ABSTRACT
Around 5–10% of healthy vaccinees lack or produce an inadequate antibody response following receipt of a standard hepatitis B vaccination regimen. Studying immune response to hepatitis B vaccination could promote researches of immunological events contributing to this poor response. To address this, we investigated follicular helper T (Tfh) cells and firstly demonstrated similar kinetics between circulating Tfh (cTfh) cells and Tfh cells derived from mice spleen after hepatitis B vaccination. And cTfh cells were positively associated with anti-HBs at one week after vaccination (D7). Furthermore, we found PBMCs stimulated by HBsAg showed preferential activation of CXCR3− Tfh cells subsets in vitro. The expression of transcription factor BCL6 in CD4+ T cell significantly differed between D7 and four weeks after vaccination (D28). However, dynamic curve of CD19+ B cells tended to rise then fall but no significant trends were observed. Our findings revealed a decrease in cTfh cells and subset skewing contribute to reduced antibody responses in immune response to hepatitis B vaccination, which indicated the importance of Tfh cell in facilitating the optimization of vaccine efficacy.
Introduction
Hepatitis B virus (HBV) infection affects approximately 257 million people worldwide, with estimated 900,000 deaths annually primarily attributed to HBV-related complications.Citation1 On account of lack of its functional cure and life-threatening complications, well-established vaccination programs with outstanding effectiveness against HBV have been considered the primary defense against HBV. Over the past three decades hepatitis B vaccination has been widely available across the world, there has been an overall downtrend in HBV prevalence. However, the global HBV infection elimination goals which were put forward by WHO remain to be achieved.Citation1,Citation2 There is epidemiological evidences around 5–10% of healthy adults lacked or produced a poor antibody response following completion of a standard three-dose vaccination regimen with anti-HB titers lower than 10 IU/L.Citation3 This would leave the population still vulnerable to HBV in areas where it is endemic. Importantly, studying immune response to hepatitis B vaccination will be of great value in ascertaining the immunological events that lead to poor responses, especially in susceptible adult populations.
The cellular mechanisms responsible for immunological responses to hepatitis B vaccine have not been fully elucidated to date. Given that hepatitis B surface antigen (HBsAg) are strict T-cell-dependent antigens, T cells are considered a prerequisite for protective antibody production in hepatitis B vaccination. When T cells are activated by diverse antigens, they differentiate into specific effector subsets to directly assist B cell in proliferation, differentiation and antibody class switching in the germinal center (GC). Therefore, T helper (Th) cells are pivotal component in clarifying the mechanisms responsible for the immune response to hepatitis B vaccination. Previous research in this area focused on traditional Th1 and Th2 cells and their cytokines.Citation4 However, their data were complicated and controversial regarding the role of Th1 and Th2 cells in the poor vaccine-induced immunity to HBV.Citation5,Citation6 Moreover, recent findings showed Th1 and Th2 cells and their cytokines only participated in the activation of B cells and antibody class switching,Citation7,Citation8 which may explain the inconsistent results in terms of the role of Th1 and Th2 cells in the poor response to vaccination.
Recently, follicular helper T (Tfh) cells, characterized by high expression of CXCR5, BCL6, PD-1, and ICOS have been clarified as a major function of CD4+ T cells in assisting with B cell differentiation, GC generation and antibody maturation via a stable interaction with B cells and have been discussed in a surprising range of human immune diseases.Citation9 Among these, some researchers investigated whether Tfh cells were cellular determinants of vaccine-induced immune responses, especially in the case of vaccine failure.Citation10 Tfh cells can be divided into three subpopulations according to the expression of CXCR3 and CCR6.Citation11 Interestingly, specific Tfh cell polarization emerged after challenge with different antigens and different subsets displayed distinct capacities to assist B cells.Citation12–14 While we previously reported initial studies exploring changes in cTfh cells and crucial microRNAs after revaccination with hepatitis B vaccination,Citation15 the role of cTfh cells subset and Tfh cells in spleen during the immune response against HBV remains unclear. It is technically challenging to obtain Tfh cells from the lymph of humans. Fortunately, circulating Tfh (cTfh) cells presented a similar phenotype and sustained B-cell responses after reactivation to be substituted in place of Tfh cells.Citation16 In brief, we aimed to identify specific changes in Tfh cells and subsets that were related to hepatitis B vaccine-induced antibody generation in the current study. These findings would provide a basis for improving poor responses and the rational design of optimized vaccines.
