ABSTRACT
Introduction
In low-income countries, Hepatitis E infection is a common cause of acute hepatitis. So far, only two recombinant vaccines (rHEV and HEV 239) have been developed against Hepatitis E virus (HEV). Of which HEV 239 is licensed in China, but is not yet available in any other country.
Objective
This study aims to discuss epidemiology, diagnosis, available vaccines for HEV, and provides an overview of 100 top-cited studies on HEV.
Methods
A bibliometric analysis was conducted on the topic “HEV” through a systematic search of the Web of Science. The keywords used were “Hepatitis E” and retrieved articles were assessed for number of attributes.
Results
The search returned a total of 3,235 publications, cited 95,858 times with h-index 129. The main finding for the 100 top-cited articles on HEV showed: number of authors ranging from 1 to 23, cited references range from 4 to 304, global citations score per year range from 6.61 to 175, and global citations score range from 148 to 791. Of the 100 top-cited studies, the authors who published most articles are Purcell (n = 18), Meng (n = 17), and Emerson (n = 15). Most The largest share of articles on HEV was contributed by United States of America (n = 49) with 12,795 citations. The National Institute of Allergy andInfectious Diseases was leading institute with greatest number of publications (n = 16), cited 3,950 times.
Conclusions
The studies conducted on HEV have increased over time. The information presented would be very useful in decision making for policy makers providing health care, and for academicians in providing a reference point for future research.
Introduction
Many pathogenic microorganisms such as bacteria, fungi, parasites, and viruses can cause different infections and diseases in humans and animals. Every year due to contagious and non-contagious diseases millions of people die around the globe.Citation1 Hepatitis E virus (HEV) has become a global public health problem causing acute hepatitis. In the past HEV has not been studied as per its risk,Citation2–4 however, in recent years the number of studies on HEV have been conducted to understand the natural history, transmission, and animal reservoirs of HEV infection.Citation4–6,The burden of infection is high in low-income countries. In recent years, increasing number of HEV cases have been reported in industrialized countries as well. In large-scale outbreaks of HEV, high morbidity and mortality have been reported, particularly in pregnant women.Citation7
Hepatitis is disease of liver which is caused by several different viruses including Hepatitis A virus (HAV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Hepatitis D virus (HDV), and HEV. Hepatitis A, B, C, and E are responsible for majority of infections and health-related complication, if not treated on time, they can lead to death.Citation8
HEV incubation period varies from 2 to 10 weeks (average 5 to 6 weeks). HEV symptomatic infection is most common among people of age15 to 40 years.Citation9
Common symptoms of HEV include fever (mild), nausea, vomiting, anorexia, abdominal and joint pain, skin rash, jaundice, dark urine, pale stools, and hepatomegaly. Acute HEV infection can get severe in rare cases and results in fulminant hepatitis (acute liver failure). The fulminant hepatitis is more frequently reported during pregnancy.Citation9These diseases can spread from one person to another by direct or indirect contact. Zoonotic diseases have also been reported in humans, which transmit from animals to humans.Citation10
In general, the HEV Infection transfers through fecal-oral route (usually through drinking contaminated water and by ingestion of poorly cooked food). HEV infection can be transmitted through other sources such as blood transfusion, zoonotic transmission, and from mother to child. However, person to person transmission of HEV infection is not that common.Citation11–13
Up until now, seven closely related genotypes of HEV (HEV-1-7) have been documented.Citation14 Large scale waterborne epidemics in developing countries are linked to HEV-1 and HEV-2. On the other hand, genotypes HEV-3 and HEV-4 are associated with sporadic cases occurring in developed countries.Citation7 The possible courses of HEV infections are presented in .
Figure 1. Possible courses of HEV infection.Citation15
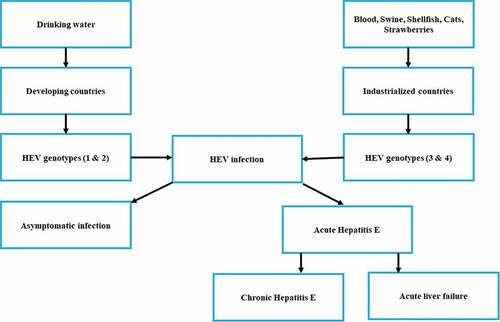
The zoonotic isolates (HEV-3 and HEV-4) as it transfers from animals to humans via the consumption of uncooked and contaminated meat.Citation4 Recent studies documented that genotype HEV-3 is associated with liver cirrhosis and chronic hepatitis.Citation16 Furthermore, the number of HEV cases among immune-compromised patients is of serious concern worldwide.Citation7
Transmission
In general, the HEV infection transmits by fecal-oral route, usually through drinking contaminated water and ingesting food such as undercooked meat). HEV infection can transmit through other sources such as blood transfusion, zoonotic transmission, and from mother to child. However, person to person transmission of HEV infection is rare.Citation11–13,Citation17Zoonotic transmission of HEV, genotype 3 and 4results from sporadic and cluster cases in humans. Many outbreaks of HEV infection due to fecal contamination of drinking water are reported in some countries in Africa and Asia.Citation18
In China, the pork industry is the largest contributor; therefore, the human population engaged in pig-farming risks exposure, as well as those who consume the animals as food. Personal hygiene combined with pork consumption influences health. Waste water contaminated with HEV can be a possible route of environmental contamination through pig slurry.
