ABSTRACT
We have evaluated the immunological response to Hepatitis B virus (HBV) booster vaccine dose in 129 adults with underlying diseases in comparison with 694 subjects at occupational risk of infection, who have previously completed the primary series and resulted with anti-HBs <10 mIU/mL. After booster dose, 60.5% of the patients with underlying diseases and 14.8% of the subjects at occupational risk resulted seronegative. By comparing two groups, rate of subjects with anamnestic response was higher in at occupational risk group respect to that at risk for medical conditions (OR: 5.99 [95%IC, 3.81–9.41], p < .001). This difference was associated to gender (males/females: OR: 0.619 [95%IC, 0.421–0.910], p = .015) and age (better response for younger people, p = .011).
Sir,
we read with great interest the article of Grazzini et al.Citation1 In this study, authors assessed the effectiveness of Hepatitis B virus (HBV) booster vaccine dose in eliciting the immunological response in seronegative (anti-HBs level <10 mIU/mL) healthcare workers (HCWs) and students of Careggi Teaching Hospital (Florence, Italy), vaccinated during childhood or adolescence. As with other Italian experiences, Citation2,Citation3 they observed that 87.8% (698/795) of the study population responded to the challenge dose. Moreover, among those persistently seronegative, 76.2% (32/42) who received the fifth dose and 60.0% (3/5) who completed the second vaccination course seroconverted.Citation1 In their final considerations, the authors highlighted the importance of receiving all the three additional vaccine doses, in order to obtain protection.
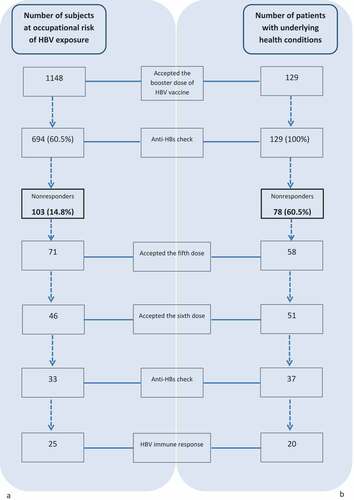
These results are in line with a similar analysis that we have retrospectively conducted in 1148 subjects at occupational risk of HBV infection (51.6% females; mean age 28.7 ± 4.81 years), followed up at San Martino’s Hospital outpatient vaccination clinic (Liguria Region, Italy). One thousand sixty-three (92.6%) students or clinicians of health disciplines and 85 (7.4%) scavengers, who have previously completed the primary series and resulted with anti-HBs concentrations <10 mIU/mL, received a single challenge HBV vaccine dose. Of them, only 694 subjects (60.4%) accepted serological testing at least 1 month after immunization (). The majority of patients were females (58.8%) and had a mean age of 28.7 ± 12.15 years. The proportion of subjects with available serological testing resulting seroprotected (anti-HBs ≥ 10 mIU/mL) was 85.2% (591/694). Four hundred sixty (66.3%) were vaccinated in the first year of life, 148 (21.3%) in adulthood and 86 (12.4%) were vaccinated in adolescence; as the results observed by Grazzini et al., we didn’t find any statistically significant difference among groups stratified by age at the time of the first vaccination course (Chi-square = 2.87; p = .24). Being female was associated with a higher rate of seroprotection with anti-HBs ≥ 10 mIU/mL (OR: 1.65 [95%IC, 1.06–2.55], p = .026).
Seventy-one (68.9%) and 46 (44.7%) of those persistently seronegative after the booster vaccine dose (N = 103) accepted the fifth dose and sixth dose, respectively. Only 71.7% (33/46) of subjects who completed the second vaccination course had post-immunization serology; 75.6% (25/33) of them responded to revaccination ().
