?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In 2000, China was declared polio-free. However, in 2018, wild poliovirus (WPV) was still endemic in two of its neighboring countries, making WPV importation and outbreak alarming possibilities. This study documents the seroprevalence of poliovirus antibodies before and after the polio vaccine switch in 2012 and 2017 in Beijing. Cross-sectional population-based serologic surveys were conducted in 2012 and 2017 in Beijing. The study subjects were selected from 10 different age groups (<1, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, and ≥40 y) using a multi-stage-stratified sampling method. Neutralizing antibody titers against poliovirus serotypes 1 (P1), 2 (P2), and 3 (P3) were assayed by World Health Organization standards. The seropositive rates (SR) and geometric mean titer (GMT) of the neutralizing antibodies were 91.71% and 1:130.26, respectively, for P1, 94.09% and 1:113.39, respectively, for P2, and 88.78% and 1:79.65, respectively, for P3 before the switch in 2012, and 87.78% and 1:108.93, respectively, for P1, and 81.67% and 1:70.56, respectively, for P3 after the switch in 2017, with a statistically significant difference for P1 and P3 between 2012 and 2017. The neutralizing antibodies for all poliovirus serotypes differed among different age and vaccination groups in both 2012 and 2017. After switching polio vaccines twice in 2014 and 2016, the P1 and P3 polio antibody levels were lower in 2017 than in 2012. The P2 antibody levels were determined from the first dose of IPV. The seroprevalence of poliovirus antibodies after adjustment of the immunization schedule of the polio vaccine on January 1, 2020, must be further monitored.
KEYWORDS:
Importance
This is the first study to document the seroprevalence of poliovirus antibodies before and after polio vaccine switch from a trivalent oral poliovirus vaccine (tOPV) immunization schedule to a combined inactivated poliovirus vaccine (IPV)/bivalent OPV1 and 3 (bOPV) sequential schedule in 2012 and 2017 in Beijing to evaluate the immunity level of the population. The study showed that the Beijing population polio antibody levels for P1 and P3 in 2017 were lower than those in 2012 after switching the polio vaccine twice in 2014 and 2016. In Beijing, a healthy population under 15 y maintained a higher polio antibody level before and after the vaccine switch, which could effectively prevent further transmission of type 1 and 3 imported wild poliovirus and vaccine-derived poliovirus. However, due to decreased levels of type 1 and 3 polio antibodies and a lower level of type 2 antibody from only a single dose of IPV after the vaccine switch, it was suggested to further monitor the seroprevalence of poliovirus antibodies after adjustment of the immunization schedule of polio vaccine on January 1, 2020.
Background
Poliovirus (PV) causes poliomyelitis and other neurologic disorders. Occasionally, it invades the central nervous system and destroys lower motor neurons, causing a clinically distinctive flaccid paralysis. It is transmitted primarily through the fecal-oral route and comprises three different serotypes: poliovirus type 1 (P1), 2 (P2), and 3 (P3).Citation1 Since the World Health Assembly launched the Global Polio Eradication Initiative in 1988, global polio eradication activities have resulted in the near elimination of the disease from six regions, with the number of cases being reduced by more than 99%, from over 350,000 cases in 1988 to as few as 33 in 2018. The number of countries in which polio was endemic decreased from 125 to 2 during the same period.Citation2 Despite the progress made, PV-free countries remain at risk of Wild poliovirus (WPV) importation. For example, in May 2014, the World Health Organization (WHO) declared the international spread of PV an emergency. Until WPV is eradicated globally, its importation and outbreaks will continue in countries that share borders with WPV-endemic countries.Citation3 Thus, it was essential for PV-free countries to maintain the high vaccination rate of polio vaccine so as to maintain the level of poliovirus antibodies.