Replication
HEV is an important human pathogen that historically has been difficult to study. Our understanding of the viral life cycle is limited.Citation19Previously published literature reported that the life cycle of HEV is largely unknown due to the lack of a robust cell-culture for Hepatitis virus.Citation20–22 Recently, the replicon and in vitro infection system became available, a lot of work has been generated on HEV positive-strand RNA viruses and sub-genomic expression of viral open reading frames.Citation22
For translation and cleavage process the virus uses common cellular mechanism. The successful propagation of the HEV in cell culture has been limited. In other viruses, the ORF 1 contains particular motifs with unknown functions. The virus uses viral RNA polymerase for the synthesis of genomic and messenger RNA through a negative-strand RNA intermediate. The sub-genomic RNA transcripts (2.0 and 3.7 kb) have been reported but the significance is not known.Citation23
Lack of standardized detection methods, sporadic and low-level virus shedding, and subclinical infections made the development of animal models difficult for HEV infection. Continued animal models development of HEV will be instrumental in understanding the many complex questions associated with HEV infection.Citation19
Vulnerable groups
HEV can cause infection at any stage of life. But during the HEV outbreaks, adolescents and adults between ages of 15 and 40 years are at a particularly high risk for infection. However, in young children the HEV infection is less likely to cause disease. HEV infection appears to be more common in men than women.Citation24Beside this, among pregnant women the HEV infection is associated with an increased likelihood of symptomatic disease, fulminant hepatic failure and death, as compared to non-pregnant women and men. In pregnant women, the mortality rate is as high as 15 to 20%.Citation25People with preexisting chronic liver disease are also at high risk of serious illness, which may lead to life threatening conditions.Citation26,Citation27 The marginalized communities living in, internally displaced people (IDP) refugees’ camps, migrants, urban slums, cross-border populations and ethnic minorities are hard to reach and therefore at increased risk of HEV and other infectious diseases.Citation28A total of 1,117 HEV suspected cases were reported during April 2014 to January 2015 among the refugees residing in the Gambella region. Of the total cases the mortality rate was 21 (1.9%).Citation29
HEV history
In 1980, HEV was first time isolated during a waterborne outbreak in India. Through the application and uses of antibody’s assays for the detection of Hepatitis A virus (HAV) and Hepatitis B virus (HBV) subsequent outbreaks across Asia, the Middle East and North Africa were recognized.Citation30,Citation31Initially HEV was designated as enterically transmitted non-A, non-B hepatitis (ET-NANBH) due to similar clinical presentations to hepatitis A and B.Citation32
Few years later, in 1983, from HEV affected patients a scientist had experimentally ingested a fecal suspension showed the symptoms of disease after 30–35 days. Study of his fecal inoculations during his infection period reported HEV through immune electron microscopy. This discovery and identification of virus brought a new revolution in the field of virology.Citation32
Early studies implied that RNA virus was the potential agent to cause ET-NANBH. Analysis of a cDNA library was performed for infectious bile sample, which resulted in a highly conserved RNA-dependent RNA-polymerase (RdRp) motif potion usually found in RNA viruses.Citation33
As a result in 1991, the scientists cloned and sequenced the HEV successfully, and found that the HEV genome contains three opening reading frames (ORF1, ORF2, and ORF3) with a size of 7.2kb. The ORF1 codes for transcription of non-structural proteins, ORF2 codes for capsid protein, and ORF3 responsible for protein of unknown origin.Citation23,Citation34 The exact function of ORF3 still remains unclear. However, numerous functions and roles have been proposed.Citation22,Citation34
In the year 2009, the HEV structure was studied through X-ray crystallography.Citation35 Later on a number of studies have been conducted on the molecular biology and genetics of HEV. It was found the virus shares some genetic similarities with rubella virus, and some physical similarities with caliciviruses. Based on those similarities, the virus was assigned to its own genus “Hepevirus” and family “Hepeviridae”.Citation14
Global statistics of HEV
HEV cases are reported from both developed and developing countries, but the HEV is more common in East and South Asia. Global statistics of HEV infection shows that, every year an estimated 20 million cases are reported. Of the reported cases approximately 3.3 million lead to symptomatic cases of hepatitis E. In 2015, HEV caused approximately 44,000 deaths, accounting for 3.3% deaths due to viral hepatitis. Of the total reported cases great majority were from developing countries.Citation9In contrast to large scale outbreaks in the developing countries, the cases in developed countries are associated with zoonotic transmission.Citation18 In Africa and Asia, the estimated prevalence of HEV ranges from 15 to 25%.Citation36
Prevalence of HEV in developed countries
In past, among developed regions there was a perception that HEV is a rare disease and is caused by visiting epidemic centers.Citation37 In Europe and other developed countries, the autochthonous hepatitis E was caused by genotype 3.Citation38 Recently, increases in HEV cases have been observed in developed/industrialized countries. The seroprevalence of HEV in developed countries ranges from <5 to >50%. The variation in seroprevalence may be attributed to the different ethnic groups, geographical location, and the type of serological assays used.Citation39
Another study reported the estimated seroprevalence of HEV in Europe ranges from0.6 to 52.5%, which increases with age. Depending on assays method the seroprevalence varied among the general population; Wantai (WT): 17%, Mikrogen (MG): 10%, MP-diagnostics (MP): 7%, DiaPro: 4%, Abbott 2%. Communities living in close contact with wild animals and swine had significantly higher seroprevalence than the general population(p < .0001).Citation38The prevalence of anti-HEV antibodies among blood donors in European countries range from 1.3% in Italy (Scotto et al., 2014) to 52% in France (Mansuy et al., 2011).Citation40,Citation41
HEV infection in kidney transplant patients
HEV infection for organ recipients can lead to chronic hepatitis and complications. Some previous studies showed an unexpectedly high prevalence of anti-HEV antibodies in hemodialysis patients.Citation42–45In hemodialysis patients, the high prevalence of HEV could be related to their impaired immunity.Citation46–48A study conducted in Italy showed, the HEV prevalence was 6% in dialysis patients, 3.3% in transplant recipient, while in general population the prevalence was 2.7%.Citation49
HEV infection in pregnancy
HEV infection is more lethal in pregnant women compared to general population. The HEV may result in severe consequences during pregnancy for both mother and fetus such as fulminant hepatic failure, vertical transmission, and maternal mortality.Citation50,Citation51 A systematic review was conducted on 23 observational studies with a sample size of 1338. The median maternal rate was 26% (IQR 17–41%), fetal 33% (IQR 19–37%) and neonatal case-fatality rate was 8% (IQR 3–20%). The overall median prevalence of fulminant hepatic failure was found to be 45.3%. The findings suggested high case-fatality rate among pregnant women and adverse outcomes on their children.Citation50
A recent study conducted on a rabbit model to study the adverse pregnancy outcomes and their prevention by Hepatitis E vaccine. The results demonstrated that different HEV genotypes infected pregnant rabbits and resulted in adverse pregnancy outcomes and as well as vertical transmission. The HEV vaccine 239, is very effective to protect the pregnant women from HEV infection and their adverse pregnancy outcomes.Citation51
A research was conducted by Li et al.Citation52 among 946 pregnant women in China. The overall positivity rate for anti-HEV IgG antibody was (74/365, 20.27%), anti-HEV IgM antibody was (15/365, 4.11%), and both anti-HEV IgM and IgG antibodies were (12/365, 3.29%). The infections were significantly higher (p <.05) in third trimester than in the first and second. The average level of alanine transaminase (ALT) was (34.49 ± 10.15), and both anti-HEV IgM and IgG positive group had the incidence of adverse pregnancy outcomes (13/18, 72.22%) that were significantly higher than other groups (p < .05). Only one sample had the HEV RNA which belonged to sub-genotype 4a.