Materials and methods
Ethics approval
This study was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (Permit Number: PJ2015034KT). Written informed consent was provided by individual participants following a clear explanation of the study process, benefits and possible adverse effects. Animal experiments were approved by the Ethical Committee for Animal Research of Guangdong Medical University (Permit Number: GDY1801060).
Human subject for cohort 1
We recruited healthy volunteers prior to the start of the experiment. All subjects met the following inclusion criteria: (1) no history of HBV, HCV or other acute or chronic infectious disease, (2) history of a three‐dose series of hepatitis B vaccination, and (3) no contraindications to hepatitis B vaccine. In total, a cohort of 16 healthy volunteers were recruited from the Community Health Service Center of Dalang, Dongguan (China). Basic information (such as age, sex, immunization history of HBV vaccine in vaccination record) was obtained in a questionnaire survey. The age and gender of volunteer were 31.5 (24–44) years and 8/8 (male/female), respectively. Seventy-five percent of people have been vaccinated for more than 10 years. One dose of 20 μg hepatitis B vaccine (recombinant hepatitis B vaccine, Engerix-B, GlaxoSmithKline, Brentford, UK) was used for intramuscular immunization of the upper arm of all eligible individuals at the Community Health Service Center at Dalang. Peripheral blood samples were collected before vaccination (D0), and one week (range 7–8 days, D7), two weeks (range 14–15 days, D14), and four weeks (range 28–29 days, D28) after vaccination. According to antibody titer at D28, we classified healthy volunteers into responders (>100 IU/L) and non-responders (<10 IU/L).
Human subjects for cohort 2
Study subjects who have history of 0-, 1-, and 6-month standard schedule vaccination but no history of HBV infection and contraindications were recruited and consented at Guangdong Medical University (Dongguan, China). We recruited 12 women whose age ranging from 19 to 25. Eligible individuals were also injected a booster hepatitis B vaccination. Among these, blood samples of 12 eligible healthy young volunteers were collected at D0, D7, and D28 for further peripheral blood mononuclear cell (PBMC) stimulation with HBsAg.
Mice
BALB/c mice (6–8-weeks old, female) were purchased from the Central Animal Facility of Southern Medical University (Guangzhou, China) and housed in the SPF-level Laboratory Animal Room (Pearl Lab Animal Sci & Tech Co., Ltd, Dongguan, China). Animals were fed a standard rodent diet and tap water ad libitum throughout the whole study. Mice (n = 9 per group) were immunized intramuscularly with 0.25, 0.5, 1 and 2 μg of hepatitis B vaccine (Shenzhen Kangtai Biological Products Co., Ltd.). Mice were euthanized by cervical dislocation. The spleen and blood were quickly removed and measured after vaccination at 7, 14 and 21 days, respectively.
Processing of blood samples and multicolor flow cytometry
Fasting venous blood samples were collected from eligible subjects. The serum was obtained (200 μL/tube) and stored at ˗80°C. Human freshly isolated PBMCs at 106/tube from different points for each individual were stained in duplicate with antibodies for T cell and B cell staining. Antibodies Anti-human CD4 PerCP-Cyanine5.5 (eBioscience), Anti-human CD185(CXCR5) PE (eBioscience), Anti-human CD278(ICOS) APC-eFluor 780 (eBioscience) were stained for T cell and Hu CD19 PE HIB19(BD Biosciences) for B cell. Similar methods have been reported by our previous study.Citation15
PBMC stimulation with HBsAg
Twelve people were recruited from the cohort. One microgram HBsAg without Aluminum adsorbent (Shenzhen Kangtai Biological Products Co., Ltd.) was added to the supernatants in the antigen stimulating group. PBMCs with or without HBsAg were cultured in RPMI-1640 (Gibco) supplemented with 10% newborn cow serum (Gibco) and 1% penicillin-streptomycin, and were incubated at a humidified 37°C incubator in 5% CO2. After 24 h stimulation, cells were incubated with the antibodies indicated above and then the expression of cTfh cells in response to stimulation with HBsAg was assessed by flow cytometry. Flow cytometry scheme was Anti-human CD4 PerCP-Cyanine5.5, Anti-human CD185(CXCR5) PE, Anti-human CD278(ICOS) APC-eFluor 780, Anti-human PE-Cyanine 7 CXCR3 from eBioscience company.
