ABSTRACT
Rotavirus, which causes acute gastroenteritis and severe diarrhea, has posed a great threat to children worldwide over the last 30 y. Since no specific drugs and therapies against rotavirus are available, vaccination is considered the most effective method of decreasing the morbidity and mortality related to rotavirus-associated gastroenteritis. To date, six rotavirus vaccines have been developed and licensed by local governments. Notably, Rotarix™ and RotaTeq™ have been recommended as universal agents against rotavirus infection by the World Health Organization; however, lower efficacies were found in less-developed and developing regions with medium and high child mortality than well-developed ones with low child mortality. For now, two promising novel vaccines, Rotavac™ and RotaSiil™ were pre-qualified by the World Health Organization in 2018. Other rotavirus vaccines in the pipeline including neonatal strain (RV3-BB) and several non-replicating rotavirus vaccines with a parenteral delivery strategy are currently undergoing investigation, with the potential to improve the performance of, and eliminate the safety concerns associated with, previous live oral rotavirus vaccines. This paper reviews the important developments in rotavirus vaccines in the last 20 y and discusses problems and challenges that require investigation in the future.
Introduction
Rotavirus (RV), an RNA virus of the family, Reoviridae, is a ubiquitous pathogen that causes acute gastroenteritis, leading to severe diarrhea and vomiting, among infants and children worldwide, particularly in low-income countries.Citation1–6 RV was first discovered in stool specimens from children with gastroenteritis by Ruth Bishop and collaborators in 1973.Citation7,Citation8 Five years later, the International Committee on Taxonomy of Viruses officially named the virus RV, because of its characteristic wheel-like appearance, observed through an electron microscope by Thomas Henry Flewet.Citation9–11
RV is highly contagious and transmitted by the fecal-oral route. It spreads primarily through contaminated food, water, environmental contamination, aerosolized viral particles in vomitus, or direct person-to-person contact within closed communities and institutions, such as daycare centers, schools, restaurants, cruise ships, and resorts, as well as hospitals and military establishments.Citation12–15 RV disease is characterized by acute onset watery diarrhea, vomiting, fever, and abdominal pain. It generally lasts 3–8 d, usually beginning 2 d after a person has been exposed.Citation16 This disease also has a winter seasonal pattern, most frequently occurring from December through June in countries with temperate climates.Citation17 Latest annual RV mortality rates for RV disease in children <5 y of age, ranging from 122,322 to 215,757 worldwide were estimated by different organizations and methods in 2013.Citation1,Citation3,Citation18 For now, no specific therapies, antibiotics or other drugs against RV are currently available and good hygiene is insufficient to control the spread of the disease. Vaccination has become the most efficient solution to control and prevent RV infection.
Due to the wide application of RV vaccines in some developed countries, the rate of hospitalization from acute gastroenteritis has decreased significantly in last decade.Citation19,Citation20 With the support of Gavi (the Vaccine Alliance) and other partners, RV vaccines were introduced to more than 110 countries like Ghana and resulted in substantial declines in hospitalizations due to RV.Citation21,Citation22 The global impact of RV vaccines from 2006 to 2019 showed the similar reductions in RV and acute gastroenteritis hospitalizations, with the largest decreases in countries with low child mortality and higher coverage, and among younger age groups.Citation23 However, there are some low- or middle-income countries in south-eastern Asia or sub-Saharan Africa still suffered a large number of cases and deaths from RV every year, which have yet to launch RV vaccine campaigns.Citation16 Besides, compared with developed regions, a significantly lower efficacy of RV vaccines has been reported in less-developed countries, which may be attributable to the high disease burden, differences in prevalent serotypes, preexisting antibodies, malnutrition, microbiome or intestinal enteropathy, the concomitant use of oral poliovirus vaccine (OPV) and other factors.Citation13,Citation24-32 Moreover, since intussusception (a bowel obstruction, in which one segment of bowel becomes enfolded within another), a serious adverse reaction, was associated with vaccination using the first licensed RV vaccine (RotaShield™), the much stricter safety evaluation was progressed in the following RV vaccines.Citation33–41 Previous reviews compared the licensed RV vaccine introductions with vaccine coverage and introduced the possible methods of raising the coverage of RV vaccines to improve the efficacy between high and low-income countries.Citation42,Citation43 In order to achieve the full immunization goal and solve the above deviation of efficacy between different countries and other issues on the safety of RV vaccines, our review will not only provide an overview of current research and development of licensed RV vaccines, including their efficacy, immunogenicity, and safety profiles, but also add the novel pre-qualified RV vaccines and other candidates in the pipeline. Besides, this review will assess the health impact of vaccination in countries where RV vaccines had been introduced for universal use and discuss hot topics in research and future directions.
RV structure and overview of RV vaccines
The mature RV virion is a non-enveloped double-stranded RNA virus with a segmented genome comprising a triple-layered protein capsid. There are six viral proteins (VPs) form the virus particle (virion) and six nonstructural proteins (NSPs) produced in cells infected by RV, which were called as VP1, VP2, VP3, VP4, VP6, VP7, and NSP1, NSP2, NSP3, NSP4, NSP5, NSP6, respectively.Citation44,Citation45 Of them, VP4 (P protein) and VP7 (G protein) in the outer layer are responsible for determining the genotype of the strain and also inducing specific neutralizing antibodies.Citation46,Citation47 The VP6 which is highly antigenic can be used to identify RV species and the NSP4 is a viral enterotoxin that induces diarrhea.Citation48,Citation49 The role of these proteins makes them key antigenic targets for designing novel RV vaccine candidates and immunotherapies.
RV vaccines can be roughly divided into four categories according to origins or compositions: non-human, animal-human reassortant, human, and non-replicating vaccines ( near here).
Figure 1. The classification of rotavirus vaccines Rotavirus vaccines can be roughly divided into four categories according to origins or compositions: non-human, animal-human reassortant, human, and other rotavirus vaccines
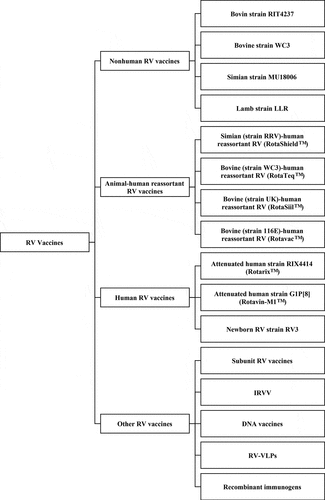
Non-human RV vaccines are those based on animal RV strains, which are heterotypic to human RV and need high titers to produce a moderate efficacy profile.Citation50–63 Further, the efficacies of the pioneering RIT 4237,Citation50–63 bovine strain WC3,Citation64–72 and simian strain RRVCitation73-82 vaccines varied significantly in different regions. In particular, lower protection of the non-human RV vaccines was found in developing countries with high natural infection burdens, and in areas with prevalent serotypes differing from the vaccine. These evidences have led to suspension of the most non-human RV vaccines.
Animal-human reassortant RV vaccines are constructed by containing the VP7 (or VP4) gene from human strains alongside the remaining animal RV genes (to attenuate RV virulence in infants). These RV vaccines were generally found to have high efficacy and immunogenicity profiles, and to provide heterotypic protection.
Human RV vaccines are derived from human RV and capable of exhibiting great immune response. This might be associated with the homotypic human strains included in the vaccines.