A recent study shows that, of the total 7160 pregnant women, 1182 were positive to anti-HEV IgG antibody and only 66 were positive to anti-HEV IgM antibody. The overall pooled prevalence among pregnant women was 16.51% (95% CI: 0.10–0.23).Citation53
Number of studies reported HEV outbreaks in pregnant women in different countries as shown in . Highest number of outbreaks occurred in India in 1987, 1988, and in 1991. The recent outbreaks of HEV in pregnant women were reported in Sudan during 2010–2011.
Table 1. HEV outbreaks (serologically confirmed) in pregnant women in different countries
HEV and HAV co-infection
TheCitation54 co-infection of both Hepatitis A virus (HAV) and HEV lead to serious complications resulting in acute viral hepatitis.Citation68 The epidemics of HAV are not very common and affect infants and young children in developing countries.Citation69 While the HEV affects the older children and young adults in the tropical regions. However, the epidemics of HEV are common.Citation68,Citation70
A cross-sectional study was conducted among 1230 individuals. The serum samples were processed for the presence of HAV and HEV antibodies. The result shows that the prevalence of HAV and HEV were 15.5, and 27.2%, respectively. The co-infection of HAV-HEV was 5.1%. Furthermore, the prevalence of HEV is higher than that of HAV. The screening of HEV especially in pregnant women and improved levels of personal hygiene among lower socio-economic communities is of great importance.Citation68
Laboratory diagnosis of HEV
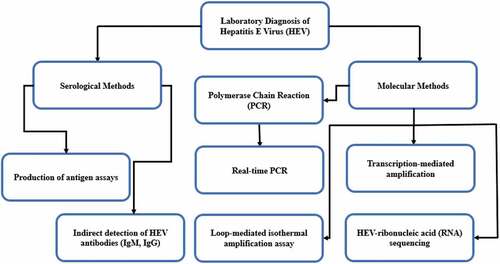
Recently, number of HEV cases linked to blood transfusion have increased and are considered as transfusion-transmitted virus. To confirm a diagnosis of HEV infection, clinical manifestations and biochemical tests not only diagnose HEV infections, but are helpful in identifying the onset and stage of infections. Therefore, to diagnose HEV infection properly, it requires optimally a combination of both molecular and serological techniques, and to monitor the treatment response in chronically HEV-infected individuals. There are several serological and molecular detection methods for HEV infection. However, the detection of HEV laboratory diagnostic techniques varies in their specificity and sensitivity. The antigen, viral nucleic acid, and viral protein can be detected by direct techniques, while the HEV specific antibodies (IgM and IgG) can help establish a diagnosis in acute and chronic infections.Citation71 Diagnostic methods of HEV are presented in .
Figure 2. HEV diagnosis methods.Citation71
Treatment of HEV
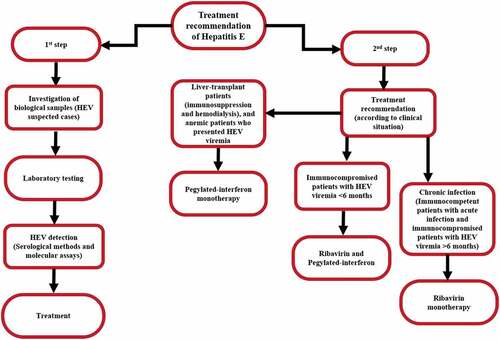
The acute HEV infection in immunocompetent individuals does not usually require antiviral therapy. However, some patients might require symptomatic treatment, but almost all patients are able to clear the disease spontaneously.Citation72A 61-year-old patient infected with HEV genotypes 3, who developed impaired liver function and severe acute hepatitis E, with no sign of liver encephalopathy was treated with ribavirin therapy. The result showed that after therapy alanine aminotransferase level (ALT) had normalized, and the level of bilirubinemia was 138 μmol/L. Furthermore, the HEV RNA was almost undetectable in the serum from same patient.Citation73 Treatment recommendation of HEV is presented in .
Figure 3. Treatment recommendations of HEV infection.Citation74
Vaccines
The mortality rate of HEV (3.3%) demands a globally available vaccine. Usually, pregnant women with HEV-1 infection have shown worse effect and have been considered to be the main target group to receive vaccination.Citation75,Citation76 Public health surveillance of overall population is also extremely important to overcome on outbreaks with improving sanitation and hygiene in endemic and non-endemic countries.Citation77,Citation78
It has been found that to develop live attenuated or inactivated vaccine by in vitro cell culture replication is not feasible.Citation79 Symptomatic relief by passive immunoprophylaxis has been achieved but not succeeded to prevent the infection. So, the focus was shifted on development of recombinant vaccine.Citation80
So far, two recombinant vaccines against HEV infection have been developed by Glaxo SmithKline, Belgium,Citation81 and Xiamen Innovax Biotech, China.Citation82
The only licensed HEV vaccine is HEV 239 vaccine (Hecolin, Xiamen Innovax Biotech, China), which was approved and registered in China in 2011 but has not yet been approved in any other country. Further clinical trials of this vaccines are ongoing in Bangladesh.Citation83,Citation84 HEV is antigenically conserved in that it presents only one known serotype that is protective for all four HEV genotypes. Thus, a group-common vaccine approach is possible.Citation85,Citation86
Prevention
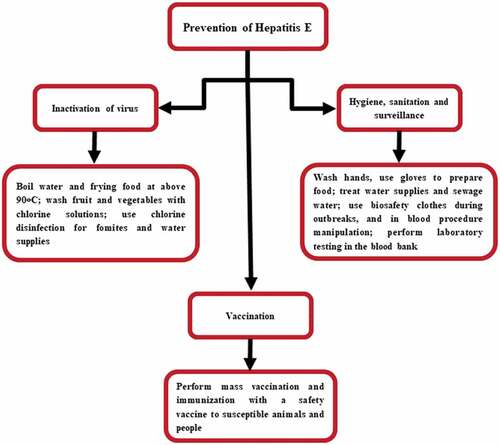
There is a dire need of “One Health” initiative to forge co-equal, all inclusive research collaboration and disease prevention measurements between physicians, veterinarians, environmentally related disciplines, and other scientific-health domains. For HEV, there is no globally approved and commercially available vaccine. Good hygiene practices, improved sanitation measures, clean drinking water, and avoidance of high-risk situations is the best ways to prevent the HEV infection. While traveling to endemic areas, special care and prevention must be observed, including boiling water or drinking bottled water, and avoiding raw or steamed shellfish such as, oysters that live in contaminated waters. Pregnant women are at high risk of infection especially those living or traveling to the endemic areas must take special care according to the standard traveling guideline to the endemic regions. The prevention strategies of HEV are presented in .