In Italy, the National Immunization Plan 2017–2019 recommends HBV vaccination to newborns and to categories of subjects with an increased risk of infection for occupational exposure or for specific behaviors. Vaccination is also recommended to adults with high-risk medical conditions (for example chronic liver disease, hemophilia, hemodialysis, psoriasis/eczematous lesions, HIV infection).Citation4
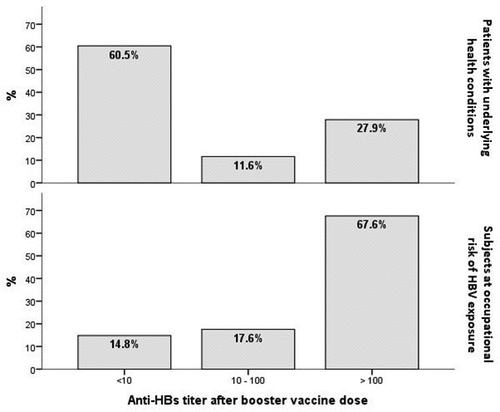
We have evaluated the immunological response rate to HBV booster vaccine dose in adults with underlying diseases in comparison with the response rate observed in the at occupational risk group previously described. A total of 129 patients who have received the primary three-doses vaccination course in the past and resulted with anti-HBs level <10 mIU/mL were included in this study. Seventy-five (58.1%) of them were HIV infected patients, 28 (21.7%) candidates to/received a solid organ transplantation (SOT), 18 (14.0%) with HCV infection, 1 of them undergoing chemotherapy, 8 (6.2%) with other underlying health conditions, 4 of them treated with immunosuppressants (). Mean age was 45.0 ± 14.98 years, 72.9% of the subjects were males. Seventy-eight (60.5%) had an anti-HBs concentration < 10 mIU/mL after the booster vaccine dose and 15 of the 51 (29.4%) patients who mounted an anamnestic response were low-responders (anti-HBs titer 10–100 mIU/mL) (). Although a second vaccination course was offered to all seronegative subjects, only 51 of them (65.4%) received all three additional doses and 37 had an available blood test after the last dose. Twenty patients (20/37, 54.0%) responded to the second vaccination course ().
Table 1. Study population according to medical condition
By comparing two groups, the rate of subjects showing an anamnestic response after the booster dose was higher in HCWs and scavengers group respect to that at risk for medical conditions (OR: 5.99 [95%IC, 3.81–9.41], p < .001) ().
Moreover, this difference between groups in the anamnestic response to HBV booster dose was also associated to gender (males vs females: OR: 0.619 [95%IC, 0.421–0.910], p = .015) and age (better response for younger people, p = .011). Differences between two groups in the rate of response was not influenced by the time elapsed between the date of last vaccine dose of the primary course and the date of booster dose (p = .57) (data available for 448 subjects at occupational risk and 92 patients with underlying diseases, respectively).
By considering the antibody titer after the second vaccination course, no difference was revealed in the rate of response between two groups (p = .83).
Our results emphasize the importance of serological testing in patients at high-risk in order to identify those non responders to the primary vaccination and to protect them through revaccination; this advice becomes even stronger in patients with underlying health conditions, who in our study have shown to be less likely to immunologically respond to the booster dose.
This aspect is also particularly relevant considering the severity of chronic HBV in immunocompromised hosts. HIV-infected people, for example, are prone to develop HBV coinfection due to the shared transmission routes and among them HBV infection is more likely to progress to chronic hepatitis and cirrhosis, Citation5-8 but the immune response to the vaccination in this population is less effective compared with HIV negative adults.Citation9-15 In HIV-infected persons the immunogenicity ranges from 18% to 72% and, in terms of magnitude and antibody persistence, is lower than in the general population.Citation5,Citation16,Citation17 Higher CD4 counts and lower HIV viral loads have been associated with improved immunological response to vaccination.Citation9,Citation18-20 Seventy-four patients (74/75, 98.7 %) of our study population had CD4-T cell count higher than 200 cells/mmc and 68 (68/75, 90.7%) had HIV-RNA below 50 copies/mL, in the 6 months preceding the booster dose.