Non-replicating vaccines with a parenteral delivery strategy have safety advantages of potentially reducing the risk of intussusception and other side effects that has been associated with some oral vaccines, since the vaccine viruses could not replicate in the gut.Citation13
Current licensed RV vaccines
Bovine (strain WC3)-human reassortant RV (RotaTeq™)
RotaTeq™ (Merck & Co., Inc., Whitehouse Station, New Jersey, USA) is a live, oral three-dose vaccine that contains five reassortant RVs (G1, G2, G3, G4, and P1A[8]) derived from human and bovine parent strains. It was approved by the US Food and drug Administration (FDA) in February 2006 and is recommended to be given to infants at 2, 4, and 6 months of ageCitation83 ( near here).
Table 1. The table of the characteristics of licensed RV vaccines
In view of the previous association of the RotaShield™ vaccine with intussusception, substantial clinical trials were conducted to evaluate the safety of RotaTeq™ with respect to intussusception. Notably, in a large RV efficacy and safety trial (REST), conducted from 2001 to 2004 in 11 countries, involving almost 70,000 infants between 6 and 12 weeks, six vaccines and five placebo recipients developed intussusception within 42 d after any dose, which did not represent a significant difference between the groupsCitation84–85 ( near here). Besides, the efficacy profile of RotaTeq™ was encouraging; efficacy after three doses was estimated at up to 74% against G1–4 RV gastroenteritis (RVGE) and 98% against severe RVGE, compared with placebo during the first RV season. Further, an overall 95% reduction in hospitalizations and emergency department visits was found, which was consistent among regions (94.7%, 94.9%, and 90.0% in Europe, USA, and the Latin American/Caribbean regions, respectively).Citation97 Similar results were found in American Indian infants from REST, indicating no difference between types of population in the same developed region.Citation86 Furthermore, 98.3% and 68.0% protection against severe RVGE and RVGE, respectively, were obtained in Europe for two RV seasons postvaccination, regardless of serotype.Citation87 Extended Finnish research proved that the protection prolonged up to 3.1 y, following the last vaccine dose.Citation88 Another sub-study demonstrated that RotaTeq™, at the end of its shelf life, remained generally well tolerated, immunogenic, and efficacious.Citation89 Moreover, delayed vaccination, with an interval between previous and subsequent doses greater than 10 weeks, had no significant effect on efficacy or immunogenicity, supporting the use of various dosing intervals.Citation90 In another developed country, Japan, RotaTeq™ showed strong potency in preventing RVGE, moderate-to-severe RVGE, and severe RVGE (74.5%, 80.2%, and 100%, respectively), consistent with prior studies.Citation91
Table 2. Selected efficacy clinical trials* of four WHO prequalified live oral RV vaccines
However, there are significantly lower vaccine efficacies of RotaTeq™ were found in developing countries or regions with high mortality rates of RV, which was significantly different from the protective levels achieved in developed regions. In two field trials of RotaTeq™ performed in sub-Saharan Africa (Ghana, Kenya, and Mali) and Asia (Bangladesh and Vietnam), the efficacy against severe RVGE of 39.3% and 48.3% for the first 2 y of life, respectively.Citation24,Citation27 Combining data from the five sites in developing countries, the efficacy against RVGE for any severity, severe, and very severe was 33.9%, 42.5% and 51.2%, respectively, which strengthened the precision of efficacy estimates and revealed rising efficacy with increased RVGE severity.Citation92–94 Further, RotaTeq™ vaccine provided 69.3% efficacy against RVGE by any serotype and was generally well tolerated in healthy Chinese infants.Citation95
After the licensure of RotaTeq™ for global use in 2006, abundant effectiveness studies and real-world impact of introducing into national vaccination programs were conducted.Citation96–102 Recent systematic review suggested that the effectiveness (VE) was 90% and 45% in countries with low and high child mortality, respectively.Citation103 Pooled VE of full series RotaTeq™ against RV-associated hospitalizations and emergency department (ED) visits were 84% (95% CI: 80–87%) in the USA.Citation104 Since post-licensure data demonstrated that RotaTeq™ was effective against RV disease under routine use, adoption of RV vaccines into expanded national programs would support the World Health Organization's (WHO) recommendation to promote their global use, especially in regions with high RV disease burden and child mortality.
Attenuated human strain RIX4414 (Rotarix™)
Rotarix™, which is based on an attenuated human RV strain, RIX4414, genotype G1P[8], developed by GlaxoSmithKline. This monovalent human-derived RV vaccine was officially recommended for infants, with a two-dose oral regimen at 2 and 4 months of age in the USA in October 2008. Since then, more than 100 countries worldwide have introduced this vaccine ( near here).
An early efficacy trial demonstrated 72% protection against RVGE and 85% against severe RVGE during the entire follow-up period.Citation105 Besides, significant protection for 2 y was demonstrated in six European countries, with efficacies of 89.8%, 84.8%, 83.1%, 72.9%, and 58.3% against RVGE caused by circulating G1, G2, G3, G4, and G9 RV types, respectively.Citation106 Similar to observations in Europe, 63.5% heterotypic protection against severe RVGE caused by the G9 strain was found in Brazil.Citation106–108 In Latin America, a two-dose regimen of Rotarix™ achieved excellent efficacy rates against any RVGE, severe RVGE, and hospitalizations, as high as 70%, 86%, and 93%, respectively.Citation109 Moreover, a large phase ш trial enrolling 63,225 healthy infants inoculated with two doses of Rotarix™ demonstrated 84.7% and 100% efficacy against severe RVGE and more severe RVGE in 11 Latin American countries and FinlandCitation110 ( near here). In addition, the following efficacy study reported an overall 80.5% efficacy against RVGE during the entire 2 y.Citation111 Moreover, a high heterotypic protection against wild-type G9 RV strains (81.8%) was also shown in Brazil.Citation112 Rotarix™ was deemed not to be associated with an increased risk of intussusception in these studies.
In Singapore, Hong Kong, and Taiwan, vaccine efficacy of Rotarix™ was 96.1% against severe RVGE, 100% against G1, and 93.6% against circulating non-G1 RV types during the first 2 y of life;Citation113 100% efficacy against severe RVGE was sustained until the third year.Citation114 Similar vaccine efficacy against severe RVGE was obtained in other developed countries in Asia, such as JapanCitation115 and Korea,Citation116 indicating that Rotarix™ offered high protection among affluent Asian urban populations.
On the contrary, Rotarix™ provided moderate protection (72%) against severe RVGE in Chinese children over two consecutive RV seasons.Citation117 Yet, one study adopting two or three dose regimens of Rotarix™ among African infants declared an overall efficacy against severe RVGE of 61.2%. Besides, a much lower efficacy was also found in Malawi than in South Africa (49.4% vs. 76.9%) for the first year,Citation25 indicating the different efficacies between Africa countries. The efficacy of Rotarix™ later reached nadir as low as 38.1% in Malawi and 59% in South Africa during two entire consecutive RV seasons.Citation118,Citation119 In India, two doses of Rotarix™ induced a moderate seroconversion rate of 58.3% and the seroconversion rate did not interfere by increasing the number of doses.Citation120,Citation121
After the licensure of Rotarix™ in 2006, abundant effectiveness studies showed the VE against RVGE hospitalization were 83%, 65–96%, 76–77% in the high-income USA,Citation104 upper-middle-income Brazil,Citation122 lower-middle-income Bolivia and El Salvador, respectively.Citation123,Citation124 Though the majority of real-world data has been generated in high- or middle-income settings, the VE in an African setting with a high prevalence of HIV infection was 57% for two doses and 40% for one dose which was lower than prior studies in middle- or high-income countries.Citation125 Recent meta-analysis concluded the previous VE studies and suggested that the overall VE was 69%Citation126 and the VE was 84%, 75%, and 57% in countries with low, medium, and high child mortality, respectively.Citation103 The analysis of real-world studies showed that Rotarix™ is effective against hospitalizations and/or ED visits due to RV infection. Though the vaccine efficacy and VE in low-income countries with high children mortality due to diarrhea was lower than in well-developed regions, Rotarix™ still saved many lives regardless of theoretic risk of intussusception.Citation127
Bovine (strain 116E)-human reassortant RV (Rotavac™)
In the 1990s, two natural bovine-human reassortant RV strains were isolated from two sites in New Delhi and Bangalore, the 116E strain and the I321 strain, respectively.Citation128,Citation129 As infants infected with either strain were found to be protected against severe disease when re-infected,Citation130 the two strains were later developed as RV vaccines. The two vaccines, administered orally as single doses of 105 fluorescence-forming units (FFU), induced 73% and 39% serum IgA seroconversion rates in the 116E and I321 groups, respectively, in 2005.Citation131 As the 116E strain was proven to be safe and robustly immunogenic than those for strain I321, clinical trials of strain 116E are further advanced. This vaccine was officially named as Rotavac™ (strain 116E, genotype G9P[11]) manufactured by Bharat Biotech International Limited in India. It was licensed in India in 2014 and launched in the public health system 2 y later ( near here). This vaccine is given in three doses to infants on the same schedule of diphtheria-tetanus-pertussis vaccine (DTP) 1, 2, and 3.