Live attenuated trivalent oral poliovirus vaccine (tOPV) was included in China’s Expanded Immunization Program (EPI) in 1978. Children were immunized with a three dose tOPV at 2, 3, and 4 months old as primary vaccination, followed by a single dose at 4 y old as a booster vaccine.Citation4 Intensified large-scale supplementary immunization activities were carried out in the 1990 s to eliminate polio. By strengthening routine immunization of polio vaccine and carrying out mass polio vaccination activities, polio vaccine vaccination rate has been continuously improved and a good immune barrier of polio antibodies has been formed.Citation5 The Western Pacific Region including China was declared WPV-free in October 2000.Citation6 As China shares borders with two countries that had endemic WPV in 2018, WPV importation has been a continuous threat to China’s polio-free status. WPV importations were explained by inadequate service delivery of poliovirus vaccine and low seroprevalence of polio antibodies.Citation7,Citation8
In Beijing, oral-attenuated polio vaccine was first used in 1959 and was included into the EPI in 1978. There has been no reported case of polio in Beijing since 1985.Citation9 Beijing, the capital of China, has frequent political, economic, and cultural cooperation with foreign countries. So its population was at risk of infection from imported WPV.Citation10–12
Polio eradication required a complete absence of WPVs and the absence of vaccine polioviruses contained in tOPV. tOPV needs to be withdrawn because in rare circumstances vaccine poliovirus may revert to establish circulating vaccine-derived poliovirus (cVDPV).Citation13 Paralysis caused by WPV is clinically indistinguishable from paralysis caused by VDPV.Citation14 Of the three types of WPV, the last case of type 2 was reported in 1999Citation15 and declared eradicated in September 2015, and the most recent case of type 3 was reported in November 2012 and declared eradicated in October 2019. The outbreaks caused by type 2 cVDPV represented the vast majority of all cVDPV.Citation16 To completely eliminate vaccine-derived polioviruses from the community, OPV withdrawal was planned in a phased manner.Citation17 To eradicate polio, WHO proposed the Polio Eradication and Endgame Strategic Plan 2013–2018, which recommended that ≥1 dose of inactivated poliovirus vaccine (IPV) should be added to the routine immunization schedule in all countries using OPV before 2014, and that tOPV should be replaced by bivalent OPV 1 and 3 (bOPV) in a synchronized manner worldwide in 2016, followed by complete OPV cessation.Citation18 In Beijing, the first dose of tOPV was withdrawn and replaced by IPV on December 25, 2014. Since the switch, IPV has been given at 2 months old, followed by tOPV at 3 and 4 months as well as 4 y old. On May 1, 2016, the national polio vaccine switch was launched, and it included the introduction of the first dose of IPV in routine immunization schedule, the replacement of tOPV by bOPV, and the recovery and destruction of tOPV. Thus, the national immunization schedule of polio was sequentially one dose of IPV at 2 months old followed by three doses of bOPV at3 and 4 months, and 4 y old.
Seroprevalence survey is a useful tool for assessing the performance of an immunization program, evaluating the status of immunity against polio, and discovering areas with low immunity and populations susceptible to polio infection. The study was designed to determine the prevalence of antibodies against poliovirus serotypes among broad age groups selected from different districts of Beijing in 2012 and 2017, before and after polio vaccine switch from tOPV immunization schedule to a combined IPV/bOPV sequential schedule in Beijing.
Materials and methods
Study population
Two cross-sectional population-based serological surveys were conducted in Beijing before and after the vaccine switch in 2012 and 2017. Of 16 districts, 7 and 8 were selected in 2012 and 2017, respectively, using a multi-stage stratified sampling method based on the geographical location and demographic characteristics. Using a systematic sampling method, 10 villages/communities were selected from each district. Individuals who had resided in the study area for at least 6 months were selected by convenience sampling. Volunteers were consecutively enrolled after written informed consent was obtained from adults and parents or guardians of children. The exclusion criteria for individuals were as follows: (1) those with serious acute illness; (2) those diagnosed or suspected of congenital immunodeficiency disorders and other serious diseases; (3) those with contraindication to venipuncture; and (4) those who refused or did not respond. All participants were stratified into 10 different age groups (<1, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, and ≥40 y) according to the pre-defined sample size. The ratio of native and migrant residents was within 0.9–1.1 in each group. The calculation of sample size was based on the formula:. The positive rates of antibodies were assumed at 50% (π = 50%) for each age group with both genders. A minimum sample size was required for each age group, with confidence level of 95% (a = 0.05) and a desired precision of 10% (δ = 10%). In 2012 and 2017, 220 (22 per group) and 250 (25 per group) subjects, respectively, were enrolled in each district.