Figure 4. Prevention and control strategies of HEV.Citation74
Objective
This study aimed to discuss the epidemiology, diagnosis, available vaccines, and provides an overview of the100 most cited studies on HEV. This study may help and provide the base line information on the distribution of studies on HEV, and to help the researchers and policy makers to understand the characteristics of research output and provide reference for future studies.
Methodology
Study design
A bibliometric method analysis was conducted.
Data sources
An electronic search was conducted on Web of Science, Clarivate Analytics for time span from inception of Web of Science till December, 2016 on the topic “HEV”, and articles were retrieved on January 01, 2020. For articles searching, following keywords were used such as “Hepatitis E” “Title” field. The records obtained were refined by document types (articles, reviews, editorials, meeting abstracts and others) from Web of Science Core Collection (ISI-Thomson Reuters Web of Knowledge, hosted by Clarivate Analytics). To identify the 100 most cited papers on HEV, all the articles were ranked by the number of citations.
Eligibility criteria
Studies conducted mainly on HEV and articles, reviews, editorials or research letters were included in the analysis. Studies identified the word “Hepatitis E” but did not deal with Hepatitis E were excluded. Furthermore, abstracts, correspondence, and errata/corrigenda were excluded.
Data extraction
On the same day of search, data were obtained by two independent investigators (Tauseef Ahmad and Taha H. Musa) to avoid any bias in data collection. The discrepancies were discussed and resolved after discussion with a third investigator. The retrieved data information was assessed for a number of attributes including title of article, author names, times cited, cites per document, most frequently cited articles, year of publication, type of paper, countries/region, institutes/organizations, and name of journal.
Data analysis
The descriptive statistical analysis was carried out in Microsoft Excel 2016 such as year of publication, authors, type of publication, number of citations, countries/regions, institutes/organizations, and journal names.
Ethics
No ethical approval was required for this study as this was an analysis of already published studies. None of the author of the included studies was contacted for further information regarding their publications.
Results
Characteristics of the total studies
A total of 3,235 publications were extracted from Web of Science (from inception to December, 2016) with a total of 95,858 citations. The average citations per publication were 29.63 times. The overall, h-index was 129.
Characteristics of 100 top-cited studies
Based on cited frequency the articles are listed in descending order. The top 100 highest cited articles were further processed for citations analysis. The major findings were; total number of authors were 470, range from 1 to 23, countries (n = 27), institutions (n = 220), journals (n = 33), local references (n = 92), global references (n = 1610), all cited references (n = 1702), cited reference range from 4 to 304, global citation score per year range from 6.61 to 175, and global citation score range from 148 to 791.
Citations analysis
The 100 top-cited studies on HEV are presented in . Overall, the included 100 top-cited studies have been cited 24,901 times (range from 148 to 791 times). Of the included studies, only 6 studies were cited more than 500 times, 45 studies were cited between 200 and 500 times, and a great number of studies (n = 49) were cited below 200 times. The first top-cited study was “A novel virus in swine is closely related to the human hepatitis E virus” authored by Meng et al. (1997), published in the “Proceedings of The National Academy of Sciences of The United States of America”. The study was cited 791 times with 32.96 average citations per year. This study opened a new avenue of research focusing xenozoonosis and xenotransplantation. This study formed the basis of further highly cited articles not only by Meng and his study group but also for others working on various aspects of pig organs transplantations in humans. It also provided a reference point for further studies on alternative animal model for HEV studies. The second top-cited was “Hepatitis-E Virus (HEV) – Molecular-Cloning and Sequencing of The Full-Length Viral Genome” published in the journal “Virology” by Tam et al. (1991), with 753 citations and 25.1 average citations per year. The findings on the genetic organization and expression strategy of HEV suggested in the article form the basis of a new era of studies on molecular cloning and protein assays. The third top-cited study published by Kamar et al. (2008), in the “New England Journal of Medicine” entitled “Hepatitis E virus and chronic hepatitis in organ-transplant recipients. The study was cited 718 times with an average per year citations 55.23. After the discovery of HEV in early 80 s most of the research for the next two decades was focused on phylogenetic analyzes and molecular diagnostics of HEV. The focus of research was shifted more toward treatment and vaccine development mostly in the last decade. The 10 top-cited studies on HEV vaccine is presented in . The most cited study was “Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial” cited 372 times.
Table 2. The 100 top-cited studies on Hepatitis E virus
Table 3. The 10 top-cited studies on HEV vaccine
Distribution of authors
Among the 100 top-cited studies, the authors who published most studies were Purcell (n = 18) with 4923 times, Meng (n = 17) with 4714 citations, and Emerson (n = 15) with 4286 citations as shown in .
Table 4. Authors with at least five studies as first author or coauthor
Distribution of institutions
Of the total included studies majority of the studies were published from The National Institute of Allergy and Infectious Diseases [NIAID, (n = 16)], cited 3950 times, followed by Virginia Polytechnic Institute and State University (n = 13) with 3124 citations as presented in .
Table 5. Institutes with at least five studies
Distribution of countries
Most of studies were published from United States of America [USA, (n = 49)] with 12795 citations, followed by United Kingdom [UK, (n = 18)] with 4344 citations, and France (n = 15) received 4247 citations as described in . In spite of the fact that the global burden of HEV is more in East and South Asia, and in Africa, the research output from these countries is not much. This may raise concerns for the health organizations to support and fund HEV research in disease burdened countries for developing effective control and prevention strategies.