Determination of serum anti-HBs by ELISA
The levels of anti-HBs were also quantitatively determined at baseline and post-vaccination using the Human HBsAb ELISA kit (Da An Gene Co. Ltd., Guangzhou, China) according to the instructions provided by the manufacturer and all standards were tested in duplicate.
Immunofluorescence (IF) staining and analysis
IF staining was used to assess the protein expression of CD4 (anti-CD4 antibody [EPR19514, Abcam]) and CXCR5 (anti-CXCR5 antibody [ab203212, Abcam]) in spleens. Tfh cells were graded quantitatively in three representative fields selected at random (40×) according to the Fromowitz standard.Citation17 The spleens were removed from mice and fixed in 4% formalin. Antigen retrieval was conducted after dewaxing of the paraffin-embedded tissue blocks. The specimens were blocked with antibody dilution buffer (2% goat serum) for 30 min. Tissue sections were then incubated with primary antibodies overnight at 4°C. After three washes, the sections were incubated with secondary antibodies for 1 h at 37°C. Then, the sections were counterstained with hematoxylin for 3 min.
For some micrographs, microscopy (OLYMPUS DP71, Japan) was performed to scan a large area of the spleen and then adjusted to a 40× magnification. After the acquisition, the images were analyzed by immunochemical staining of anti-CD4 and anti-CXCR5 antibodies on the same sections. CXCR5 and CD4 double positive cells were identified as Tfh cells. Tfh cells were graded quantitatively by two independent observers in three representative fields selected at random (40×) according to the Fromowitz standard, which depends on both the intensity and percentage of positively stained cells.Citation17 In brief, intensity was graded according to the scale: 0 = absent, 1 = weak, 2 = moderate, 3 = strong; the percentage of CD4-positive cells was graded as: 0 = < 25%, 1 = 26–50%, 2 = 51–75%, 3 = >75%; and the number of CXCR5-positive cells were graded as 0 = <5, 1 = 5–10, 2 = 11–15, 3 = > 15). The final staining score was the summation of the intensity and percentage scores. An overall score ≤4 was defined as low Tfh cell expression, ≥5 but ≤7 was defined as intermediate expression, and ≥8 was defined as high expression.
Cell isolation and real-time quantitative PCR
Similar methods of cell isolation have been reported by our previous study.Citation15 cDNA was generated with the Transcriptor First Strand cDNA Synthesis kit (Roche, Mannheim, Germany) on an ABI 7500 PCR instrument (Applied Biosystems) in a total volume of 10 μL. The optimum temperature cycling protocol for the amplification of genes and mature miRNAs was 95°C for 15 s, 56°C for 30 s and 60°C for 30 s for 40 reaction cycles. The housekeeping genes ACTB were used as endogenous. The results were calculated using the 2−ΔCt method. Detailed information on the primer pairs is shown in .
Table 1. Primers
Statistical analysis
Differences between multiple time points were examined using One-Way Repeated Measures Analysis of Variance. When data did not show a Gaussian distribution, Friedman test was used to analyze repeated measures data in mice experiment.Citation18 For comparison between two continuous populations, paired two-tailed Student’s t-tests were performed as appropriate. The levels of anti-HBs were quantitatively analyzed based on Arithmetic mean. Correlations between variables were carried out using Pearson’s or Spearman correlation coefficients as appropriate. A Wilcoxon signed-rank test was applied when data did not show a Gaussian distribution. All statistical analyses were performed with the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). A P value of less than .05 was considered statistically significant (*, P < .05; **, P < .01; ***, P < .001).