Questionnaire survey
Questionnaire surveys were conducted to collect data on general demographic information, history of polio vaccine immunization, and history of polio disease through a face-to-face interview. Information on children below than 14 y was extracted from the Beijing Immunization Program Management Information System or child immunization record books. History of vaccination against polio was obtained by questioning participants older than 14 y. History of polio disease was collected by using questionnaires to ask participants whether they had been diagnosed with polio by a medical institution.
Blood sample collection and antibody detection
A nurse collected a 2 ml venous blood sample from each participant by venipuncture into a vacutainer tube. Samples were immediately placed in an icebox and transported to the laboratory. Within 24 h of sample collection, samples were centrifuged for 10 min at 1500–2000 rpm, and then each sample serum (>1 ml) was stored at −20°C in a deep freezer. All samples were tested at the WHO polio reference laboratory of Beijing Centers for Disease Control and Prevention (CDC). Neutralizing antibodies against P1, P2, and P3 were detected in 2012 by a microneutralization assay with authentic Sabin strains in accordance with WHO guidelines.Citation19 Using the same method in 2017, only P1 and P3 neutralizing antibodies were detected as all P2 samples were stored and destroyed after national implementation of the polio vaccine switch on May 1, 2016. Serum samples were completely inactivated at 56°C for 30 min, diluted from 1:4 to 1:1 024, and then incubated in duplicate wells for 3 h at 36°C with 50% tissue culture infective doses (TCID50) of poliovirus antigen. After a 7-d incubation, the highest dilution of serum that protected 50% of the cultures was recorded. A serum sample was considered positive if the neutralizing antibody level was present at a dilution of 1:8. The detection of serological antibody level in the population is a routine monitoring work carried out by the Beijing Center for Disease Control and Prevention, which is used to evaluate the population immunity levels. The informed consent was obtained. This investigation did not apply for ethical review.
Statistical analysis
The database was set up using Epidata 3.1 software. Data analysis was performed using SPSS 19.0 software. Seropositivity rate (SR) was calculated for each group. The geometric mean titer (GMT) of neutralizing antibodies was used to describe the concentrations as neutralizing antibody after the logarithm transformation conformed to normal distribution. Bar graph represented antibody positive rate of different polio types, and linear graph represented GMT of different polio types. The chi-square test was used to compare the seropositivity rates among different groups. The differences in the levels of neutralizing antibodies between different groups were tested by t-test or analysis of variance (ANOVA). A p value <.01 was considered statistically significant.
Results
Study population
In 2012 and 2017, 1676 and 2144 subjects were enrolled, respectively, with ratios of male to female 1:1.05 (817:859) and 1:1.08 (1032:1112) and the ratios of native and migrant residents 1:1.00 (840:836) and 1:1.00 (1074:1070), respectively. The number of participants per age group ranged from 160 to 177 (9.55%–10.56%) in 2012 and 208 to 218 (9.70%–10.17%) in 2017. The geographical distribution of the subjects was analyzed according to geographical location in Beijing as follows: In 2012, 684 (40.81%) in urban region, 724 (43.20%) in suburb region, and 268 (15.99%) in outer suburb; and in 2017, 780 (36.38%) in urban region, 864 (40.30%) in suburb region, and 500 (23.32%) in outer suburb. Comparing population distribution in 2012 and 2017, there was no statistically significant difference in population based on gender, resident status, and age (gender: χ2 = 1.141, P = .707; resident status: χ2 = 0, P = .987; age: χ2 = 1.294, P = .998). Contrarily, the difference between their geographical distributions was statistically significant (χ2 = 31.863, P < .001).()
Table 1. Demographic data of the study population in Beijing in 2012 and 2017
Polio antibody seroprevalence
The overall SR of neutralizing antibodies for P1, P2, and P3 was 91.71%, 94.09%, and 88.78%, respectively, in 2012, and 87.78% and 81.67% for P1 and P3, respectively, in 2017. With regard to seropositivity to all the serotypes, 1378 (82.22%) participants were seropositive to a combination of P1, P2, and P3 in 2012, while 1625 (75.79%) participants were seropositive to a combination of P1 and P3 in 2017. The antibody SR for P1 and P3 was significantly higher in 2012 than in 2017 (P1:χ2 = 15.437, P < .001, P3:χ2 = 36.905, P ≤ 0.001) (, ).