Table 6. Countries with at least five studies
Distribution of journals
showsthe journal with 5 or more than 5 published studies among 100 top-cited articles. The “Journal of General Virology” was the leading journal with 12 publications with 2,573 citations.
Table 7. Leading journals in the field in Hepatitis E research
Distribution of keywords
showed the hotspots keywords; the hotspots keywords included Hepatitis (n = 100), and Virus (n = 75).
Table 8. Top ten hotspots keywords
Distribution of document types
Number of articles were found to be 73 (with 18254 citations), and review were 16 (4271 citations) as shown in .
Table 9. Distribution of document types
Distribution of published years
The highest number of studies published in 2007 (n = 10) with 1949 citations, and in 2008 (n = 09) cited 2761 times as shown in .
Table 10. Publication frequency per year
Discussion
In the last two decades, greater number of studies has been published on the HEV antibodies prevalence in different human populations. However, in recent years, the HEV cases have increased up to 70% in some industrialized countries. The heterogeneity of HEV prevalence may be attributed to different factors such as assays methods, and geographical regions.Citation39
Recently, various fields tried to identify the “citation classics” in that field, where the top 100 cited articles in that field identified.Citation87 The present study represents the analysis of citation classics in the field of hepatitis E, a disease widely prevalent in both, developed and developing countries. A large number of studies (research articles, systematic review/meta-analysis) on diagnosis, treatment, control and prevention of HEV have been conducted by researchers across the globe. However, despite the importance of bibliometric studies as a way of exploring research quantum, directions and collaborations between researchers and medical practitioners, very few studies have been performed focused on bibliometric analysis on HEV; to the best of our knowledge no bibliometric analysis was performed on the 100 top cited studies on HEV research. Therefore, this study was conducted to determine the citation analysis and their principal characteristics in details. A steady increase in HEV publications was observed over the last few years, owing to increase in the global burden of this disease. An estimated 20 million HEV infections are reported worldwide by WHO, resulting in a large number of deaths.Citation9Therefore, understanding the characteristics of published studies on HEV may be worthwhile for several reasons. Our findings showed that 100 top-cited studies were cited 24901 times, reflecting their impact and importance. There were 6 studies that were cited more than 500 times in the literature.
Previous studies done to analyze hepatitis literature found a steady increase in publications on hepatitis in general, and on hepatitis E specifically in the last few decades.Citation88,Citation89Wani et al.Citation88 performed a bibliometric analysis on all articles on hepatitis E using Web of Science, and the authors advised for further projects on hepatitis E. USA, UK, and Germany are the top countries publishing on Hepatitis in general.Citation89 Wani et al.Citation88 found that USA, India, and China are the top three countries publishing on HEV in general. Our study found that USA, UK, and France are the countries publishing most from HEV top-cited articles. This trend of publishing is not in consonance with the global geographical distribution of disease as it is more common in low- and middle-income countries where the disease occurs both as outbreaks and as sporadic cases. This study accentuates the need to establish research collaborations between researchers of developed countries and researchers in resource limited countries. The researchers, especially from resource limited countries need to be encouraged by providing technical and financial assistance to focus more on HEV research to prevent and control the disease outbreaks.
Quality and coverage of journals play an important role in dissemination of research to all stakeholders. The top journal publishing top articles on hepatitis E was “Journal of General Virology” as found in our study, “Journal of Medical Virology” was the journal with the highest number of publications on Hepatitis E.Citation6 Both of these journals do not levy any publication charges although most of the journals charge quite heavy fee for publication/article processing. This highlights the need that the other quality journals may make waiver policies to attract quality publications from developing countries. Quality research may be carried out in low/middle income countries on local levels but it never gets published in international journals for lack of funds and is not disseminated to a wider audience. This study will not only provide a referral point for potential researches but will also help in planning and policy-making regarding prevention and control of HEV.
Study limitations
This study has several limitations. In our study we only extracted data from the articles indexed in Web of Science Core Collection database, and on the other hand articles in other databases such as Scopus and PubMed have missed which may affect final findings. Furthermore, quality of the top-cited articles was not assessed which may influence the interpretation of the findings. In addition, the data generated in this article was through electronic search rather than selecting and collecting manually, therefore the findings of our study may subject to bias. The manual selection of studies and extraction of data is required for systematic reviews/meta-analysis.
Translational value
The results presented in this article may be very useful for researchers and scientists to focus their research objectives in more appropriate domain in the field of HEV, and most importantly it can provide direction to guide health care decision making policies. Furthermore, it will help the academicians and teachers to provide the quality bibliographic references for learning and teaching purpose.
Highlights
This study provides an overview on the epidemiology, diagnosis, available vaccines, and 100 top-cited studies on HEV.
HEV is considered as the leading cause of enterically transmitted viral hepatitis infection in both developing and developed countries.
So far, only two recombinant vaccines (rHEV, Belgium and HEV 239, China) have been developed against HEV but they are not available for commercial use yet.
Most of the studies on HEV are published from developed countries.
This study emphasizes the need of establishing research collaborations between researchers of developed countries and low-income countries.
The researchers, especially from resource limited countries, need to be encouraged by providing technical and financial assistance to focus more on HEV research to prevent and control the disease outbreaks.
Authors contributions
Conceptualization: TA; Methodology: TA. Data collection: TA and THM. Formal analysis: TA and THM. Writing—original draft: TA. Review and editing: TA, SN, SASA, MK, and HJ. Funding acquisition: HJ. All the authors read and approved the final manuscript for publication.
Acknowledgments
The authors acknowledge Southeast University, China for providing online access to the Web of Science, Core Collection database.
Disclosure of potential conflicts of interest
All the authors agreed and declare no conflict of interests.
Additional information
Funding
References
- Ritchie H, Roser M Causes of Death. Published online at OurWorldInData.org. 2020. Retrieved from: https://ourworldindata.org/causes-of-death [accessed: 06 April, 2020]
- Purcell RH, Emerson SU. Hepatitis E virus, p. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE editors. Fields virology. 4th. Philadelphia (Pa): Lippincott Williams and Wilkins; 2001. p. 3051–61.
- Aggarwal R, Naik S. Epidemiology of hepatitis E: current status. J Gastroenterol Hepatol. 2009;24(9):1484–93. doi:10.1111/j.1440-1746.2009.05933.x.
- Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161(1):23–30. doi:10.1016/j.virusres.2011.01.016.
- Meng XJ, Shivaprasad HL, Payne C. Hepatitis E virus infections. In: Saif M, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry. 12th. Ames, Iowa: Blackwell Publishing Press; 2008. p. 443–52.
- Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res. 2010;41(6):46. doi:10.1051/vetres/2010018.
- Kamar N, Bendall R, Legrand-Abravanel F, Xia N-S, Ijaz S, Izopet J, Dalton HR. Hepatitis E [published correction appears in. Lancet. 2012 Aug 25;380(9843):730]. Lancet. 2012;379(9835):2477–2488. doi:10.1016/S0140-6736(11)61849-7.
- World Health Organization (WHO). What is Hepatitis? Retrieved from: https://www.who.int/features/qa/76/en/ [accessed:06 April, 2020]
- World Health Organization (WHO), Hepatitis E. 8 July 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e [accessed: 07 April, 2020]
- Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Zoonotic Diseases. Page last reviewed: July 14, 2017. Retrieved from: https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html [accessed: 06 April, 2020].
- Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol. 2012;56(2):500–02. doi:10.1016/j.jhep.2011.06.021.
- Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy ITR, Kitchen A, Patel P, Poh J, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384(9956):1766–73. doi:10.1016/S0140-6736(14)61034-5.
- Matsui T, Kang JH, Matsubayashi K, Yamazaki H, Nagai K, Sakata H, Tsuji K, Maguchi H. Rare case of transfusion-transmitted hepatitis E from the blood of a donor infected with the hepatitis E virus genotype 3 indigenous to Japan: viral dynamics from onset to recovery. Hepatol Res. 2015;45(6):698–704. doi:10.1111/hepr.12390.
- Smith DB, Simmonds P, Jameel S, Emerson SU, Harrison TJ, Meng X-J, Okamoto H, Van der Poel WHM, Purdy MA; Members Of The International Committee On The Taxonomy Of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2015;96(5):1191–92. doi:10.1099/vir.0.000115.
- Hartl J, Wehmeyer MH, Pischke S. Acute Hepatitis E: two Sides of the Same Coin. Viruses. 2016;8(11):299. doi:10.3390/v8110299.
- Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358(8):859–60. doi:10.1056/NEJMc0708687.
- Modiyinji AF, Amougou‐Atsama M, Monamele CG, Nola M, Njouom R. Seroprevalence of hepatitis E virus antibodies in different human populations of Cameroon. J Med Virol. 2019;91(11):1989–94. doi:10.1002/jmv.25545.
- Meng XJ. Zoonotic and foodborne transmission of hepatitis E virus. Semin Liver Dis. 2013;33(1):41–49. doi:10.1055/s-0033-1338113.
- Kenney SP, Meng XJ. Hepatitis E Virus: animal Models and Zoonosis. Annu Rev Anim Biosci. 2019;7:427–48. doi:10.1146/annurev-animal-020518-115117.
- Niikura M, Takamura S, Kim G, Kawai S, Saijo M, Morikawa S, Kurane I, Li T-C, Takeda N, Yasutomi Y, et al. Chimeric recombinant hepatitis E virus-like particles as an oral vaccine vehicle presenting foreign epitopes. Virology. 2002;293(2):273–80. doi:10.1006/viro.2001.1240.
- Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci. 2008;33(4):451–64. doi:10.1007/s12038-008-0064-1.
- Ahmad I, Holla RP, Jameel S. Molecular virology of hepatitis E virus. Virus Res. 2011;161(1):47–58. doi:10.1016/j.virusres.2011.02.011.
- Tam AW, Smith MM, Guerra ME, Huang -C-C, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185(1):120–31. doi:10.1016/0042-6822(91)90760-9.
- Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604.
- Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, Hill V, Okware S, Hu D, Holmberg S, et al. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis. 2010;50(7):1006–10. doi:10.1086/651077.
- Kumar A, Aggarwal R, Naik SR, Saraswat V, Ghoshal UC, Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004;23:59–62.
- Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46(3):387–94. doi:10.1016/j.jhep.2006.09.016.
- World Health Organization. Waterborne Outbreaks of Hepatitis E: recognition, Investigation and Control. Geneva, Switzerland: World Health Organization; 2014. ISBN: 9789241507608
- Browne LB, Menkir Z, Kahi V, Maina G, Asnakew S, Tubman M, Elyas HZ, Nigatu A, Dak D, Maung UA, et al. Notes from the field: hepatitis E outbreak among refugees from South Sudan - Gambella, Ethiopia, April 2014-January 2015. MMWR Morb Mortal Wkly Rep. 2015;64(19):537.
- Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68(6):818–24. doi:10.1016/0002-9343(80)90200-4.
- Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980;2(8200):876–79. doi:10.1016/s0140-6736(80)92045-0.
- Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20(1):23–31. doi:10.1159/000149370.
- Reyes GR, Huang CC, Tam AW, Purdy MA. Molecular organization and replication of hepatitis E virus (HEV). Arch Virol Suppl. 1993;7:15–25. doi:10.1007/978-3-7091-9300-6_2.
- Bradley DW. Hepatitis E virus: a brief review of the biology, molecular virology, and immunology of a novel virus. J Hepatol. 1995;22(1 Suppl):140–45. Holla RP, Ahmad I, Ahmad Z, Jameel S. Molecular virology of hepatitis E virus. Semin Liver Dis. 2013;33(1):3–14. doi:10.1055/s-0033-1338110
- Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, Zhang J, Tao YJ. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci U S A. 2009;106(31):12992–97. doi:10.1073/pnas.0904848106.
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–97. doi:10.1002/hep.25505.
- Dalton HR, Bendall R, Ijaz S, Banks M. E: hepatitis an emerging infection in developed countries. Lancet Infect Dis. 2008;8(11):698–709. doi:10.1016/S1473-3099(08)70255-X.
- Hartl J, Otto B, Madden RG, Webb G, Woolson K, Kriston L, Vettorazzi E, Lohse A, Dalton H, Pischke S, et al. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses. 2016;8(8):211. doi:10.3390/v8080211.
- Capai L, Falchi A, Charrel R. Meta-Analysis of Human IgG anti-HEV Seroprevalence in Industrialized Countries and a Review of Literature. Viruses. 2019;11(1):84. doi:10.3390/v11010084.