Results
cTfh cells from healthy vaccinees peaked at 7 days following hepatitis B vaccination
We recruited a cohort of subjects to study T cell change after hepatitis B vaccination (). Based on Tfh cells function in antibody formation, it was important to gain insight into whether cTfh cells were the main functional CD4+ T cell subset in hepatitis B antibody production. Firstly, total cTfh cells were gated as CXCR5+CD4+ cells, and longitudinally tracking cTfh cells found a significant difference in dynamic changes and presented peaks at D7 (P = .005) (). Blood cTfh cells expressing ICOS were propose
Figure 1. Kinetics study from healthy adults following hepatitis B vaccination. (A)
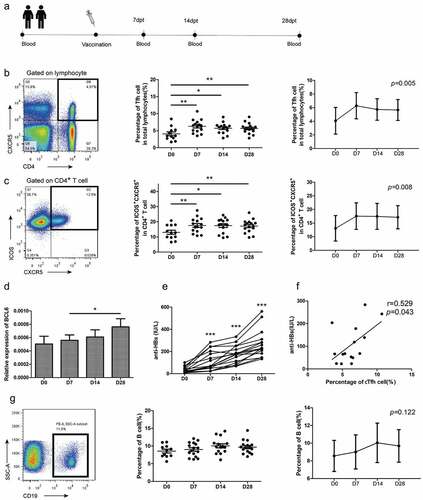
Our kinetics study also showed the generation of protective antibody responses presented a general upward trend from baseline to D28 (P < .001) and all enrolled volunteers were responders with antibody level higher than 100 IU/L (). To study CD19+ B cells, referred to as inactivated B cells, we utilized antibodies against CD19 during the development of immune responses to hepatitis B vaccination to study dynamic changes in B cells. The results showed the dynamic curve tended to rise then fell but no significant trends were observed. However, the cTfh cells that emerged in blood were positively associated with anti-HBs at D7 but not other time points ().
Immunization with HBsAg induces significant levels of Tfh expression in the spleens of mice
Although growing evidence shows cTfh cells share functional properties with Tfh cells during vaccine responses,Citation19–21 we also measured Tfh expression in the spleens of mice to verify cTfh cells as a convenient alternative after specific hepatitis B vaccination (). The results showed anti-HBs showed an upward trend with increased immunization with high antigen doses at D7 in the different antigen dose groups, demonstrating a dose-response relationship. It was intriguing that 1 μg group contributed the highest level of anti-HBs compared with the other dosage groups and presented significant peaks at D14 (). Subsequently, we used a well-established immunological marker of the concomitant expression of CD4, CXCR5 in the same spleen sections to represent Tfh cells by immunohistochemistry (). Given that Tfh cells were involved in antibody generation in lymphoid organs, it was important to further probe this similarity with lymphoid Tfh cells. These cells significantly increased up to D7 and then showed a general downward trend in 1 μg group ().
Figure 2. Immunization with HBsAg induces significant levels of Tfh expression in spleen of mice. (A)
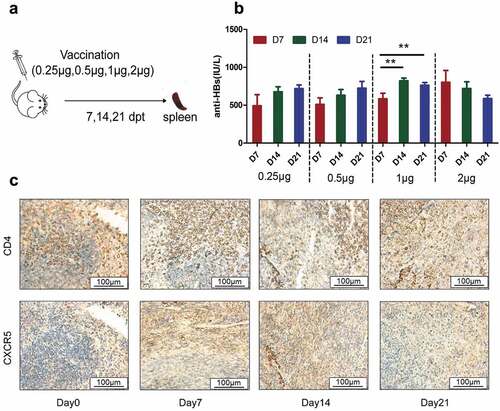
Table 2. The expression of cTfh cell with 1 μg HBsAg in mice
HBsAg stimulation of PBMCs from young vaccinees contributed to the generation of antibody by preferentially activating CXCR3− Tfh cells in vitro
To validate our observations, we obtained PBMC samples from a cohort of young volunteers to explore whether HBsAg-specific cTfh cells would change upon antigenic challenge in vitro. The kinetics study showed the anti-HBs presented a general upward trend from baseline to D28 and 11 people were responders. (data not shown). A previous study reported cTfh cells also potently induced antibody production in vitro.Citation22 For this analysis, the total and activated cTfh cells in HBsAg-induced PBMC cultures also showed a significant increase at different time points, with the exception of total cTfh cells at D28. It is intriguing that the analyzed different cell population particularly increased response to stimulation at D0 compared to later time points ().