Table 2. Poliovirus-antibody seropositive rate according to different characteristics in 2012 and 2017
Figure 1. Antibody seropositivity of single serotype and combined serotypes in 2012 and 2017.Seropositive rate: P1:χ2 = 15.437, P < .001, P3:χ2 = 36.905, P < .001; Geometric mean titer: P1:t = 3.202, P = .001, P3:t = 2.180, P = .029
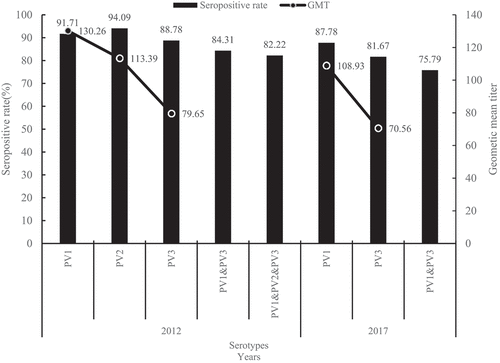
The GMT of neutralizing antibodies for P1, P2, and P3 was 1:130.26, 1:113.39, and 1:79.65 in 2012, and 1:108.93 and 1:70.56 for P1 and P3 in 2017. The GMT of neutralizing antibodies for P1 and P3 was significantly higher in 2012 than in 2017 (P1:t = 3.202, P = .001, P3:t = 2.180, P = .029) (, ).
Table 3. GMTs of poliovirus-antibody according to different characteristics in 2012 and 2017
Antibody levels in different populations
In 2012 and 2017, the SR and GMT for all tested PV antibodies did not significantly differ with gender. The antibody SR for P1 among female participants was significantly higher in 2012 than in 2017 (χ2 = 16.240, P < .001), and SR for P3 among both males and females was also significantly higher in 2012 than in 2017 (Male:χ2 = 19.873, P < .001; Female:χ2 = 17.332, P < .001) ().
In 2012 and 2017, there was no significant difference in SR and GMT for all tested PV antibodies between different residents. The antibody SR for P1 among native residents was significantly higher in 2012 than in 2017 (χ2 = 9.989, P = .002), while the antibody SR for P3 among both native and migrant residents was significantly higher in 2012 than in 2017 (Native: χ2 = 24.467, P < .001; Migrant: χ2 = 13.317, P < .001).()
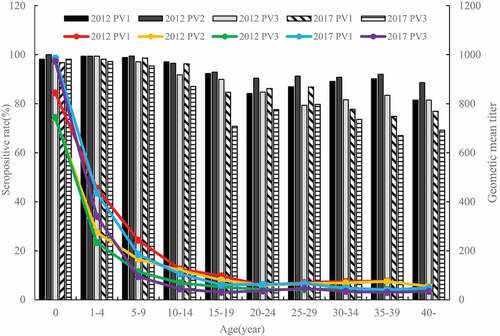
Among different age groups, the SR and GMT for neutralizing antibodies against P1, P2, and P3 differed in 2012, and those for neutralization antibodies against P1 and P3 differed in 2017 (All P values less than 0.001). In 2012, the antibody seroprevalences for P1, P2, and P3 were higher among children aged 0–14 with SR of 91.76%-100% and GMT of 1:69.72–1:844.07, compared to adults above 15 y with SR of 79.38%-92.90% and GMT of 1:41.68–1:98.05, with statistically significant difference. Similarly, in 2017, the antibody seroprevalences for P1 and P3 were also significantly higher among children aged 0–14 with SR of 87.04%-98.62% and GMT of 1:44.82–1:985.67 than among adults above 15 y with SR of 66.98%-86.79% and GMT of 1:30.69–1:67.44. For the different age groups, the results showed that the antibody SR for P1 in the 30–39 y group was significantly higher in 2012 than in 2017 (30–34: χ2 = 8.653, P = .003; 35–39: χ2 = 14.384, P < .001), while the antibody SR for P3 among the 15–19 age groups and over 35 y groups was significantly higher in 2012 than in 2017 (15–19: χ2 = 21.029, P < .001; 35–39: χ2 = 13.078, P < .001; 40: χ2 = 7.296, P = .007) (, ).