- Scotto G, Martinelli D, Centra M, Querques M, Vittorio F, Carri PD, Tartaglia A, Campanale F, Bulla F, Prato R, et al. Epidemiological and clinical features of HEV infection: a survey in the district of Foggia (Apulia, Southern Italy). Epidemiol Infect. 2014;142(2):287–94. doi:10.1017/S0950268813001167.
- Mansuy JM, Bendall R, Legrand-Abravanel F, Sauné K, Miédouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17(12):2309–12. doi:10.3201/eid1712.110371.
- Dalekos GN, Zervou E, Elisaf M, Germanos N, Galanakis E, Bourantas K, Siamopoulos KC, Tsianos EV. Antibodies to hepatitis E virus among several populations in Greece: increased prevalence in an hemodialysis unit. Transfusion. 1998;38(6):589–95. doi:10.1046/j.1537-2995.1998.38698326339.x.
- Sylvan SP, Jacobson SH, Christenson B. Prevalence of antibodies to hepatitis E virus among hemodialysis patients in Sweden. J Med Virol. 1998;54(1):38–43. doi:10.1002/(SICI)1096-9071(199801)54:1<38::AID-JMV6>3.0.CO;2-Q.
- Stefanidis I, Zervou EK, Rizos C, Syrganis C, Patsidis E, Kyriakopoulos G, Sdrakas L, Tsianas N, Rigopoulou EI, Liakopoulos V, et al. Hepatitis E virus antibodies in hemodialysis patients: an epidemiological survey in central Greece. Int J Artif Organs. 2004;27(10):842–47. doi:10.1177/039139880402701005.
- Mitsui T, Tsukamoto Y, Hirose A, Suzuki S, Yamazaki C, Masuko K, Tsuda F, Endo K, Takahashi M, Okamoto H, et al. Distinct changing profiles of hepatitis A and E virus infection among patients with acute hepatitis, patients on maintenance hemodialysis and healthy individuals in Japan. J Med Virol. 2006;78(8):1015–24. doi:10.1002/jmv.20657.
- Cohen G, Haag-Weber M, Hörl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–S82.
- Litjens NH, Huisman M, van den Dorpel M, Betjes MG. Impaired immune responses and antigen-specific memory CD4+ T cells in hemodialysis patients. J Am Soc Nephrol. 2008;19(8):1483–90. doi:10.1681/ASN.2007090971.
- Edey M, Barraclough K, Johnson DW. Review article: hepatitis B and dialysis. Nephrology (Carlton). 2010;15(2):137–45. doi:10.1111/j.1440-1797.2009.01268.x.
- Scotto G, Aucella F, Grandaliano G, Martinelli D, Querques M, Gesuete A, Infante B, Carri PD, Massa S, Salatino G, et al. Hepatitis E in hemodialysis and kidney transplant patients in south-east Italy. World J Gastroenterol. 2015;21(11):3266–73. doi:10.3748/wjg.v21.i11.3266.
- Bergløv A, Hallager S, Weis N. Hepatitis E during pregnancy: maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat. 2019;26(11):1240–48. doi:10.1111/jvh.13129.
- Li M, Li S, He Q, Liang Z, Wang L, Wang Q, Wang L. Hepatitis E-related adverse pregnancy outcomes and their prevention by hepatitis E vaccine in a rabbit model. Emerg Microbes Infect. 2019;8(1):1066–75. doi:10.1080/22221751.2019.1643260.
- Li M, Bu Q, Gong W, Li H, Wang Li, Li S, Sridhar S, Woo P CY, Wang L. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China [published online ahead of print, 2019 Feb 27]. J Matern Fetal Neonatal Med. 2019;1–5. doi:10.1080/14767058.2019.1582630.
- Ahmad T, Hui J, Musa TH, Behzadifar M, Baig M. Seroprevalence of hepatitis E virus infection in pregnant women: a systematic review and meta-analysis. Ann Saudi Med. 2020;40(2):136–46. doi:10.5144/0256-4947.2020.136.
- Shrestha S. Hepatitis E in Nepal. Kathmandu Univ Med J. 2006;4:530–44.
- Dilawari JB, Singh K, Chawla YK, Ramesh GN, Chauhan A, Bhusnurmath SR, Sharma TR, Sokhey CS. Hepatitis E virus: epidemiological, clinical and serological studies of north Indian epidemic. Indian J Gastroenterol. 1994;13:44–48.
- Khuroo MS, Hepatitis E. the enterically transmitted non-A, non-B hepatitis. Indian J Gastroenterol. 1991;10:96–100.
- Bile K, Isse A, Mohamud O, Mushahwar IK, Magnius LO, Bile K, Isse A, Mohamud O. Contrasting roles of rivers and wells as sources of drinking water on attack and fatality rates in a hepatitis E epidemic in Somalia. Am J Trop Med Hyg. 1994;51(4):466–74. doi:10.4269/ajtmh.1994.51.466.
- Lubis I. Outbreak of hepatitis E in West Kalimantan. Cermin Dunia Kedokteran. 1994;95:43‐46.
- Mast E, Polish LB, Favorov MO, Khudyakova NS, Collins C, Tukei PM, Koptich D, Khudyakov YE, Fields HA, Margolis HS. Hepatitis E among refugees in Kenya: minimal apparent person‐to‐person transmission, evidence for age‐dependent disease expression, and new serological assays. In: Kishioka K, Suzuki H, Mishiro S, Oda T, editors. Viral Hepatitis and Liver Disease. Tokyo, Japan: Springer‐Verlag; 1994. 375‐378. doi:10.1007/978-4-431-68255-4
- Coursaget P, Buisson Y, Enogat N, Bercion R, Baudet J-M, Delmaire P, Prigent D, Desramé J. Outbreak of enterically-transmitted hepatitis due to hepatitis A and hepatitis E viruses. J Hepatol. 1998;28(5):745–50. doi:10.1016/s0168-8278(98)80222-5.
- Rab MA, Bile MK, Mubarik MM, Asghar H, Sami Z, Siddiqi S, Dil AS, Barzgar MA, Chaudhry MA, Burney MI. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg. 1997;57(2):151–57. doi:10.4269/ajtmh.1997.57.151.