Figure 3. Tfh cells subsets expression after HBsAg stimulation of PBMCs from young adults in vitro. (A-D)
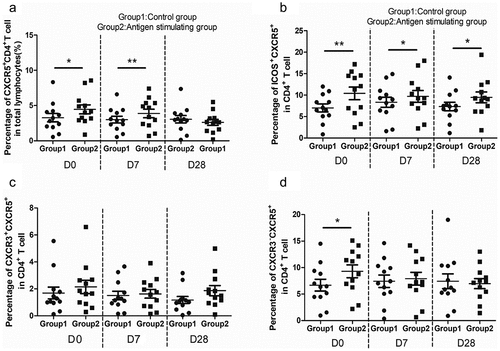
Subsequently, CXCR3 expression in cTfh cells was assessed. Importantly, we demonstrated that Tfh2 and 17-polarized CXCR3˗Tfh cells showed an increase at D0, whereas Tfh1-polarized CXCR3+Tfh did not increase after in vitro stimulation (), which implied that Tfh cells might differentiate into fully functional effector CXCR3−Tfh subsets upon activation with HBsAg in accordance with long-lived antibody responses.
Discussion
Over the past decades, the currently available safe and effective hepatitis B vaccine and standard vaccination schedules have made an overall decline in infection rates. Despite this, 5–10% of individuals do not exhibit an antibody response and the precise cellular events contributing to low hepatitis B vaccination remain largely unknown. Given the importance of Tfh cells in antibody responses, some researchers have explored the role of Tfh cells in vaccine responses. Tfh cells have been explored in response to hepatitis B vaccine but only in HIV-1-infected children receiving ART with impaired CD4+T cells, and contradictory results were obtained in the cTfh cell profile.Citation23,Citation24 In this study, we explored the cTfh cell and subsets profile in response to hepatitis B vaccination among healthy volunteers, which provided clues for further low-responsiveness to hepatitis B vaccination study. To our best knowledge, our study is the first to report the specific contribution of Tfh cells in spleen and subsets to the immune response to hepatitis B vaccination. Our findings provided evidence of promising targets with which to improve vaccine efficacy against HBV.
Obviously, it is a challenge to characterize lymphoid Tfh cells longitudinally in healthy humans at different time points, thus we analyzed dynamic alterations in CXCR5+CD4+ T cells in mice spleens to demonstrate the role of Tfh cells in antibody titers following hepatitis B vaccination, as previously described for human blood Tfh cells as substitutes for specific Tfh responses in lymphoid tissues.Citation25 We determined the optimal dosage (1 μg) of HBsAg after different dosage vaccines to explore the role of Tfh cell. Interestingly, anti-HBs levels declined with time after vaccination of 2 μg HepB vaccine, which is different from the trends in other dosage groups. This phenomenon may be attributed to the superior ability of high dose vaccine to activate Tfh cells which caused quickly memory immune response to assist B cell in antibody production and peak of antibody level to shift to an earlier date.Citation10,Citation26For further analysis, significant changes in both CXCR5+CD4+ T cells and antibodies after vaccination and the similar dynamics and peaks between cTfh cells and lymphoid Tfh cells supported the idea CXCR5+CD4+ T cells could act as a convenient alternative to lymphoid Tfh cells in analysis of the immunology induced by specific hepatitis B vaccine. Previous similar study also showed significant increase in Tfh cell after revaccinationCitation27 which supported significant kinetics of frequency of cTfh and lymphoid Tfh cells after vaccination. However, some studies reported inconsistency result in the frequencies of cTfh cells among different level of anti‐HBs titers responders.Citation27,Citation28 The inconsistency may be due to study design, such as study participants, doses of vaccine, and history vaccination. Our study was based on healthy young people with history vaccination and Xing M group provided a full course of vaccination to chronic hepatitis patients without history vaccinationCitation27,Citation28 and Li X et al. studied efficacy vaccination in 0- to 8-year-old children.Citation27 It was also possibly that cTfh cells function besides number played an important role in low-responsiveness to hepatitis B vaccination.Citation29 Further study in large cohorts need to clarify role of cTfh cells for low-responsiveness to hepatitis B vaccination.
