 2014–15 birth cohort is likely due to the shorter follow up time compared to other cohorts. The 2014–15 cohort was only followed for 3 years and therefore the opportunity for this study to capture data on catch-up vaccinations was limited in this cohort. This overall improvement, in terms of vaccine coverage, may be attributed to a series of government initiatives. Firstly, investment in routine immunization services has been increasing over time, supporting improved vaccine procurement and the subsidization of grass-roots vaccination activities.Citation18–20 In 2015 over 70% of funding to support the EPI was provided by the government, a huge increase from only 33% in 2004.Citation18,Citation21 Secondly, the childhood immunization information management system (CIIMS) has been constantly improving since 2004. CIIMS is an important tool for strengthening the performance of immunization programs in China, as it consolidates vaccination records from multiple vaccination clinics, automates vaccination notices for each child registered, and allows the identification of pockets of unvaccinated individuals or groups. In 2010, 87.49% of Chinese children aged ≤6 years were registered in CIIMS.Citation22
Vaccination timeliness, the proportion of children receiving a vaccine at the recommended age, ranged from 48.1%–83.2% for traditional EPI vaccines for children <12 months. Among these, timeliness of the initial HepB dose at the time of birth was highest, closely followed by the time-of-birth BCG dose, which reached close to 90% in the 2014–2015 birth cohort. The high rate of timeliness for the HepB birth dose has been achieved due to increasing rates of hospital delivery, and due to the development of obstetric neonatal vaccination clinics.Citation23,Citation24 Despite the same recommended timing and same birth settings, we observed a small delay in birth dose BCG vaccines compared to HepB. This may be due to the manner in which the BCG vaccine is procured, as typically one vial of vaccine contains five doses. Vaccination staff may delay BCG vaccination to avoid vaccine waste, for example when there are fewer than five neonates to be vaccinated at one moment in time.Citation23
Another vaccine that this study found to be experiencing suboptimal delivery was the MCV. Across all birth cohorts, only 56.0% of children in our sample received their first dose of MCV (MCV-1) at the age of 8 months, and only 35.4% received their booster MCV-2 at the age of 18 months. However, between cohorts vaccination timeliness has improved, reaching a maximum of 70.9% for MCV-1 and 61.5% for MCV. Despite these improvements, timeliness remains worryingly poor considering resurgent outbreaks of measles have been reported frequently in recent years.Citation25 As such, we suggest that additional efforts toward improving the timely administration of the MCV are necessary to prevent potential measles outbreaks and to continue to work toward the goal of measles elimination.
In line with previous findings from studies in both developed and developing countries,Citation26–30 our study found vaccination delays were more prominent in relatively new EPI-vaccines and for vaccines scheduled for administration at ≥12 months. Across all vaccines in both of these categories, the proportion of children receiving a vaccine on time was low, with the highest proportion reaching only 35.4% for the second dose of MCV at 18 months of age.
With regards to new EPI vaccines, there are two possible reasons for vaccination delay in the first few years following introduction. Firstly, the cost of running the EPI has greatly increased throughout the years with expanding numbers of routine vaccinations. As such, the previous economic model for sustaining the EPI which relied on the sales revenue from non-EPI vaccines to partially support EPI operating costs, was not sustainable. Further, limited government investment was insufficient to address funding gaps in operating costs. With limited budgets, EPI logistical work (such as recruiting/training personnel and ensuring operational cold chain systems) could not be organized adequately within required timeframes. Subsequently, existing EPI workers prioritize immunization services for traditional, established EPI vaccines, over newly introduced EPI vaccines.Citation20,Citation31,Citation32 Secondly, insufficient manufacturer production capacity for new vaccines often leads to vaccine shortages, which may exacerbate existing delays. However, this is unlikely to be a major source of vaccination delay in our study, as the disparity between traditional EPI and new EPI vaccination timeliness existed even after many years of vaccine implementation.