- Goumba AI, Konamna X, Komas NP. Clinical and epidemiological aspects of a hepatitis E outbreak in Bangui, Central African Republic. BMC Infect Dis. 2011 Published 2011 Apr 14;11:93. doi:10.1186/1471-2334-11-93.
- Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, Nizou J-Y, Nicand E, Guerin PJ. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42(12):1679–84. doi:10.1086/504322.
- Teshale EH, Howard CM, Grytdal SP, Handzel TR, Barry V, Kamili S, Drobeniuc J, Okware S, Downing R, Tappero JW, et al. Hepatitis E epidemic, Uganda. Emerg Infect Dis. 2010;16(1):126–29. doi:10.3201/eid1601.090764.
- International Centre for Diarrhoeal Disease Research, Bangladesh. Outbreak of hepatitis E in a low income urban community in Bangladesh. Health Sciences Bulletin 2009;7:14‐20.
- International Centre for Diarrhoeal Disease Research, Bangladesh. Hepatitis E outbreak in Rajshahi City Corporation. Health Science Bulletin 2010;8:12‐18.
- Rayis DA, Jumaa AM, Gasim GI, Karsany MS, Adam I. An outbreak of hepatitis E and high maternal mortality at Port Sudan, Eastern Sudan. Pathog Glob Health. 2013;107(2):66–68. doi:10.1179/2047773213Y.0000000076.
- Agarwal S, Anuradha SA, Sahoo AB, Duggal N. Seroprevalence of Hepatitis A Virus (HAV) and Hepatitis E Virus (HEV) Co-infection in the Patients Presenting with Acute Viral Hepatitis Attending a Tertiary Care Hospital in North India. J Commun Dis. 2017;49(3):57–60. doi:10.24321/0019.5138.201723.
- Arankalle VA, Chadha MS, Chitambar SD, Walimbe AM, Chobe LP, Gandhe SS. Changing epidemiology of hepatitis A and hepatitis E in urban and rural India (1982-98). J Viral Hepat. 2001;8(4):293–303. doi:10.1046/j.1365-2893.2001.00279.x.
- Emerson SU, Purcell RH. Running like water–the omnipresence of hepatitis E. N Engl J Med. 2004;351(23):2367–68. doi:10.1056/NEJMp048285.
- Al-Sadeq DW, Majdalawieh AF, Mesleh AG, Abdalla OM, Nasrallah GK. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Microbiol. 2018;67(4):466–80. doi:10.1099/jmm.0.000706.
- Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology. 2012;142(6):1388–1397.e1. doi:10.1053/j.gastro.2012.02.014.
- Gerolami R, Borentain P, Raissouni F, Motte A, Solas C, Colson P. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol. 2011;52(1):60–62. doi:10.1016/j.jcv.2011.06.004.
- Melgaço JG, Gardinali NR, de Mello VDM, Leal M, Lewis-Ximenez LL, Pinto MA. Hepatitis E: update on Prevention and Control. Biomed Res Int. 2018;2018:5769201. doi:10.1155/2018/5769201.
- Goumba CM, Yandoko-Nakouné ER, Komas NP. A fatal case of acute hepatitis E among pregnant women, Central African Republic. BMC Res Notes. 2010 Published 2010 Apr 15;3:103. doi:10.1186/1756-0500-3-103.
- Kumar A, Devi SG, Kar P, Agarwal S, Husain SA, Gupta RK, Sharma S. Association of cytokines in hepatitis E with pregnancy outcome. Cytokine. 2014;65(1):95–104. doi:10.1016/j.cyto.2013.09.022.
- Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O’Riordan J, Boland F, Harritshøj L, et al. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22(16):30514. doi:10.2807/1560-7917.ES.2017.22.16.30514.
- Lapa D, Capobianchi MR, Garbuglia AR. Epidemiology of Hepatitis E Virus in European Countries. Int J Mol Sci. 2015 [Published 2015 Oct 27];16(10):25711–43. doi:10.3390/ijms161025711.
- Li SW, Zhao Q, Wu T, Chen S, Zhang J, Xia NS. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother. 2015;11(4):908–14. doi:10.1080/21645515.2015.1008870.
- Trabelsi K, Kamen A, Kallel H. Development of a vectored vaccine against hepatitis E virus. Vaccine. 2014;32(24):2808–11. doi:10.1016/j.vaccine.2014.02.041.
- Shrestha MP, Scott RM, Joshi DM, Mammen MP, Thapa GB, Thapa N, Myint KSA, Fourneau M, Kuschner RA, Shrestha SK, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356(9):895–903. doi:10.1056/NEJMoa061847.
- Zhu FC, Zhang J, Zhang XF, Zhou C, Wang -Z-Z, Huang S-J, Wang H, Yang C-L, Jiang H-M, Cai J-P, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376(9744):895–902. doi:10.1016/S0140-6736(10)61030-6.
- Riedmann EM. Chinese biotech partnership brings first hepatitis E vaccine to the market. Hum Vaccin Immunother. 2012;8(12):1743–44. doi:10.4161/hv.23373.
- Zaman K, Dudman S, Stene-Johansen K, Qadri F, Yunus M, Sandbu S, Gurley ES, Overbo J, Julin CH, Dembinski JL, et al. HEV study protocol : design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open. 2020;10(1):e033702. doi:10.1136/bmjopen-2019-033702.
- Park SB. Hepatitis E vaccine debuts. Nature. 2012;491(7422):21–22. doi:10.1038/491021a.
- Wu X, Chen P, Lin H, Hao X, Liang Z. Hepatitis E virus: current epidemiology and vaccine. Hum Vaccin Immunother. 2016;12(10):2603–10. doi:10.1080/21645515.2016.1184806.
- Loomes DE, van Zanten SV. Bibliometrics of the top 100 clinical articles in digestive disease. Gastroenterology. 2013;144(4):673–676.e5. doi:10.1053/j.gastro.2013.02.013.
- Wani ZA, Kharadi AH, Ganaie M. Bibliometric analysis of ‘Hepatitis E’ literature. IJIDT. 2017;7(4):242–46. doi:10.5958/2249-5576.2017.00032.2.
- Sangam SL, Arali UB, Patil CG, Rousseau R. Growth of the hepatitis literature over the period 1976–2015: what can the relative priority index teach us? Scientometrics. 2018;115(1):351–68. doi:10.1007/s11192-018-2668-z.