ICOS expression is involved in Tfh generation and GC formation upon antigen challenge and is often used to define activated cTfh cells.Citation30 We also assessed ICOS+CXCR5+CD4+ T cells and found an increased frequency of ICOS-expressing cTfh cells, with similar results being reported with other types of vaccine.Citation13,Citation31 It is possible that the rapid increase in the total and activated cTfh cells in blood undergoing revaccination reflects a rapid memory immune response to vaccination by activating preexisting memory cTfh cells.Citation32 In addition, although the total as well as the activated cTfh cells from healthy volunteers appeared to peak at D7 post vaccination and were substantially lower at D14 and D28 following hepatitis B vaccination, which was in line with our previous results,Citation15 cTfh cells still sustained expression at a higher level during our observation period. Xing M et al.Citation28 also reported ICOS+ pTfh cells underwent expansion only in healthy responder group, which supported role of ICOS+pTfh cells in inducing anti‐HBs production. BCL6 expression significantly differed between D7 and D28, supporting a specific transcription factor in Tfh cell differentiation. Moreover, the cTfh cells that emerged in blood were positively associated with anti-HBs at D7, indicating that cTfh cells may involve in the specific response to hepatitis B vaccination. It was widely recognized that GC-Tfh cell formed from pre-Tfh cell at D7 and memory Tfh cell recalled just for 3–4 days.Citation33 Other studies also reported association between cTfh and vaccine response in this pivotal time point.Citation13,Citation34 However, we did not find a similar association at other time-points. The possible reason is that Tfh cell level peaked at D7 have played a dominant role in antibody generation. When all Tfh cell from healthy people maintained a high level, association at D28 was weakened. It was might that pivotal time point lacked in previous study leading to a result that no significant difference of cTfh cells was found among non, low and high responders.Citation28 These observations supported our work extending toward the specific role of cTfh cells in the low-responsiveness to hepatitis B vaccination.
Further determining the major subset of Tfh cells is a key step in elucidating the role of Tfh cells and their differentiation in the development of immune responses to hepatitis B vaccine. Tfh2 and 17-polarized CXCR3˗Tfh cells emerged as the subsets with superior capacity than others in cTfh cell pools to facilitate B cell differentiation and maturation.Citation12,Citation35 CXCR3+Tfh cells such as Tfh1 cell just provided insufficient help to memory B cells which was consistent with limited efficacy of influenza vaccination.Citation36 The increase in CXCR3˗Tfh cells after HBsAg stimulation in vitro indicated the hepatitis B antibody response might also be dependent on the skew of the cTfh subset and the increase in Tfh cell number alone may not be sufficient to improve the antibody response in line with similar findings regarding Tfh cell numbers in mice.Citation37
Although there is a clear link between Tfh cells and antibody responses with T cell-dependent vaccines, particularly involving T–B cell interactions, few studies have reported the simultaneous role of B cell in specific antibody production.Citation12,Citation24,Citation34 Our study reported the role of B cell and no dynamic change in B cell was established. However, memory B cell, antibody-producing plasma cell directly affected the production of antibodies. The lack of evidence about memory B cell subsets limited association between Tfh cell and potential B cell subsets in our study. Whether memory B cell, plasma cell, not total B cell, play a part in the hepatitis B vaccine remained to be classified. Further studies should be explored B cell subsets and interactions of T-B cell in poor immune response in hepatitis B vaccination.
Our study has some limitations. Based on ambispective cohort study, we could not ensure that enrolled volunteers injected the same vaccines in the primary vaccination during childhood or adulthood or same time interval between primary vaccination and revaccination, which could influence on T-cell responses. Then we only carried out a preliminary study on the role of B cells, and the memory B cell subset, interactions between Tfh cells and B cells in the immune response to hepatitis B vaccine remain to be explored. To obtain further insight, we dynamically analyzed the immunological parameters rather than focusing on a stationary time point like previous research. In addition, considering the frequency and quantity of blood sampling required, eligible volunteers were limited to adults. Although adults are at highest risk of HBV, whether there was an age-related factor affecting expression changes and our correlations are fully applicable to infants and children with vaccination failure needs to be further investigated.
In conclusion, our results provide the first evidence Tfh cells and CXCR3− Tfh cells subset are, at least in part, responsible for immune responses to hepatitis B vaccination. Importantly, our findings indicate the potential benefit of preferentially promoting the generation of highly functional Tfh cell subsets could help improve antibody responses to hepatitis B vaccine.
Acknowledgments
General: We would like to express special gratitude to all the personnel who supported or helped with this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hutin Y, Nasrullah M, Easterbrook P, Nguimfack BD, Burrone E, Averhoff F, Bulterys M. Access to treatment for Hepatitis B virus infection - worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:773–77. doi:10.15585/mmwr.mm6728a2. PMID:30025413.