Vaccine efficacy of Rotavac™ against severe RVGE was 56.3% reported in the first year of life, and 48.9% in the second year of life in a multi-center phase III trial,Citation132,Citation133 which is comparable to the results for Rotarix™ and RotaTeq™ in developing countries ( near here). However, the incidence of confirmed intussusception was 94/100,000 child-years among vaccinees and 71/100,000 child-years among the placebo group.Citation134 Although there was no statistical difference between the treatments, further investigation of the association between vaccination and intussusception is ongoing, using a surveillance system to monitor the safety profile.Citation135,Citation136 Further, since there is no evidence of potential interference of co-administration with other childhood vaccines, a field trial assessed and confirmed the hypothesis that co-inoculation avoided interference with the immune response to the antigens contained in childhood vaccines.Citation137,Citation138 In order to examine the role of buffer on the performance of RV vaccines, a phase Ⅳ study assessed whether Rotavac™ without buffer is as effective as the version with buffer added. The results suggested Rotavac™ was well tolerated and immunogenic, in terms of highest geometric mean titer and the proportion of seroconversion, in the absence of citrate-bicarbonate buffer.Citation139 Overall, Rotavac™ was successfully prequalified by the WHO in January 2018, and these favorable clinical trial results warrant further global marketing and development, expanded the choice of products, improved the global supply of vaccines, and helped to reduce costs.
Bovine (strain UK)-human reassortant RV (RotaSiil™)
The development of Rotarix™ can be traced to an earlier bovine-human RV reassortant tetravalent (BRV-TV) vaccine, incorporating 10 genes from the UK bovine strain and the human RV G1, 2, 3, or 4.Citation140 The efficacy of BRV-TV was approximately 69% against any RVGE, 79% against moderately RVGE, and 88% against severe RVGE during the first RV epidemic season;Citation141 Though the vaccine showed no protective efficacy against any RVGE during the second RV epidemic season, the efficacy against severe RVGE was sustained and the number of cases was very small. Subsequently, given the emergence of the novel, epidemiologically important serotype G9, the Serum Institute of India Ltd. (SIIL) collaborated with the national institute of allergy and infectious diseases (NIAID) to develop the lyophilized RotaSiil™ vaccine, containing RV serotypes G1, G2, G3, G4, and G9, which is the only heat-stable RV vaccine available worldwide (storage for 2 y at 37°C; 6 months at 40°C)Citation142( near here). This vaccine is also given in three doses to infants on the same schedule of DTP 1, 2, and 3. RotaSiil™ was found to be safe, immunogenic, and well tolerated in phase Ⅰ&Ⅱ clinical trials.Citation143 In contrast, RotaSiil™ vaccine efficacies of 34.5%, 66.7%, and 78.8%, against all RVGE, severe RVGE, and very severe RVGE, respectively, were found among infants in a phase ш trial in NigerCitation144 ( near here). Only one confirmed case of intussusception (1/2042) occurred in vaccine group, 542 d after receiving the third dose of RotaSiil™, and was deemed unrelated to vaccination.Citation145 Similarly, efficacy of RotaSiil™ against severe RVGE and very severe RVGE was 39.5% and 54.7%, respectively, over the entire follow-up period of 2 y in India.Citation146 In 2016, RotaSiil™ became the second local RV vaccine licensed in India, and the fourth WHO prequalified RV vaccine in September 2018. This novel vaccine shared the same favorable advantages with Rotavac™.
Lamb strain LLR
As the only developed and licensed live RV vaccine in China, the live monovalent oral Lanzhou lamb RV vaccine (LLR) was derived from an ovine RV (G10P[12]) in 1985, which was passaged 37 times in calf kidney cells by the Lanzhou Institute of Biological ProductsCitation147 (). The vaccine, licensed early in 2000 in China, is a liquid formulation, with buffer containing 105.5 infectious particles per dose, and recommended for children on the following schedule: one dose annually for those 2 months to 3 y old, then one dose for those 3–5 y old.Citation147 At the end of 2014, a total of 60 million doses of LLR had been distributed to children in China;Citation148 However, little data were reported about the safety, immunogenicity, and efficacy of LLR as it has not been confirmed by designed clinical trials. In 2002–2004, a post-licensure case–control study demonstrated that one dose of LLR vaccine was moderately effective (73.3% protection) in preventing RV disease requiring hospitalization.Citation147 However, the following study proved that one or two doses of the LLR vaccine conferred partial protection against RV disease, with 43.8% or 44.6% efficacy, respectively, during 2009–2011.Citation149 Similarly, the overall protection of 35.0% was subsequently identified from October 2011 to March 2012.Citation148 This may be attributed to the current used regimen of LLR was too complicated to follow, which leads to insufficient coverage of the LLR vaccine in this study. Besides, since the LLR vaccine has not been included into the National Immunization Program and the price is relatively expensive (about 18.4 USD per dose, 74 USD for four doses) in China and people are lack of the awareness of RV, lots of parents are not willing to complete the recommended immunization regimen.Citation147 According to the report, only 25.3% of children aged 2–59 months received at least one dose of LLR vaccine in Guangzhou (a highly developed urban region in China).Citation150
Attenuated human strain G1P[8] (Rotavin-M1™)
The licensed live, oral, RV vaccine (Rotavin-M1™), manufactured by POLYVAC-Vietnam, is derived from an attenuated G1P[8] strain recovered in 2003 from a child hospitalized for acute gastroenteritisCitation151,Citation152( near here). Before the licensure of Rotavin-M1™ in 2014, because the prices of the two available international RV vaccines were unaffordable for Vietnamese children, the government of Vietnam pursued a policy to encourage local bio-companies to address this need. The safety of Rotavin-M1™ was established with no serious adverse events or intussusception were recorded in infants, relative to Rotarix™.Citation152 Infants aged 6–12 weeks received either two- or three-dose schedules of low titer (106.0 FFU/dose) or high titer (106.3 FFU/dose) vaccines, separately from the OPV. The highest IgA seroconversion rate (73%, 95%CI 58–88%) was achieved in the two-dose schedule with high-dose RV vaccine administrated 2 months apart. Since no more data are available for this vaccine, further post-marketing studies should focus on exploring effectiveness, and the possible association of interference between Rotavin-M1™ and other vaccines administered concomitantly.