Antibody levels in different immunization history
Compared to participants who did not receive polio vaccine and those with unknown polio vaccination history, those with polio vaccination had significantly higher levels of SR and GMT for each PV serotype in 2012 and 2017 (All P < .001). The results suggested that SR and GMT for P1, P2, and P3 did not increase with increased polio vaccine dose ().
Discussion
Although China was declared WPV-free in 2000, the population could be at risk of infection through WPV importation or circulation of vaccine-derived PV that threatens global eradication.Citation20 When maintaining a polio-free state, the level of polio antibody in the healthy population directly reflects the level of immune barrier. Therefore, in this study, we evaluated the seroprevalence of neutralizing antibodies for PV in 10 different age groups (<1, 1–4, 5–9,10-14, 15–19, 20–24, 25–29, 30–34, 35–39, and ≥40 y) before and after polio vaccine switch in 2012 and 2017 in Beijing. This is the first study to document the change of polio antibodies since the introduction of IPV in 2014 and the replacement of tOPV by bOPV in 2016 in Beijing. A five-year interval survey of polio antibody level in a healthy population in Beijing played an important role in predicting whether there was a potential risk of transmitting poliovirus in the population.
This study noted that the SR of neutralizing antibodies against P1, P2, and P3 was between 89% and 94%, and the GMT was between 1:80 and 1:130 in a healthy population in 2012 before polio vaccine switch. The SR range of P1, P2, and P3 antibodies in all age groups was from 81% to 100%. In 2017, the SR of P1 and P3 antibodies in healthy population was 82%and 88% and GMT was 1:71 and 1:109, respectively. The SR range of P1 and P3 antibodies in all age groups was from 69% to 99%. The five-year interval survey results of polio antibody level showed a significant decline before and after polio vaccine switch, including the introduction of IPV in 2014 and replacement tOPV by bOPV in 2016, indicating that the adjustment of polio vaccine immunization strategy in Beijing had some effect on the level of neutralizing antibodies for P1 and P3. Sutter documented that in developed countries, polio outbreak could be prevented with population polio neutralizing antibody levels of 66–80%. However, in developing countries with suboptimal sanitation and hygiene, wild poliovirus transmission and outbreaks could occur with population immunity levels as high as 94–97%. The neutralizing polio antibody level higher than 97% for all poliovirus types was a contributing factor in preventing polio infection.Citation21 Therefore, the higher level of immunity against polio outbreak and transmission with the seropositive rate of 82–94% has long been established among broad age healthy populations in Beijing with better sanitation and hygiene. Particularly, for polio-susceptible students under 15 y old and preschool children, the positive rate and GMT were maintained at a high level with SR of 87–100% and GMT of 1:45–1:986. However, the polio antibody decreased to a lower level after polio vaccine switch, especially in people above 15 y old with SR of 67–87% and GMT of 1:31–1:67, which may be due to the absence of indirect immunization after IPV replacement of tOPV. Until polio is eradicated globally, Beijing, which has frequent international communications, remains at risk of poliovirus importation. In an outbreak of wild poliovirus in Xinjiang province of China in 2011, a survey indicated that 4.0% of the sample population had no antibodies against the three poliovirus serotypes.Citation22 Several other serological studies also revealed that the polio outbreaks caused by WPV1 were likely due to the immunity gap, as reported in the Democratic Republic of Congo in 2010–2011Citation23 and in Northern Nigeria in 2012–2013.Citation24 Furthermore, not only wild poliovirus but also cVDPV may spread across countries and cause disease in susceptible populations.Citation25 There was no significant difference in the SR and GMT of P1, P2, and P3 antibodies between men and women, as well as native and migrant residents. Therefore, the conclusion that gender and resident status had an impact on the level of antibodies in the population could not be drawn in this study.