The serological evaluation after the third dose is so important among these patients as their poor response to the primary vaccine series.Citation10 In case of anti-HBs non-protective levels after an initial vaccination course, a single challenge dose seems to be able to implement the proportion of subjects with protective antibody concentrations, Citation10,Citation21,Citation22 but in another study just a small increase was reported.Citation10,Citation23 However, revaccination is effective in increasing the response to the vaccineCitation10,Citation22 and have been observed as 36–85%.Citation9,Citation19,Citation24,Citation25 Some experts recommend deferring the second vaccination course up to antiviral therapy has been started and an adequate CD4 count has been reached.Citation9 Some studies have demonstrated the efficacy of high-dose rechalling vaccination, Citation9,Citation26 while other studies have not confirmed these findings.Citation9,Citation27 Of note, non-responders to the primary vaccination course who responded to revaccination lost HBV protective antibody level more rapidly respect to those who responded to the first three doses; therefore, these patients could take more advantage by serological follow-up.Citation9,Citation24,Citation28
For patients with other underlying diseases, similar to the HIV-infected patients, the seroconvertion rates to HBV vaccination are affected by impaired immunity. Immunization of patients with liver cirrhosis with the standard dose has been shown to be ineffectiveCitation29-31 with a response rate that ranged from 16% to 79%.Citation29-31 In order to improve the immunogenicity double-dose vaccine or accelerated dose schedules have been used.Citation29,Citation32-35 Because of the poor responsiveness to vaccination, as for HIV-infected patients, serological testing after the primary course is advised in immunocompromised patients to check if revaccination is necessary.Citation29,Citation36,Citation37 Has been demonstrated that a second vaccination course could increase the response rates in cirrhotic patients who are unresponsive to the primary vaccination.Citation29
HBV vaccination efficacy could also be compromised by immunosuppression. Patients not treated with immunosuppressant had a higher probability to obtain a better immune response to the vaccination than those on immunomodulatory (RR 1.33; 95% CI 1.08–1.63) or anti-TNF therapy (RR 1.57; 95%-CI 1.19–2.08).Citation38 Timeliness of the serological testing is crucial especially in those candidates to long-term immunosuppressive, before the start of the treatment.
Twenty-eight patients of our study population were solid organ transplanted or candidates to transplantation, of whom 24 for liver dysfunction (85.7%). HBV immunization before organ transplantation is recommended for all nonimmune patients as reduce the risk of infection from hepatitis B virus core antibody-positive donorsCitation39 and because of the high risk of severe HBV infection after transplantation.Citation40 However, the seroconversion rates in these patients are suboptimal.Citation40 In addition, antibody levels in those who have responded to the primary course drop rapidly and up to 35% of patients become seronegative after liver transplant.Citation40,Citation41 Vaccination before transplantation is to be preferred because of the better efficacy profile. The majority of our study cohort (20/28, 71.4%) received the HBV booster dose after transplantation.
In conclusion, our study confirms the importance of assessing the need for revaccination in order to quickly protect nonresponders. Consistent with the evidence by Grazzini et al.Citation1 we observed a poor compliance to revaccination among both at risk for medical conditions and at risk for occupational exposure study groups (only 65.4% and 44.7% of them accepted all three additional doses proposed, respectively). It becomes even more crucial in fragile and immunocompromised patients, in which a greater lack of antibody response after the challenge dose has been observed. These data underline the importance of offering adequate counseling and addressing the misperception of risk and uncertainties about the vaccine effectiveness.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Grazzini M, Arcangeli G, Mucci N, Bonanni P, Bini C, Bechini A, Boccalini S, Tiscione E, Paolini D. High chance to overcome the non-responder status to hepatitis B vaccine after a further full vaccination course: results from the extended study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother. 2020;16(4):949–54. doi:10.1080/21645515.2019.1680082.
- Dini G, Toletone A, Barberis I, Debarbieri N, Massa E, Paganino C, Bersi F, Montecucco A, Alicino C, Durando P. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: A cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum Vaccin Immunother. 2017 Feb;13(2):440–44. doi:10.1080/21645515.2017.1264788.
- Stefanati A, Bolognesi N, Sandri F, Dini G, Massa E, Montecucco A, Lupi S, Gabutti G. Long-term persistency of hepatitis B immunity: an observational cross-sectional study on medical students and resident doctors. J Prev Med Hyg. 2019 Sep 30;60(3):E184–E190. doi:10.15167/2421-4248/jpmh2019.60.3.1315.