Delayed vaccination leaves a pool of susceptible children in the population, increasing the risk of potential outbreaks of vaccine preventable diseases. The risk of outbreaks is amplified further when coupled with low vaccination coverage rates. It is not within the scope of this study to identify the determinants of vaccination delay. However, our results suggest that the timely vaccination of children should be considered an important measure of EPI performance, particularly with regards to newly introduced EPI vaccines and vaccines scheduled for ≥ 12 months of age.
In general, the vaccination coverage of non-EPI vaccines was similar to that of previous studies conducted in other developing, rural areas. Compared to the results of an existing meta-analysis of vaccination coverage in rural areas, the coverage rate estimated for the first dose of the Hib vaccine in this study (46.0%) is roughly comparable to the pooled coverage rate (41.6%). For VarV however, the coverage in our study (32.0%) is considerably lower than the meta-analysis reported pooled coverage rate (59.3%).Citation33,Citation34 Further, although the vaccination coverage of ORV and PCV7 found in this study was lower than the national 2012 coverage estimates in China, the estimates in our study were similar to others conducted in similar rural areas.Citation35,Citation36
Over time most non-EPI vaccines were demonstrated to have been slowly increasing their coverage, with the exception of the ORV where coverage decreased across all birth cohorts. This may be due to the adverse, rare side effects (e.g. intussusception) associated with the ORV, causing service providers to be less likely to recommend the ORV and parents less willing to vaccinate their children against rotavirusCitation37.
Currently, the central government of China is considering the introduction of the Hib, varicella vaccine and other new vaccines into the EPI. The sluggish increase in uptake and vaccination coverage observed in new, non-EPI vaccines in this study suggests that research into the determinants of vaccination uptake/refusal is required. Investigations into these determinants, prior to new vaccine implementation, may improve the delivery and uptake of newly available vaccines in China.
There were several limitations to this study. Firstly, the study restricted analyses to children with available vaccination data, which may lead to an overestimation of vaccination coverage, as children with vaccination data are more likely to have been vaccinated. Secondly, the findings of this study cannot be generalized to all rural areas in China, as the present study used participants from a previous sero-survey, and did not recruit with random sampling. Thirdly, due to the limited demographic data available for the study population, it was not possible to evaluate the potential factors that determine incomplete or delayed vaccination. Despite these limitations, this study offers a general picture of the vaccination status of children in this rural area of China. The study also gives insight into improvements in EPI and non-EPI vaccine delivery in the region over recent years, and highlights areas where the immunization program could be strengthened further in the future.
In conclusion, this study demonstrates the success and improvement of the current immunization program in China, but some challenges remain to be addressed. To prevent outbreaks of vaccine-preventable diseases, providers should increase efforts to achieve and maintain high levels of vaccination coverage, particularly for vaccine doses scheduled after 12 months of age. Additionally, timely administration of vaccines should be reinforced as an important measure of EPI performance. We recommend that future changes in vaccine practice and policy should primarily focus on improving the coverage and timeliness of vaccines introduced after 2008, and/or scheduled for administration ≥12 months of age. Finally, we further recommend that as the EPI in China expands to include new vaccines, specific determinants of vaccination uptake should be explored in advance of new vaccine roll-out.
Disclosure of potential conflicts of interest
H.Y. has received funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company and Shanghai Roche Pharmaceutical Company.
Supplemental Material
Download MS Word (31.8 KB)Acknowledgments
The authors would like to thank all of the health workers from Anhua County Center for Disease Control who conducted the interviews and data collection. Likewise, we acknowledge Mr. Fang, MJ from Hunan Provincial Center for Disease Control for technical assistance developing this paper.
Supplementary material
Supplemental data for this article can be accessed on the online at http://dx.doi.org/10.1080/21645515.2020.1772620..
Additional information
Funding
References
- Wang H, An Z, Yin Z. Achievements in prevention and control of seven infectious diseases targeted by the national immunization program in China across 70 years. Chin J Vaccines and Immun. 2019;25:359–67.