The study showed that there was a decline in SR and GMT of polio antibodies with age. This was consistent with what had been detected in Korea.Citation26 The level of GMT rapidly declined in the first few years after the completion of basic immunization. After the age of 15, GMT tended to be stable and maintained at a certain level. Studies have shown that intestinal immunity to poliovirus waned over time. The SR of antibodies in polio-susceptible children under 15 y maintained a higher level, which is likely attributable to the EPI requiring children to receive 4 doses of polio vaccine. As recommended in the WHO polio vaccine position paper,Citation27 four doses of tOPV were administrated where only tOPV schedule was used before OPV Switch in April 2016. Where a sequential IPV-bOPV schedule was used after OPV Switch, the initial administration of one or two doses of IPV should be followed by ≥2 doses of bOPV to ensure both sufficient levels of protection in the intestinal mucosa and a decrease in the burden of VAPP. In 2017, the SR of antibodies for P1 and P3 among children under 15 y was 87%-99%. The SR of antibodies in adults above 15 y decreased slightly with the increase in age with SR of 67%-87%. In 2012, the SR of antibodies against P1, P2, and P3 in children under 15 y was 92%–100% and 79%–92% in the adults over 15 y old. There was no statistically significant change of polio antibody levels in children under 15 y caused by polio vaccine switch, while in adults over 15 y, the levels significantly decreased due to polio vaccine switch.
The antibody SR and GMT of persons with 0–1 dose polio vaccine and with unknown immunization history were lower. They included three children under 14 with 0 dose of polio vaccine; one was younger than the initial immunization age and the others had contraindications. Some adult participants gave inaccurate recall, and they were considered as unvaccinated or having unknown vaccination history.
After the polio vaccine immunization strategy switch on May 1, 2016, the level of P2 antibody was determined from the first dose of IPV. The sequential immunization program of polio vaccine carried out by Lu et al. in Beijing showed that after the first dose of IPV, the GMT of P2 neutralizing antibody was only 1:37.77, and the SR was 96.4%.Citation28 As the following bOPV vaccine only contained type 1 and 3 polio vaccine, the level of P2 antibody gradually decreased with time. Other studies showed that the positive conversion rate of serum neutralizing antibodies for P2 after the first dose of IPV at the age of 6–8 months was lower, 32%-39%.Citation29,Citation30 In 2017 and 2018, 96 and 71 cases of cVDPV2 occurred in nine countries.Citation31 Therefore, once type 2 polio vaccine-associated virus was imported, the risk of polio serotype 2 VDPV and its epidemiology may occur.
There were several limitations in this study. First, the participants in the final step at the survey site were not selected randomly, which may result in selection bias. Secondly, polio neutralizing antibodies of type 2 could not be detected in 2017 due to the destruction of all type 2 poliovirus samples in Provincial Polio Network Laboratory after national implementation of the polio vaccine switch on May 1, 2016. The changes in type 2 antibody levels could not be analyzed and compared between 2012 and 2017.
In conclusion, the study showed that the polio antibody levels for P1 and P3 in the Beijing population were lower in 2017 than 2012 after switching polio vaccine twice in 2014 and 2016. The polio antibody levels of a healthy population under 15 y of age in Beijing were maintained at a higher level before and after the polio vaccine switch, which could effectively prevent further transmission of type 1 and 3 imported wild polioviruses and vaccine-derived poliovirus. However, because of decreased levels of type 1 and 3 polio antibody and lower level of type 2 polio antibody from only a single dose of IPV after polio vaccine switch, China has adjusted the polio vaccine immunization schedule from a single dose of IPV followed by three doses of bOPV to two doses of IPV followed by two doses of bOPV since January 1, 2020. The main purpose was to increase the level of type 2 antibody. It was necessary to monitor the seroprevalence of poliovirus antibodies regularly.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We are grateful to the efforts made by staff of district centers for disease control and prevention in Beijing involved in this study, including Dongcheng District CDC, Xicheng CDC, Chaoyang District CDC, Fengtai District CDC, Shijingshan District CDC, Haidian District CDC, Mentougou District CDC, Fangshan District CDC, Tongzhou District CDC, Shunyi District CDC, Changping District CDC, Daxing District CDC, Huairou District CDC, Miyun District CDC, and Yanqing District CDC. We also thank all participants who took part in the study and provided the samples.