- Italian Ministry of Health. National immnunization prevention plan 20172019. Published on the Italian Official Gazette; 2017 February 18 [accessed 2020 Mar 31]. http://www.gazzettaufficiale.it/eli/id/2017/02/18/17A01195/sg
- Nicolini LA, Magne F, Signori A, Di Biagio A, Sticchi L, Paganino C, Durando P, Viscoli C. Hepatitis B virus vaccination in HIV: immunogenicity and persistence of seroprotection up to 7 years following a primary immunization course. AIDS Res Hum Retroviruses. 2018 Nov;34(11):922–28. doi:10.1089/aid.2017.0070.
- Alter MJ. Epidemiology of viral hepatitis and HIV coinfection. J Hepatol. 2006;44:S6–S9. doi:10.1016/j.jhep.2005.11.004.
- Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, Zilmer K, Vella S, Kirk O, Lundgren JD for the EuroSIDA Group. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS Lond Engl. 2005;19:593–601. doi:10.1097/01.aids.0000163936.99401.fe.
- Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter cohort study (MACS). Lancet. 2002;360:1921–26. doi:10.1016/S0140-6736(02)11913-1.
- Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-infected adult: a review of the current recommendations, part I. Infect Dis Ther. 2017;6(3):303–31. doi:10.1007/s40121-017-0166-x.
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013;2014(58):309–18.
- van den Berg R, van Hoogstraten I, van Agtmael M. Non-responsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev. 2009;11:157–64.
- Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS. 2009;20:595–600. doi:10.1258/ijsa.2009.009126.
- Collier AC, Corey L, Murphy VL, Handsfield HH. Antibody to human immunodeficiency virus (HIV) and suboptimal response to hepatitis B vaccination. Ann Intern Med. 1988;109:101–05. doi:10.7326/0003-4819-109-2-101.
- Paitoonpong L, Suankratay C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis. 2008;40:54–58. doi:10.1080/00365540701522975.
- Pasricha N, Datta U, Chawla Y, Singh S, Arora SK, Sud A, Minz RW, Saikia B, Singh H, James I, et al. Immune responses in patients with HIV infection after vaccination with recombinant hepatitis B virus vaccine. BMC Infect Dis. 2006;6:65. doi:10.1186/1471-2334-6-65.
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore A, Bell B, Hennessy T, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200(9):1390–96. doi:10.1086/606119.
- Lopes VB, Hassing RJ, de Vries-sluijs TE, El Barzouhi A, Hansen BE, Schutten M, de Man RA, van der Ende ME. Longterm response rates of successful hepatitis B vaccination in HIV-infected patients. Vaccine. 2013;31:1040–44. doi:10.1016/j.vaccine.2012.12.047.
- Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, Peel S, Agan BK. Hepatitis B vaccine responses in a large US military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731–38. doi:10.1016/j.vaccine.2009.04.016.
- Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12:966–76. doi:10.1016/S1473-3099(12)70243-8.
- Okulicz JF, Mesner O, Ganesan A, O’Bryan TA, Deiss RG, Agan BK. Hepatitis B vaccine responsiveness and clinical outcomes in HIV controllers. PLoS One. 2014;9:1–8. doi:10.1371/journal.pone.0105591.
- Abzug MJ, Warshaw M, Rosenblatt HM, Levin M, Nachman S, Pelton S, Borkowsky W, Fenton T. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis. 2009;200:935–46. doi:10.1086/605448.
- Irungu E, Mugo N, Ngure K, Njuguna R, Celum C, Farquhar C, Dhanireddy S, Baeten JM. Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J Infect Dis. 2013 Feb 1;207(3):402–10. doi:10.1093/infdis/jis695.
- Keet IP, van Doornum G, Safary A, Coutinho RA. Insufficient response to hepatitis B vaccination in HIV-positive homosexual men. AIDS. 1992;6:509–10.
- Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, Wendling MJ, Vetter D, Nicolle M, Kempf-Durepaire G, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects HIV-1 Viral Load Vacc. 2000;18:1161–65.