RV vaccines in the pipeline
Newborn RV strain RV3-BB
The Australian RV3-BB strain as a potential vaccine is isolated from infants in the first month of life and has completed Phase Ⅰ&Ⅱ clinical trials.Citation153–157 In the phase Ⅰ trial, one dose of RV3-BB (serotype G3P[6]) was safe and well tolerated. However, low-level immune responses were observed in infants.Citation158 The phase Ⅱ clinical trial adopted a three-dose regimen (6.5 × 105 FFU/mL), but immune response was still unsatisfactory (46% infants), partially because of limited replication of RV3 in the small intestine, which may be solved by increasing the dose of vaccine. Among the responders, RV3 provided partially heterotypic protection (54%) against challenge by the predominant G1P [8] community strain during the subsequent winter epidemic.Citation154 In order to increase the infectivity of RV3, a single, higher titer (8.3 × 106 FFU/mL) dose of second-generation RV3-BB vaccine was developed and well tolerated in vaccinees, without any associated adverse reactions in 2013.Citation155 The immune response to this advanced vaccine was not effected by maternal antibodies and this second-generation vaccine was highly immunogenic, with a positive cumulative response of up to 90% in a phase Ⅱ trial in New Zealand.Citation156,Citation159 These favorable data support progression of RV3-BB into efficacy trials. Recently, an overall heterotypic protection of 63% in the neonatal-schedule and infant-schedule groups combined was observed in Indonesia.Citation157,Citation160 Although RV3-BB is efficacious in preventing severe RVGE, further development and assessment are required to determine whether or not RV3-BB can offer protection against a range of circulating RV strains.Citation161
Subunit RV vaccines
Since subunit RV vaccines induced specific neutralizing antibodies in preclinical studies,Citation162–165 the P2-VP8-P[8] protein, consisting of a truncated VP8 subunit from the Wa strain (G1P[8]) of human RV, fused with the P2 epitope from tetanus toxin expressed in Escherichia coli, was finally produced at the Walter Reed Army Institute of Research, Pilot Bioproduction Facility (Silver Spring, MD, USA). This subunit RV was found to be well tolerated, with promising immunogenicity, when administered intramuscularly to adults in a clinical trial.Citation166 All vaccine recipients demonstrated significant IgA responses, and all but one demonstrated IgG responses. Later, this parenteral RV vaccine was also found to be immunogenic in infants, providing an alternative approach for RV disease prevention. Infants also received three doses of the Rotarix™ vaccine at 4, 8, and 12 weeks after the third study injection. The results suggested that P2-VP8-P[8] did not negatively affect the subsequent immune response to Rotarix™, indicating a possibility for concomitant use of parenteral and oral vaccines to achieve optimal protection against severe RV disease.Citation167 Based on these trial data, a trivalent P2-VP8 (P[4], P[6], and P[8]) subunit vaccine is undergoing testing at three sites in South Africa.Citation167 A liquid formulation of P2-VP8 is currently undergoing human clinical trials.Citation168 Additionally, another non-live subunit vaccine candidate consisting of human RV inner-capsid rVP6 protein and norovirus virus-like particles (VLPs) showed 65% protection against heterologous RV challenge regardless of delivery route in preclinical mouse model, which indicating the use of highly conserved VP6 protein could induce non-serotype-specific antibody responses.Citation169 Based on these results, subunit RV vaccine was considered as alternatives for RV immunization.
Other candidate vaccines
Inactivated RV vaccines (IRVV) are origin from weakened or killed RV strains like human RV strain CDC-9, G2P[8], G3P[8], G9P[8] and G1P[8] and ZTR-68 by stimulating antibodies against RV infection.Citation170–178 IRVV protected animal models through different challenging routes like oral or intramuscular, microneedle delivery for skin vaccination in preclinical trials. According to several studies, intramuscular inoculation with three doses of IRVV could elicit high serum IgG antibody and neutralizing antibody responses in gnotobiotic piglets,Citation171 which is similar to the results of mice model.Citation170,Citation172,Citation175
The DNA vaccines, RV virus-like particles (RV‐VLPs) and recombinant proteins in vitro and in vivo models were found to induce homologous and cross‐reactive immune responses.Citation179–188 Of them, RV‐VLPs, which was first reported in the 1980s, are superior to the conventional vaccines because they are conformationally similar to the parent virus and thus able to stimulate both the cellular and humoral immune responses to a greater extent.Citation189,Citation190 These promising data offer a great experimental basis and research direction for the development of RV‐VLPs to reach a reasonable protective immunization level against RV in the future.
Except the above candidates, there are several other RV vaccines in the development with little published data. Firstly, the Instituto Butantan in Brazil developed a live-attenuated bovine-human reassortant RV vaccine containing five viral antigens (G1, G2, G3, G4 and G9). This vaccine showed a good profile of safety and immunogenicity in a phase I study.Citation191 Second, a live-attenuated bovine-human reassortant RV tetravalent vaccine including antigens against four serotypes was developed by the Sanofi affiliate, Shantha Biotechnics in India. A phase I/II study showed three doses of vaccine were safe, tolerated and immunogenicity in healthy Indian infants.Citation192 Third, PATH and China National Biotec Group/Wuhan Institute of Biological Products co-manufacture a novel bovine and human hexavalent reassortant RV vaccine candidate.Citation193 Fourth, a new version of the LLR vaccine, based on the original monovalent vaccine (a tervalent human-lamb reassortant vaccine, combining human RV serotypes G2, G3, G4 with lamb RV genesCitation194) was developed and is undergoing clinical trials, whose results are yet to be published. Moreover, a novel liquid formulation of bovine RV pentavalent vaccine (LBRV-PV), stored at 2–8°C, like traditional vaccines, was well tolerated in adults, without safety concerns, indicating the possibility that it could become an alternative RV candidate vaccine, providing end-users with a convenient choice of a ready to use vaccine.Citation195
Conclusions and future perspectives
In the last 30 y, we have seen significant progress in the development of RV vaccines. There are six licensed RV vaccines available and a number of RV vaccine candidates undergoing development. Generally, data from current clinical trials and surveillance systems indicate that Rotarix™ and RotaTeq™ are effective against RVGE, and significantly reduced acute hospitalization associated with RV. Also, a cost-effectiveness study in Kenya predicted that cumulatively, over the first 5 y of life, the estimated vaccination costs of US 1,782,761, USD using Rotarix™ or RotaTeq™, would lead to savings of 48,585 disability adjusted life years.Citation196 Besides, another modeling study estimated that, RV vaccination would prevent nearly 600,000 deaths in Gavi countries and save approximately 484.1 USD million from the government perspective and 878.0 USD million from the societal perspective over the period 2018–27.Citation197 The high cost-effectiveness of RV vaccines provides a strong argument for their widespread use, particularly in low-income, high-burden settings.Citation62,Citation198-200 Moreover, the Rotavac™ and RotaSiil™ RV vaccines may provide additional choice in low-income regions, with several great advantages, including heat stability, low cost, and cold-chain footprint.