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
References
- World Health Organization. 10 facts about the eradication of poliomyelitis [EB/OL]. 2017 April 25. https://www.who.int/features/factfiles/polio/en/
- Global Wild Poliovirus 2014-2019. 2019. Accessed 9 November 2019 http://polioeradication.org/wp-content/uploads/2016/08/weekly-polio-global-update-03Aug19.pdf
- Moturi EK, Porter KA, Wassilak SG, Tangermann RH, Diop OM, Burns CC, Jafari H. EIS officer, CDC. Progress toward polio eradication - worldwide, 2013-2014. MMWR Morb Mortal Wkly Rep. 2014;63:468–72.
- Luo HM, Yu WZ, Wen N, Wang HB, Dong CX, Fan CX, Li L. Application of poliomyelitis vaccine and recommendation on switch of immunization strategies in China. Chin J Vaccines Immunization. 2014;20:172–76.
- Zhang LF, Wen N, Cao LS, Su QR, Fan CQ, Yu WZ, Zheng JS, Li L, Wang HQ, HM L. Comparison of poliomyelitis vaccine routine immunization data from different sources in 8 provinces (municipalities) in China. Chin J Vaccines Immunization. 2015;21:400–04.
- Yu WZ, Wen N, Zhang Y, Wang HB, Fan CX, Zhu SL, Xu WB, Liang XF, Luo HM, Li L. Poliomyelitis eradication in china: 1953–2012. J Infect Dis. 2014;210(Suppl 1):S268–74. doi:10.1093/infdis/jit332.
- Wang HB, Zhu SL, Zheng JS. Sero-survey of polio antibodies during wild poliovirus outbreak in Southern Xinjiang Uygur Autonomous Region, China. PLoS One. 2014;9:e800691–8.
- Luo HM, Zhang Y, Wang XQ, Yu WZ, Wen N, Yan DM, Wang HQ, Wushouer F, Wang HB, Xu AQ, et al. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Engl J Med. 2013;369(21):1981–90. doi:10.1056/NEJMoa1303368.
- Zhao D, Ma R, Zhou T, Yang F, Wu J, Sun H, Liu F, Lu L, Li X, Zuo S, et al. Introduction of inactivated poliovirus vaccine and impact on vaccine-associated paralytic poliomyelitis-Beijing, China, 2014–2016. MMWR Morb Mortal Wkly Rep. 2017;66(49):1357–61. doi:10.15585/mmwr.mm6649a4.
- Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication—Myanmar,1996–1999. MMWR Morb Mortal Wkly Rep. 1999;48(42):967–71.
- Centers for Disease Control and Prevention (CDC). Importation of wild poliovirus into Qinghai Province—China, 1999. MMWR Morb Mortal Wkly Rep. 2000;49(6):113–14.
- Wen N, Luo HM, Wang HB, Cui FQ, Zheng H, Wang FZ, Zhang Y, Fan CX, Hao LX, Ma C, et al. Study on risk assessment and response of wild poliovirus importation after polio imported into Xinjiang Uygur Autonomous Region, 2011. Chin J Vaccines Immunization. 2013;19:193–98.
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi:10.1146/annurev.micro.58.030603.123625.
- Jenkins HE, Aylward RB, Gasasira A, Donnelly CA, Mwanza M, Corander J, Garnier S, Chauvin C, Abanida E, Pate MA, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med. 2010;362(25):2360–69. doi:10.1056/NEJMoa0910074.