- Yu W, Lee L, Liu Y, Scherpbier R, Wen N, Zhang G, Zhu X, Ning G, Wang F, Li Y, et al. Vaccine-preventable disease control in the People’s Republic of China: 1949–2016. Vaccine. 2018;36(52):8131–37. doi:10.1016/j.vaccine.2018.10.005.
- Lance ER. China’s Expanded Program on Immunization success, challenge and healthier children. Chin J Vaccines and Immun. 2013;19:473–79.
- Liang X, Wu Z. Implementation of EPI for 30 years to protect hundreds of millions of people’s health. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:4–6.
- Cao L, Zheng J, Cao L, Yuan P, Cui J, Wang H, Li L. Immunization coverage of national immunization program vaccines at the township level, based on a survey conducted by the county level, China, 2013. Chin J Vaccines and Immun. 2014;20:486–91.
- Zheng J, Cao L, Guo S, Zhang X, Lu L, Wang L, Hu Y, Cao L, Yuan P, Cui J, et al. Immunization coverage of the national immunization program vaccines at the township level, based on a survey conducted by provincial CDCs in China, 2013. Chin J Vaccines and Immun. 2014;20(6):492–98.
- Ran Z. Examination and application of vaccination certificates among children in kindergartens and schools. Chin J Vaccines and Immun. 2015;21:444–49.
- Dombkowski KJ, Lantz PM, Freed GL. The need for surveillance of delay in age-appropriate immunization. Am J Prev Med. 2002;23:36–42.
- Rodewald L, Maes E, Stevenson J, Lyons B, Stokley S, Szilagyi P, Cao L. Immunization performance measurement in a changing immunization environment. Pediatrics. 1999;103:889.
- The People’s Government of Anhua County. Insight into Anhua. Yiyang (China): Office of county Party committee; 2019. [accessed 2019 Dec 14 Jan 1]. http://www.anhua.gov.cn/1/2/content_586499.html
- Wei X, Yang J, Gao L, Wang L, Liao Q, Qiu Q, Luo K, Yu S, Zhou Y, Liu F, et al. The transfer and decay of maternally-transferred antibody against enterovirus A71, and dynamics of antibody due to later natural infections: a longitudinal, paired mother-neonate cohort study in China (accepted). Lancet Infect Dis. 2020.
- R Core Team. Version 3.6.1 [software]. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna (Austria); 2019 July 31. https://www.R-project.org/.
- Zhang W, Liu S, Ceng Z, Xu Q, Song L, Li Z, Xiao Z, Zhang Y. Evaluation on immunization coverage rate of children from one to seven years old based on immunization information system in Shandong province. Xian Dai Yu Fang Yi Xue. 2015;42:3693–3697, 3729.
- Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L, Cao L. Using the immunization information system to determine vaccination coverage rates among children aged 1–7 years: a report from Zhejiang Province, China. Int J Environ Res Public Health. 2014;11(3):2713–28. doi:10.3390/ijerph110302713.
- Ran Z Investigation and evaluation of the current situation and effect of the verification of the vaccination certificate of the children in school [dissertation]. Beijing (China): China Center for Disease Control and Prevention; 2016.
- Cai B, Zhang C, Zhao M, Li N, Yu E, Li Q, Tang X, Zheng L, Li S, Yang B, et al. Analysis of coverage rates of vaccines in national immunization program in Hubei province. J Prev Med Inf. 2011;27(8):640–42.
- Zhang M, Ran Z, Zheng J, Cao L, Guo S, Zhou L, Liu F, Han Y, Yuan P. Impact of immunization certificate examination on coverage rates of national immunization program vaccines among children entering kindergarten and school. Chin J Vaccines and Immun. 2016;22:606–10.
- Yu W, Lu M, Wang H, Rodewald L, Ji S, Ma C, Li Y, Zheng J, Song Y, Wang M, et al. Routine immunization services costs and financing in China, 2015. Vaccine. 2018;36(21):3041–47. doi:10.1016/j.vaccine.2018.04.008.