There are several issues that remain to be addressed for these licensed RV vaccines. First, although licensed RV vaccines are generally tolerated and safe in clinical trials, the occurrence of intussusception caused by RotaShield™ decreased the acceptability of RV vaccines for parents and impeded RV research progress.Citation201 Since safety is considered the principal parameter for developing any new vaccine, the unexpected intussusception postvaccination with RV vaccines should be monitored closely. For now, abundant post-marketing surveillance data for RotaTeq™ had been reported from Australia and the United States.Citation202–205 Australian investigators suggested that the risk of intussusception remained low and occurred primarily in the first week after the first immunization. In the United States, researchers suggested that RotaTeq™ is not associated with intussusception. Besides, another study confirmed that the risk of intussusception after administration of Rotarix™ vaccine was not higher than the background risk of intussusception in seven lower-income sub-Saharan African countries.Citation206 Though data available with Rotarix™ and RotaTeq™ in these regions are insufficient to exclude any risk associated with RV vaccines, but this is outweighed by the large benefits of vaccination. Hence, further active surveillance studies still need to be progressed to determine whether RV vaccines are causally with intussusception or not. Though there are no post-marketing surveillance data available for other licensed RV vaccines at present, the performance during clinical trials indicated that there is no significant increase of risk associated with intussusception. To date, the cause of intussusception post-vaccination remains unknown, necessitating further investigation and surveillance. Apart from the above intussusception, another possible safety issue was the virus shedding which was common post-vaccination with live RV vaccines and may occasionally generate new or unusual RV strains by reassorting. It also can lead to passive transmission in healthy children. The duration of shedding and amounts of virus affect the risk of infection within the unvaccinated infants, which deserves research attention. Hence, strains infecting humans should be closely monitored, using modern sequencing technologies and phylogenetic analyses.
Second, the licensed RV vaccines exhibit great efficacy worldwide; Nevertheless, efficacies in resource-limiting settings were lower than those observed in industrialized settings potentially due to higher disease burden and mortality. Although the reason for this difference has not been thoroughly elucidated, it is possibly associated with preexisting antibodies and differences between circulating RV strains and those used for RV vaccine development. Additionally, other factors such as OPV, concomitant infections, and gut microbiome/intestinal enteropathy might also have a role in the discrepancy. To date, most RV infections globally were attributed to G1P[8], G2P[4], G3P[8], and G4P[8] in the past 30 y;Citation207 however, rare strains like G2P[6], G9P[4], G12P[6], G12 P[8] recently contributed to a larger proportion of RV infections as the results of circulating genotypes varied by years and regions.Citation208–210 Strain diversity, resulting from point mutations, genetic reassortment, genome rearrangement, and interspecies transmission, increases the difficulty in developing corresponding RV vaccines as backup.Citation211–213 Though mass RV vaccine introduction in the future may lead to gradual replacement of circulating genotypes detected in the pre-vaccine period by another in the post-vaccine period, several pre – and post-licensure studies have shown that Rotarix™, RotaTeq™, and Rotavac™ provide significant protection against severe RV disease caused by a variety of strains in high – and middle-income countries, suggesting that their efficacy/effectiveness may not be serotype-specific.Citation97,Citation98,Citation106,Citation139
Third, the simultaneous administration of current RV vaccines with most other vaccines in a routine immunization program showed no significant interference with the protective immune responses or safety profiles of the respective vaccines.Citation214–220 Nonetheless, potential interference was recorded on co-administration of Rotarix™ with monovalent, bivalent, or trivalent OPV, compared with inactivated polio vaccine. This interference highlights the need for separating RV vaccines and OPV in the EPI schedule and requires further investigation to explore the mechanism and emerging risk.Citation29,Citation221 Therefore, once a novel RV vaccine is introduced, we should not only consider its safety and efficacy profiles, but also the potential risk of interference with co-administrated vaccines.
Fourth, RV vaccines are recommended for healthy infants, but they are forbidden to infants with human immunodeficiency virus (HIV) infection in the United States at present. RV vaccines are particularly important to specific groups, such as malnourished children, premature infants and infants with acquired immunodeficiency syndrome, who are especially vulnerable to RV. However, clinical trials targeting malnourished children, premature infants and children with acute diarrhea, intussusception, cancer, or HIV infection are exceedingly rare. According to several studies, RotaTeq™ can prevent 73.0% of RVGE and reduce hospitalizations and ED visits in premature infants by 100%.Citation83,Citation222 Moreover, one study suggested that breastfed and non-breastfed infants were equally protected from the severe consequences of RVGE.Citation223 However, other studies found that the immune response was not enhanced by withholding breastfeeding and IgA seroconversion in infants immediately breastfed tended to be higher than in those withheld from a feeding.Citation224,Citation225 Since whether breastfeeding plays a protective role in RV infection is controversial, a meta-review systematically found that there may be no direct correlation between RV diarrhea and breastfeeding.Citation226,Citation227 In addition, satisfactory immune responses were mounted postvaccination with Rotarix™ or RotaTeq™ in HIV-positive infants, without aggravating their immunologic or HIV-related parameters; however, the small number of vaccinees did not provide sufficient power to fully assess safety in HIV-infected infants, which therefore requires further evaluation.Citation228,Citation229
Fifth, an additional challenge toward increasing the impact of RV vaccination is the need for improving coverage, which still lags behind coverage of DTP and other routine vaccines.Citation42,Citation230–231 Though the estimated national RV vaccine coverage increased since vaccine introduction in USA in 2013–2015,Citation104 lower than that of other routine childhood vaccines. Even though RV vaccines have been routinely implemented in major developed countries for a decade, poor medical infrastructure, a lack of refrigerators or dependable electrical supply, and related economic issues in impoverished settings interfere with the implementation of RV vaccines. Since heat stability of the RV vaccine is important for its successful application in areas with less developed cold-chains, development of stable RV vaccines, such as the bovine-human RV reassortant vaccine, RotaSiil™, are necessary for their use in varying local conditions. Besides, as the people most at risk for RV are usually those least able to pay for vaccines, some local vaccine manufacturers in China and India have launched domestic programs for RV vaccine development. Considering the high RV disease burden in low-income settings where have not launched the RV vaccine program yet, great efforts are needed and necessary for improving coverage and encourage the development of suitable vaccines for native populations at an affordable cost.
In conclusion, the current licensed RV vaccines were safe and effective in settings with low child mortality due to diarrhea, but less effective was found in settings with medium and high child mortality. How to improve protection and expand coverage requires further investigation. The ideal RV vaccine should be an efficacious and heat-stable RV vaccine at an affordable price. The future development and introduction of RV vaccines will still require substantial investment in human resources and materials, given the extraordinary performance of introducing RV in developed regions. Hopefully, the introduction of an effective RV vaccine worldwide is promising to reduce the morbidity and mortality of RV-associated gastroenteritis, the disease impact and severe diseases among children <5 y old in the next few years.
Abbreviations
| RV | = | Rotavirus |
| OPV | = | Oral poliovirus vaccine |
| VPs | = | Viral proteins |
| NSPs | = | Nonstructural proteins |
| P | = | VP4 |
| G | = | VP7 |
| RotaTeq™ | = | Bovine (strain WC3)-human reassortant RV vaccine |
| FDA | = | Food and Drug Administration |
| RVGE | = | RV gastroenteritis |
| ED | = | Emergency department |
| WHO | = | World Health Organization |
| Rotarix™ | = | Attenuated human strain RIX4414 vaccine |
| Rotavac™ | = | Bovine (strain 116E)-human reassortant RV vaccine |
| FFU | = | Fluorescence-forming units |
| RotaSiil™ | = | Bovine (strain UK)-human reassortant RV vaccine |
| BRV-TV | = | Bovine-human RV reassortant tetravalent vaccine |
| SIIL | = | Serum Institute of India Ltd. |
| NIAID | = | National institute of allergy and infectious diseases |
| LLR | = | Lanzhou lamb RV vaccine |
| Rotavin-M1™ | = | Attenuated human strain G1P[8] RV vaccine |
| RV3-BB | = | Newborn RV strain G3P[6] RV vaccine |
| IRVV | = | Inactivated RV vaccine |
| RV‐VLPs | = | RV virus-like particles |
| LBRV-PV | = | Liquid formulation of bovine RV pentavalent vaccine |
| HIV | = | Human immunodeficiency virus |
Disclosure of potential conflicts of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–s15. doi:10.1086/605025. PMID:19817620.