- WHO. Global Hepatitis Report. 2017:1–83.
- Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006;78:169–77. doi:10.1002/jmv.20524. PMID:16372285.
- Kardar GA, Jeddi-Tehrani M, Shokri F. Diminished Th1 and Th2 cytokine production in healthy adult nonresponders to recombinant hepatitis B vaccine. Scand J Immunol. 2002;55:311–14. 11940238. doi:10.1046/j.1365-3083.2002.01057.x.
- Larsen CE, Xu J, Lee S, Dubey DP, Uko G, Yunis EJ, Alper CA. Complex cytokine responses to hepatitis B surface antigen and tetanus toxoid in responders, nonresponders and subjects naive to hepatitis B surface antigen. Vaccine. 2000;18:3021–30. 10825606. doi:10.1016/S0264-410X(00)00084-0.
- Wataya M, Sano T, Kamikawaji N, Tana T, Yamamoto K, Sasazuki T. Comparative analysis of HLA restriction and cytokine production in hepatitis B surface antigen-specific T cells from low- and high-antibody responders in vaccinated humans. J Hum Genet. 2001;46:197–206. doi:10.1007/s100380170089. PMID:11322660.
- Kaech SM. T-bet in Tfh cells: now you see me, now you don’t. J Exp Med. 2018;215:2697–98. doi:10.1084/jem.20181878. PMID:30337467.
- Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. 2016;34:335–68. doi:10.1146/annurev-immunol-041015-055605. PMID:26907215.
- Crotty S. Follicular Helper T. Cell Biology: a decade of discovery and diseases. Immunity. 2019;50:1132–48. doi:10.1016/j.immuni.2019.04.011. PMID:31117010.
- Linterman MA, Hill DL. Can follicular helper T cells be targeted to improve vaccine efficacy?. F1000Res. 2016;5: doi:10.12688/f1000research.7388.1. PMID:26989476.
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi:10.1016/j.immuni.2010.12.012. PMID:21215658.
- Farooq F, Beck K, Paolino KM, Phillips R, Waters NC, Regules JA, Bergmann-Leitner ES. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci Rep. 2016;6:27944. doi:10.1038/srep27944. PMID:27323685.
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra132. doi:10.1126/scitranslmed.3005191. PMID:23486778.
- Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–42. doi:10.1016/j.it.2014.06.002. PMID:24998903.
- Xu X, Li Y, Liang Y, Yin M, Yu Z, Zhang Y, Huang L, Ni J. MiR-18a and miR-17 are positively correlated with circulating PD-1(+)ICOS(+) follicular helper T cells after hepatitis B vaccination in a chinese population. BMC Immunol. 2018;19:25. doi:10.1186/s12865-018-0263-y. PMID:30055570.
- Asrir A, Aloulou M, Gador M, Perals C, Fazilleau N. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun. 2017;8:847. doi:10.1038/s41467-017-00843-7. PMID:29018187.
- Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, Grimson R, Lundy J. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18:1268–75. 3315956. doi:10.1016/S0046-8177(87)80412-4.
- Sheldon MR, Fillyaw MJ, Thompson WD. The use and interpretation of the Friedman test in the analysis of ordinal-scale data in repeated measures designs. Physiother Res Int. 1996;1:221–28. doi:10.1002/pri.66. PMID:9238739.
- Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, Kurupati RK, Kannan S, Ertl H, Schmader KE, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol. 2014;193:3528–37. doi:10.4049/jimmunol.1302503. PMID:25172499.
- Aljurayyan A, Puksuriwong S, Ahmed M, Sharma R, Krishnan M, Sood S, Davies K, Rajashekar D, Leong S, McNamara PS, et al. Activation and induction of antigen-specific T Follicular Helper cells play a critical role in live-attenuated influenza vaccine-induced human mucosal anti-influenza antibody response. J Virol. 2018;92. doi:10.1128/jvi.00114-18. PMID:29563292.
- Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, et al. Circulating Th1-cell-type Tfh cells that exhibit impaired B Cell help are preferentially activated during Acute Malaria in Children. Cell Rep. 2015;13:425–39. doi:10.1016/j.celrep.2015.09.004. PMID:26440897.