- Zheng Z, Huang L, Huang Y, Zhuo J. Analysis on financing impact to the national expanded program for immunization in Guangxi Zhuang autonomous region. Chin J Vaccines and Immun. 2010;16:65–68.
- Zheng H, Liu Q, Guan X, Wang J, Ao R, Qi Q, Ma Q. Analysis on expanded programme on immunization in Sichuan province in 2010. Chin J Vaccines and Immun. 2012;18:158–61.
- Yu W, Jin S, Cui G, Yu J, Wang J, Tao Z. Study on financing of expanded program on immunization in some regions of China. Chin J Vaccines and Immun. 2005;11(4):292–97. doi:10.3969/j..1006-916X.2005.04.012.
- Jiang X, Cao L. Implementation and application progress of immunization information management systems. Chin J Vaccines and Immun. 2014;20:79–84.
- Cai B, Li N, Zhang C, Wang L, Tang X, Luo Y. Implementation and effect evaluation of obstetric neonatal immunization information management system. Chin J Vaccines and Immun. 2014;20:141–45.
- Cui F, Puer H, Hadler S, Liang X. Analysis of hospital delivery rate and neonatal vaccination coverage rate of hepatitis B vaccine in different regions of China. Chin J Vaccines and Immun. 2007;013(001):1–3.
- Ma C, Su Q, Hao L, Wen N, Fan C, Cao L, Zhang Y, Wang H, Luo H, Wang H, et al. Surveillance and response to measles outbreaks in China, 2009–2015. Chin J Vaccines and Immun. 2014;20(3):193–99.
- Babirye JN, Engebretsen IM, Makumbi F, Fadnes LT, Wamani H, Tylleskar T, Nuwaha F. Timeliness of childhood vaccinations in Kampala Uganda: a community-based cross-sectional study. PLoS One. 2012;7(4):e35432. doi:10.1371/journal.pone.0035432.
- Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7):e14. doi:10.1136/jech.2010.124651.
- Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, Van Damme P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine. 2014;32(2):284–89. doi:10.1016/j.vaccine.2013.10.084.
- Le Polain WO, Schellenberg JR, Manzi F, Mrisho M, Shirima K, Mshinda H, Alonso P, Tanner M, Schellenberg DM. Timeliness and completeness of vaccination and risk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int Health. 2013;5(2):139–47. doi:10.1093/inthealth/iht006.
- Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26(31):3805–11. doi:10.1016/j.vaccine.2008.05.031.
- Xi J, Cao L, Zheng J. The current situation and management strategy of human resources in China’s immunization program. Chin J Public Health Manage. 2014;30:372–73.
- Cao L, Wang H, Zheng J. National immunization coverage survey in China after integrated more vaccines into EPI since 2008. Chin J Vaccines and Immun. 2012;18:419–24.
- Li W, Yin G, Kong Y, Wang Q. Meta-analysis of the vaccination rate of Haemophilus influenzae type B (Hib) in Chinese children. Vaccine. 2017;34:69–73.
- Liu A, Sun T. Meta-analysis of vaccination rate of varicella vaccine in Chinese children. Chin J Vaccines and Immun. 2017;23:698–704.
- Jiang Y, Yin H, Shi Y, Yuan Y, Cao W, Ceng Q, Chang C. Immunization status of extra EPI vaccines and its influencing factors among children aged 1–6 years in Chongqing. Chin J Health Educ. 2013;29:605–07.
- Wang Z, Wang W, Guo W, Xu J, Li J. Survey on vaccination rate of second class vaccine and influencial factors in children in rural areas of nine counties in Henan province. Prog Microbio Immunol. 2013;41:53–61.
- CDC. Addition of history of intussusception as a contraindication for rotavirus vaccination. MMWR Morb Mortal Wkly Rep. 2011;60(41):1427.