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet (London, England). 2010;375:1969–87. doi:10.1016/s0140-6736(10)60549-1. PMID:20466419.
- Tate JE, Burton AH, C B-P, Parashar UD, Global R. Global, Regional, and national estimates of rotavirus mortality in Children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–s105. doi:10.1093/cid/civ1013. PMID:27059362.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of Rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172:958–65. doi:10.1001/jamapediatrics.2018.1960. PMID:30105384.
- Bern C, Martines J, de Zoysa I, Glass RI. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ. 1992;70:705–14. PMID:1486666.
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. doi:10.3201/eid0905.020562. PMID:12737740.
- Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–83. doi:10.1016/s0140-6736(73)92867-5. PMID:4127639.
- Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974;1:149–51. doi:10.1016/s0140-6736(74)92440-4. PMID:4129719.
- Flewett TH, Bryden AS, Davies H, Woode GN, Bridger JC, Derrick JM. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet (London, England). 1974;2:61–63. doi: PMID:4137164.
- Flewett TH, Woode GN. The rotaviruses. Arch Virol. 1978;57:1–23. doi:10.1007/bf01315633. PMID: 77663.
- Matthews RE. Third report of the international committee on taxonomy of viruses. Classification and nomenclature of viruses. Intervirology. 1979;12:129–296. doi:10.1159/000149081. PMID: 43850.
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O’Ryan M, Kang G, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. doi:10.1038/nrdp.2017.83. PMID: 29119972.
- Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018;20:223–33. doi:10.1007/s40272-018-0283-3. PMID: 29388076.
- Mahmud-Al-Rafat A, Muktadir A, Muktadir H, Karim M, Maheshwari A, Ahasan MM. Rotavirus epidemiology and vaccine demand: considering Bangladesh chapter through the book of global disease burden. Infection. 2018;46:15–24. doi:10.1007/s15010-017-1082-4. PMID: 29047020.
- Sadiq A, Bostan N, Yinda KC, Naseem S, Sattar S. Rotavirus: genetics, pathogenesis and vaccine advances. Rev Med Virol. 2018;28:e2003. doi:10.1002/rmv.2003. PMID: 30156344.
- World Health Otganization. Rotavirus vaccines- WHO position paper -. January 2013. [ accessed 2020 Apr 3]. https://www.who.int/wer/2013/wer8805.pdf?ua=1
- Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–96. doi:10.1093/ije/dyn260. PMID: 19056806.
- Clark A, Black R, Tate J, Roose A, Kotloff K, Lam D, Blackwelder W, Parashar U, Lanata C, Kang G, et al. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. PLoS One. 2017;12:e0183392. doi:10.1371/journal.pone.0183392. PMID: 28892480.
- Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. National estimates of reductions in acute gastroenteritis-related hospitalizations and associated costs in US Children after implementation of Rotavirus vaccines. J Pediatric Infect Dis Soc. 2018;7:257–60. doi:10.1093/jpids/pix057. PMID: 28992205.
- Hallowell BD, Parashar UD, Curns A, DeGroote NP, Tate JE. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination - United States, 2000-2018. MMWR Morb Mortal Wkly Rep. 2019;68:539–43. doi:10.15585/mmwr.mm6824a2. PMID:31220058.
- Mwenda JM, Parashar UD, Cohen AL, Tate JE. Impact of rotavirus vaccines in sub-Saharan African countries. Vaccine. 2018;36:7119–23. doi:10.1016/j.vaccine.2018.06.026. PMID: 29914848.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the global Rotavirus surveillance network. Lancet Glob Health. 2019;7:e893–e903. doi:10.1016/s2214-109x(19)30207-4. PMID: 31200889.
- Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020. doi:10.1093/infdis/jiaa081. PMID: 32095831.
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376:606–14. doi:10.1016/s0140-6736(10)60889-6. PMID: 20692030.
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi:10.1056/NEJMoa0904797. PMID: 20107214.
- Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, Baek LJ, Parashar U, Glass RI, Jiang B. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–23. PMID: 20442687. doi:10.1097/INF.0b013e3181e232ea.
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi:10.1016/S0140-6736(10)60755-6. PMID: 20692031.
- Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30(Suppl 1):A30–5. doi:10.1016/j.vaccine.2011.11.093. PMID: 22520134.
- Ramani S, Mamani N, Villena R, Bandyopadhyay AS, Gast C, Sato A, Laucirica D, Clemens R, Estes MK, O’Ryan ML. Rotavirus serum IgA immune response in Children receiving Rotarix coadministered with bOPV or IPV. Pediatr Infect Dis J. 2016;35:1137–39. doi:10.1097/inf.0000000000001253. PMID: 27254033.
- Taniuchi M, Platts-Mills JA, Begum S, Uddin MJ, Sobuz SU, Liu J, Kirkpatrick BD, Colgate ER, Carmolli MP, Dickson DM, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine. 2016;34:3068–75. doi:10.1016/j.vaccine.2016.04.080. PMID: 27154394.
- Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, et al. Significant correlation between the infant Gut Microbiome and Rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215:34–41. doi:10.1093/infdis/jiw518. PMID: 27803175.
- Harris V, Ali A, Fuentes S, Korpela K, Kazi M, Tate J, Parashar U, Wiersinga WJ, Giaquinto C, de Weerth C, et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2018;9:93–101. doi:10.1080/19490976.2017.1376162. PMID: 28891751.
- Bernstein DI, Glass RI, Rodgers G, Davidson BL, Sack DA. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus vaccine efficacy group. Jama. 1995;273:1191–96. doi:10.1001/jama.1995.03520390051032. PMID: 7707626.
- Migasena S, Simasathien S, Samakoses R, Pitisuttitham P, Sangaroon P, van Steenis G, Beuvery EC, Bugg H, Bishop R, Davidson BL, et al. Simultaneous administration of oral rhesus-human reassortant tetravalent (RRV-TV) rotavirus vaccine and oral poliovirus vaccine (OPV) in Thai infants. Vaccine. 1995;13:168–74. doi:10.1016/0264-410x(95)93131-r. PMID: 7625111.
- Lanata CF, Black RE, Flores J, Lazo F, Butron B, Linares A, Huapaya A, Ventura G, Gil A, Kapikian AZ. Immunogenicity, safety and protective efficacy of one dose of the rhesus rotavirus vaccine and serotype 1 and 2 human-rhesus rotavirus reassortants in children from Lima, Peru. Vaccine. 1996;14:237–43. doi:10.1016/0264-410x(95)00132-k. PMID: 8920706.
- Linhares AC, Gabbay YB, Mascarenhas JD, de Freitas RB, Oliveira CS, Bellesi N, Monteiro TA, Lins-Lainson Z, Ramos FL, Valente SA. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belem, Brazil. Bull World Health Organ. 1996;74:491–500. PMID: 9002329.
- Rennels MB, Glass RI, Dennehy PH, Bernstein DI, Pichichero ME, Zito ET, Mack ME, Davidson BL, Kapikian AZ. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines–report of the national multicenter trial. United States Rotavirus vaccine efficacy group. Pediatrics. 1996;97:7–13. PMID: 8545227.
- Perez-Schael I, Guntinas MJ, Perez M, Pagone V, Rojas AM, Gonzalez R, Cunto W, Hoshino Y, Kapikian AZ. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–87. doi:10.1056/nejm199710233371701. PMID: 9337376.
- Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 1999; 48:1–20. PMID: 10219046.
- Centers for Disease Control and Prevention (CDC). Intussusception among recipients of rotavirus vaccine–United States. 1998-1999; MMWR Morb Mortal Wkly Rep. 1999; 48:577–81. PMID: 10428095.