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. 11104798. doi:10.1084/jem.192.11.1553.
- Bekele Y, Amu S, Bobosha K, Lantto R, Nilsson A, Endale B, Gebre M, Aseffa A, Rethi B, Howe R, et al. Impaired phenotype and function of T Follicular Helper cells in HIV-1-infected Children receiving ART. Medicine (Baltimore). 2015;94:e1125. doi:10.1097/md.0000000000001125. PMID:26166114.
- Bekele Y, Yibeltal D, Bobosha K, Andargie TE, Lemma M, Gebre M, Mekonnen E, Habtewold A, Nilsson A, Aseffa A. T follicular helper cells and antibody response to Hepatitis B virus vaccine in HIV-1 infected children receiving ART. Sci Rep. 2017;7:8956. doi:10.1038/s41598-017-09165-6. PMID:28827754.
- Brenna E, Davydov AN, Ladell K, McLaren JE, Bonaiuti P, Metsger M, Ramsden JD, Gilbert SC, Lambe T, Price DA, et al. CD4(+) T Follicular Helper cells in Human tonsils and blood are clonally convergent but divergent from non-Tfh CD4(+) cells. Cell Rep. 2020;30:137–52. e135 doi:10.1016/j.celrep.2019.12.016. PMID:31914381.
- Pilkinton MA, Nicholas KJ, Warren CM, Smith RM, Yoder SM, Talbot HK, Kalams SA. Greater activation of peripheral T follicular helper cells following high dose influenza vaccine in older adults forecasts seroconversion. Vaccine. PMID:27919633. 2017;35:329–36. doi:10.1016/j.vaccine.2016.11.059.
- Li X, Xu Y, Dong Y, Yang X, Ye B, Wang Y, Chen Y. Monitoring the efficacy of infant hepatitis B vaccination and revaccination in 0- to 8-year-old children: protective anti-HBs levels and cellular immune responses. Vaccine. PMID:29588118. 2018;36:2442–49. doi:10.1016/j.vaccine.2018.03.044.
- Xing M, Feng Y, Yao J, Lv H, Chen Y, He H, Wang Z. Induction of peripheral blood T follicular helper cells expressing ICOS correlates with antibody response to hepatitis B vaccination. J Med Virol. 2020;92:62–70. doi:10.1002/jmv.25585. PMID:31475733.
- Pallikkuth S, de Armas LR, Rinaldi S, George VK, Pan L, Arheart KL. Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: results from the FLORAH study. Plos Biol. 2019;17:e3000257. doi:10.1371/journal.pbio.3000257. PMID:31100059.
- Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh cell differentiation. Front Immunol. 2016;7:520. doi:10.3389/fimmu.2016.00520. PMID:27933060.
- Abudulai LN, Fernandez S. Production of IgG antibodies to pneumococcal polysaccharides is associated with expansion of ICOS+ circulating memory T follicular-helper cells which is impaired by HIV infection. PLos One. 2017;12:e0176641. doi:10.1371/journal.pone.0176641. PMID:28463977.
- Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur J Immunol. 2012;42:1981–88. doi:10.1002/eji.201242540. PMID:22730020.
- Song W, Craft J. T follicular helper cell heterogeneity: time, space, and function. Immunol Rev. 2019;288:85–96. doi:10.1111/imr.12740. PMID:30874350.
- Bentebibel SE, Khurana S, Schmitt N, Kurup P, Mueller C, Obermoser G, Palucka AK, Albrecht RA, Garcia-Sastre A, Golding H, et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6:26494. doi:10.1038/srep26494. PMID:27231124.
- Matsui K, Adelsberger JW, Kemp TJ, Baseler MW, Ledgerwood JE, Pinto LA. Circulating CXCR5(+)CD4(+) T Follicular-like Helper cell and Memory B cell responses to Human Papillomavirus Vaccines. PLoS One. 2015;10:e0137195. doi:10.1371/journal.pone.0137195. PMID:26333070.
- Koutsakos M, Nguyen THO, Kedzierska K. With a little help from T Follicular Helper Friends: humoral Immunity to influenza vaccination. J Immunol. 2019;202:360–67. doi:10.4049/jimmunol.1800986. PMID:30617117.
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–53. doi:10.1016/j.immuni.2010.07.015. PMID:20691615.