- De Vries-Sluijs TE, Hansen BE, van Doornum GJ, Springeling T, Evertsz N, de Man R, van der Ende M. A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J Infect Dis. 2008;197:292–94. doi:10.1086/524690.
- Psevdos G, Kim JH, Groce V, Sharp V. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS. 2010;24:403–07. doi:10.1089/apc.2009.0340.
- Rey D, Piroth L, Wendling MJ, Miailhes P, Michel M-L, Dufour C, Haour G, Sogni P, Rohel A, Ajana F, ANRS HB04 B–BOOST study group, et al. Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): a multicentre, open-label, randomised controlled trial. Lancet Infect Dis. 2015;15:1283–91. doi:10.1016/S1473-3099(15)00220-0.
- Cruciani M, Mengoli C, Serpelloni G, Lanza A, Gomma M, Nardi S, Rimondo C, Bricolo F, Consolaro S, Trevisan M, et al. Serologic response to hepatitis B vaccine with high dose and increasing number of infections in HIV infected adult patients. Vaccine. 2009;27:17–22. doi:10.1016/j.vaccine.2008.10.040.
- Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27(6):e1942. doi:10.1002/rmv.1942.
- Roni DA, Pathapati RM, Kumar AS, Nihal L, Sridhar K, Tumkur Rajashekar S. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv Virol. 2013;2013:196704. doi:10.1155/2013/196704.
- Villeneuve E, Vincelette J, Villeneuve JP. Ineffectiveness of hepatitis B vaccination in cirrhotic patients waiting for liver transplantation. Can J Gastroenterol. 2000;14(Suppl B):59b‐62b. doi:10.1155/2000/548206.
- Engler SH, Sauer PW, Golling M, Klar EA, Benz C, Stremmel W, Kallinowski B. Immunogenicity of two accelerated hepatitis B vaccination protocols in liver transplant candidates. Eur J Gastroenterol Hepatol. 2001;13:363‐367. doi:10.1097/00042737-200104000-00010.
- Kallinowski B, Benz C, Buchholz L, Stremmel W. Accelerated schedule of hepatitis B vaccination in liver transplant candidates. Transplant Proc. 1998;30:797‐799. doi:10.1016/S0041-1345(98)00053-0.
- Arslan M, Wiesner RH, Sievers C, Egan K, Zein NN. Double‐dose accelerated hepatitis B vaccine in patients with end‐stage liver disease. Liver Transpl. 2001;7:314‐320. doi:10.1053/jlts.2001.23069.
- Bonazzi PR, Bacchella T, Freitas AC, Osaki KT, Lopes MH, Freire MP, Machado MCC, Abdala E. Double‐dose hepatitis B vaccination in cirrhotic patients on a liver transplant waiting list. Braz J Infect Dis. 2008;12:306‐309. doi:10.1590/S1413-86702008000400009.
- Mast EE, Weinbaum CM, Fiore AE, Burton N, Cox-Ganser J, Damon S, Falk H, Fridkin S, Garbe P, McGeehin M, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006;55:1‐33.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine‐preventable diseases. Washington (DC): Public Health Foundation; 2011. p. 12.
- Manser CN, Maillard MH, Rogler G, Schreiner P, Rieder F, Bühler S Vaccination in patients with inflammatory bowel diseases. Digestion. 2020 Jan 22 2020:1–11. [published online ahead of print].
- Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery RK, Koval C, Lease ED, Pillai A, Doucette KE, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015 May;15(5):1162–72. doi:10.1111/ajt.13187.
- Ljungman P. Vaccination of immunocompromised patients. Clin Microbiol Infect. 2012;18(Suppl 5):93–99. doi:10.1111/j.1469-0691.2012.03971.x.
- Horlander JC, Boyle N, Manam R, Schenk M, Herring S, Kwo PY, Lumeng L, Chalasani N. Vaccination against hepatitis B in patients with chronic liver disease awaiting liver transplantation. Am J Med Sci. 1999;318:304–07. doi:10.1016/S0002-9629(15)40643-3.