- Abramson JS, Baker CJ, Fisher MC, Gerber MA, Meer HC, Murray DL, Overturf GD, Prober CG, Rennels MB, Saari TN, et al. Possible association of intussusception with Rotavirus vaccination. American academy of pediatrics. Committee on infectious diseases. Pediatrics. 1999;104:575. PMID: 10469790.
- Abou-Nader AJ, Sauer MA, Steele AD, Tate JE, Atherly D, Parashar UD, Santosham M, Nelson EAS. Global rotavirus vaccine introductions and coverage: 2006-2016. Hum Vaccin Immunother. 2018;14:2281–96. doi:10.1080/21645515.2018.1470725. PMID: 29787334.
- Carvalho MF, Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother. 2019;15:1237–50. doi:10.1080/21645515.2018.1520583. PMID: 30215578.
- Estes MK, Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989;53:410–49. doi:10.1128/MMBR.53.4.410-449.1989. PMID: 2556635.
- Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–51. doi:10.1053/j.gastro.2009.02.076. PMID: 19457420.
- Hoshino Y, Jones RW, Kapikian AZ. Characterization of neutralization specificities of outer capsid spike protein VP4 of selected murine, lapine, and human rotavirus strains. Virology. 2002;299:64–71. doi:10.1006/viro.2002.1474. PMID: 12167342.
- Pesavento JB, Crawford SE, Estes MK, Prasad BV. Rotavirus proteins: structure and assembly. Curr Top Microbiol Immunol. 2006;309:189–219. doi:10.1007/3-540-30773-7_7. PMID: 16913048.
- Bishop RF. Natural history of human rotavirus infection. Arch Virol Suppl. 1996;12:119–28. doi:10.1007/978-3-7091-6553-9_14. PMID: 9015109.
- Hyser JM, Estes MK. Rotavirus vaccines and pathogenesis: 2008. Curr Opin Gastroenterol. 2009;25:36–43. doi:10.1097/MOG.0b013e328317c897. PMID: 19114772.
- Vesikari T, Isolauri E, Delem A, D’Hondt E, Andre FE, Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983;2:807–11. doi:10.1016/s0140-6736(83)90734-1. PMID: 6137646.
- Delem A, Lobmann M, Zygraich N. A bovine rotavirus developed as a candidate vaccine for use in humans. J Biol Stand. 1984;12:443–45. doi:10.1016/s0092-1157(84)80068-2. PMID: 6098585.
- Vesikari T, Isolauri E, D’Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–81. doi:10.1016/s0140-6736(84)92323-7. PMID: 6143964.
- Vesikari T, Isolauri E, Delem A, d’Hondt E, Andre FE, Beards GM, Flewett TH. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985;107:189–94. doi:10.1016/s0022-3476(85)80123-2. PMID: 3894608.
- Vesikari T, Ruuska T, Bogaerts H, Delem A, Andre F. Dose-response study of RIT 4237 oral rotavirus vaccine in breast-fed and formula-fed infants. Pediatr Infect Dis. 1985;4:622–25. doi:10.1097/00006454-198511000-00005. PMID: 3001659.
- De Mol P, Zissis G, Butzler JP, Mutwewingabo A, Andre FE. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;2:108. doi:10.1016/s0140-6736(86)91643-0. PMID: 2873370.
- Maldonado Y, Hestvik L, Wilson M, Townsend T, O’Hare J, Wee S, Yolken R. Safety and immunogenicity of bovine rotavirus vaccine RIT 4237 in 3-month-old infants. J Pediatr. 1986;109:931–35. doi:10.1016/s0022-3476(86)80271-2. PMID: 3537248.
- Vesikari T, Rautanen T, Isolauri E, Delem A, Andre FE. Immunogenicity and safety of a low passage level bovine rotavirus candidate vaccine RIT 4256 in human adults and young infants. Vaccine. 1987;5:105–08. doi:10.1016/0264-410x(87)90055-7. PMID: 3037812.
- Lanata CF, Black RE, Del Aguila R, Gil A, Verastegui H, Gerna G, Flores J, Kapikian AZ, Andre FE. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989;159:452–59. doi:10.093/infdis/159.3.452. PMID: 2536789.
- Zoppi G, Mantovanelli F, Pittschieler K, Delem A, Teuwen DE. Response to RIT 4237 oral rotavirus vaccine in human milk, adapted-and soy-formula fed infants. Acta Paediatr Scand. 1989;78:759–62. doi:10.1111/j.1651-2227.1989.tb11139.x. PMID: 2556883.
- Ruuska T, Vesikari T, Delem A, Andre FE, Beards GM, Flewett TH. Evaluation of RIT 4237 bovine rotavirus vaccine in newborn infants: correlation of vaccine efficacy to season of birth in relation to rotavirus epidemic period. Scand J Infect Dis. 1990;22:269–78. doi:10.3109/00365549009027047. PMID: 2164706.
- Santosham M, Letson GW, Wolff M, Reid R, Gahagan S, Adams R, Callahan C, Sack RB, Kapikian AZ. A field study of the safety and efficacy of two candidate rotavirus vaccines in a native American population. J Infect Dis. 1991;163:483–87. doi:10.1093/infdis/163.3.483. PMID: 1995721.
- Vesikari T, Ruuska T, Delem A, Andre FE, Beards GM, Flewett TH. Efficacy of two doses of RIT 4237 bovine rotavirus vaccine for prevention of rotavirus diarrhoea. Acta Paediatr Scand. 1991;80:173–80. doi:10.1111/j.1651-2227.1991.tb11830.x. PMID: 1852084.
- Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–61. doi:10.1016/0264-410x(93)90026-t. PMID: 8382419.
- Clark HF, Furukawa T, Bell LM, Offit PA, Perrella PA, Plotkin SA. Immune response of infants and children to low-passage bovine rotavirus (strain WC3). Am J Dis Child. 1986;140:350–56. doi:10.1001/archpedi.1986.02140180084030. PMID: 3006476.
- Clark HF, Borian FE, Bell LM, Modesto K, Gouvea V, Plotkin SA. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988;158:570–87. doi:10.1093/infdis/158.3.570. PMID: 2842405.
- Bernstein DI, Kacica MA, McNeal MM, Schiff GM, Ward RL. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antiviral Res. 1989;12:293–300. doi:10.1016/0166-3542(89)90056-9. PMID: 2561335.
- Garbag-Chenon A, Fontaine JL, Lasfargues G, Clark HF, Guyot J, Le Moing G, Hessel L, Bricout F. Reactogenicity and immunogenicity of rotavirus WC3 vaccine in 5-12 month old infants. Res Virol. 1989;140:207–17.. doi:10.1016/s0923-2516(89)80098-6. PMID: 2547237.
- Bernstein DI, Smith VE, Sander DS, Pax KA, Schiff GM, Ward RL. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990;162:1055–62. doi:10.1093/infdis/162.5.1055. PMID: 2172394.
- Clark HF, Borian FE, Modesto K, Plotkin SA. Serotype 1 reassortant of bovine rotavirus WC3, strain WI79-9, induces a polytypic antibody response in infants. Vaccine. 1990;8:327–32. doi:10.1016/0264-410x(90)90089-5. PMID: 2168607.
- Clark HF, Borian FE, Plotkin SA. Immune protection of infants against rotavirus gastroenteritis by a serotype 1 reassortant of bovine rotavirus WC3. J Infect Dis. 1990;161:1099–104. doi:10.1093/infdis/161.6.1099. PMID: 2161038.