- Centers for Disease Control and Prevention (CDC). Progress toward the global interruption of wild poliovirus type 2 transmission, 1999. MMWR Morb Mortal Wkly Rep. 1999;48(33):736–738, 747.
- Wang HB, Luo HM, Li L, Fan CX, Hao LX, Ma C, Su QR, Yang H, Reilly KH, Wang HQ, et al. Vaccine-derived poliovirus surveillance in China during 2001-2013: the potential challenge for maintaining polio free status. BMC Infect Dis. 2017;17(1):742–49. doi:10.1186/s12879-017-2849-z.
- Blake IM, Pons-Salort M, Molodecky NA, Diop OM, Chenoweth P, Bandyopadhyay AS, Zaffran M, Sutter RW, Grassly NC. Type 2 poliovirus detection after global withdrawal of trivalent oral vaccine. N Engl J Med. 2018;379(9):834–45. doi:10.1056/NEJMoa1716677.
- Zipursky S, Vandelaer J, Polio Endgame: BA. Lessons learned from the immunization systems management group. J Infect Dis. 2017;216(suppl_1):S9–S14. doi:10.1093/infdis/jiw592.
- World Health Organization. Manual for the virological investigation of polio[EB/OL]. WHO/EPI/GEN 1997.
- Zarocostas J. Imports of polio to China and western Africa threaten global eradication. BMJ. 2011;343(343):d6186. doi:10.1136/bmj.d6186.
- Opare JKL, Odoom JK, Akweongo P, Afari EA1, Pappoe M. Poliovirus antibody levels and lameness among individuals in three regions of Ghana. Hum Vaccin Immun. 2019; 15(9):2050-2059.
- Wang HB, Yu WZ, Wang XQ, Wushouer F, Wang J-P, Wang D-Y, Cui F-Q, Zheng J-S, Wen N, Ji Y-X. An outbreak following importation of wild poliovirus in Xinjiang Uyghur Autonomous Region, China, 2011. BMC Infect Dis. 2015;15(1):34–42. doi:10.1186/s12879-015-0761-y.
- Patel MK, Konde MK, Didi-Ngossaki BH, Ndinga E, Yogolelo R, Salla M, Shaba K, Everts J, Armstrong GL, Daniels D, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010-2011. Clin Infectious Dis. 2012;55(10):1291–98. doi:10.1093/cid/cis714.
- Centers for Disease Control and Prevention (CDC). Polio field census and vaccination of underserved populations - Northern Nigeria, 2012-2013. MMWR Morb Mortal Wkly Rep. 2013;62(33):663–65.
- Patel M, Orenstein W. A world free of polio–the final steps. N Engl J Med. 2016;374(6):501–03. doi:10.1056/NEJMp1514467.
- Kim HJ, Hwang S, Lee S, Kwon Y, Park K, Park YJ, Bae GR, Lee SW, Jeong YS, Hyeon JY. A national cross-sectional study for poliovirus seroprevalence in the Republic of Korea in 2012: implication for deficiency in immunity to polio among middle-aged people. BMC Infect Dis. 2015;15(1):164. doi:10.1186/s12879-015-0894-z.
- World Health Organization. Polio vaccines: WHO position paper.[EB/OL]. 2016 https://www.who.int/immunization/documents/positionpapers/en/
- Lu L, Li X, Zhang H, Liu D, Zhang Z, Wang H, Liu F, Ning Z, Li J, Pang X. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants. Vaccine. 2015;33(36):4653–58. doi:10.1016/j.vaccine.2014.08.091.
- Hird TR, Grassly NC, Andino R. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4):e1002599. doi:10.1371/journal.ppat.1002599.
- Simasathien S, Migasena S, Beuvery C, van Steenis G, Samakoses R, Pitisuttitham P, Vesikari T. Comparison of enhanced potency inactivated poliovirus vaccine (EIPV) Versus standard oral poliovirus vaccine (OPV) in Thai infants. Scand J Infect Dis. 1994;26(6):731–38. doi:10.3109/00365549409008643.
- Circulating Vaccine-derived Poliovirus(cVDPV). [EB/OL]. 2019. http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/.