- Ward RL, Sander DS, Schiff GM, Bernstein DI. Effect of vaccination on serotype-specific antibody responses in infants administered WC3 bovine rotavirus before or after a natural rotavirus infection. J Infect Dis. 1990;162:1298–303. doi:10.1093/infdis/162.6.1298. PMID: 2172404.
- Georges-Courbot MC, Monges J, Siopathis MR, Roungou JB, Gresenguet G, Bellec L, Bouquety JC, Lanckriet C, Cadoz M, Hessel L, et al. Evaluation of the efficacy of a low-passage bovine rotavirus (strain WC3) vaccine in children in Central Africa. Res Virol. 1991;142:405–11. doi:10.1016/0923-2516(91)90008-q. PMID: 1663261.
- Anderson EL, Belshe RB, Bartram J, Crookshanks-Newman F, Chanock RM, Kapikian AZ. Evaluation of rhesus rotavirus vaccine (MMU 18006) in infants and young children. J Infect Dis. 1986;153:823–31. doi:10.1093/infdis/153.5.823. PMID: 3009633.
- Losonsky GA, Rennels MB, Kapikian AZ, Midthun K, Ferra PJ, Fortier DN, Hoffman KM, Baig A, Levine MM. Safety, infectivity, transmissibility and immunogenicity of rhesus rotavirus vaccine (MMU 18006) in infants. Pediatr Infect Dis. 1986;5:25–29. doi:10.1097/00006454-198601000-00005. PMID: 3003715.
- Rennels MB, Losonsky GA, Levine MM, Kapikian AZ. Preliminary evaluation of the efficacy of rhesus rotavirus vaccine strain MMU 18006 in young children. Pediatr Infect Dis. 1986;5:587–88. doi:10.1097/00006454-198609000-00019. PMID: 3020521.
- Flores J, Perez-Schael I, Gonzalez M, Garcia D, Perez M, Daoud N, Cunto W, Chanock RM, Kapikian AZ. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987;1:882–84. doi:10.1016/s0140-6736(87)92858-3. PMID: 2882289.
- Christy C, Madore HP, Pichichero ME, Gala C, Pincus P, Vosefski D, Hoshino Y, Kapikian A, Dolin R. Field trial of rhesus rotavirus vaccine in infants. Pediatr Infect Dis J. 1988;7:645–50. doi:10.1097/00006454-198809000-00009. PMID: 2845349.
- Perez-Schael I, Garcia D, Gonzalez M, Gonzalez R, Daoud N, Perez M, Cunto W, Kapikian AZ, Flores J. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990;30:219–29. doi:10.1002/jmv.1890300315. PMID: 2160516.
- Rennels MB, Losonsky GA, Young AE, Shindledecker CL, Kapikian AZ, Levine MM. An efficacy trial of the rhesus rotavirus vaccine in Maryland. The clinical study group. Am J Dis Child. 1990;144:601–04. doi:10.1001/archpedi.1990.02150290095037. PMID: 2330930.
- Tajima T, Thompson J, Wright PF, Kondo Y, Tollefson SJ, King J, Kapikian AZ. Evaluation of a reassortant rhesus rotavirus vaccine in young children. Vaccine. 1990;8:70–74.doi:10.1016/0264-410x(90)90181-k. PMID: 2156387.
- Vesikari T, Rautanen T, Varis T, Beards GM, Kapikian AZ. Rhesus Rotavirus candidate vaccine. Clinical trial in children vaccinated between 2 and 5 months of age. Am J Dis Child. 1990;144:285–89. doi:10.1001/archpedi.1990.02150270035021. PMID: 2154925.
- Jalil F, Zaman S, Carlsson B, Glass RI, Kapikian AZ, Mellander L, Hanson LA. Immunogenicity and reactogenicity of rhesus rotavirus vaccine given in combination with oral or inactivated poliovirus vaccines and diphtheria-tetanus-pertussis vaccine. Trans R Soc Trop Med Hyg. 1991;85:292–96. doi:10.1016/0035-9203(91)90061-3. PMID: 1653474.
- Goveia MG, Rodriguez ZM, Dallas MJ, Itzler RF, Boslego JW, Heaton PM, DiNubile MJ. Safety and efficacy of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatr Infect Dis J. 2007;26:1099–104. doi:10.1097/INF.0b013e31814521cb. PMID: 18043445.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi:10.1056/NEJMoa052664. PMID: 16394299.
- Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, Cook JR, Koch G, Heaton P. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis. PMID: 18162243. 2007;11(Suppl 2):S29–35. doi:10.1016/s1201-9712(07)60019-8.
- Grant LR, Watt JP, Weatherholtz RC, Moulton LH, Reid R, Santosham M, O’Brien KL. Efficacy of a pentavalent human-bovine reassortant rotavirus vaccine against rotavirus gastroenteritis among American Indian Children. Pediatr Infect Dis J. 2012;31:184–88. doi:10.1097/INF.0b013e3182435afe. PMID: 22252206.
- Vesikari T, Itzler R, Karvonen A, Korhonen T, Van Damme P, Behre U, Bona G, Gothefors L, Heaton PM, Dallas M, et al. RotaTeq, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine. 2009;28:345–51. doi:10.1016/j.vaccine.2009.10.041. PMID: 19879226.
- Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq(R), in Finnish infants up to 3 years of age: the Finnish extension study. Eur J Pediatr. 2010;169:1379–86. doi:10.1007/s00431-010-1242-3. PMID: 20559656.
- Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, Bauder J, Boslego JW, Heaton PM. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics. 2007;119:11–18. doi:10.1542/peds.2006-2058. PMID: 17200266.
- Goveia MG, Suprun L, Itzler RF, McFetridge R, Dallas MJ, Kuter BJ. Efficacy and safety of pentavalent human-bovine reassortant rotavirus vaccine when administered with greater than 10 weeks between doses. Pediatr Infect Dis J. 2010;29:263–65. doi:10.1097/INF.0b013e3181be6257. PMID: 19949360.
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schodel F, Brown ML, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother. 2013;9:1626–33. doi:10.4161/hv.24846. PMID: 23732903.
- Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, Hille D, Ciarlet M, Neuzil KM. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine. 2012;30(Suppl 1):A24–9. 10.1016/j.vaccine.2011.08.124. PMID: 22520132
- Sow SO, Tapia M, Haidara FC, Ciarlet M, Diallo F, Kodio M, Doumbia M, Dembele RD, Traore O, Onwuchekwa UU, et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine. 2012;30(Suppl 1):A71–8. doi:10.1016/j.vaccine.2011.11.094. PMID: 22520140.
- Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, Rivers SB, Levine MM, Laserson KF, Feikin DR, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub-Saharan Africa. Vaccine. 2012;30(Suppl 1):A79–85. doi:10.1016/j.vaccine.2012.01.022. PMID: 22520141.
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, Wei D, Fu B, Liao X, Chu J, et al. Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double-blind, placebo-controlled trial. Vaccine. 2017;35:5897–904. doi:10.1016/j.vaccine.2017.08.081. PMID: 28935470.
- Chang WC, Yen C, Wu FT, Huang YC, Lin JS, Huang FC, Yu HT, Chi CL, Lin HY, Tate JE, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J. 2014;33:e81–6. doi:10.1097/inf.0000000000000105. PMID: 24569388.
- Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar UD. Effectiveness and impact of rotavirus vaccines in the United States - 2006-2012. Expert Rev Vaccines.. 2014;13:365–76. doi:10.1586/14760584.2014.877846. PMID: 24392657.
- Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, Staat MA, Halasa NB, Weinberg GA, Szilagyi PG, Chappell J, et al. Long-term consistency in Rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US Children, 2012-2013. Clin Infect Dis. 2015;61:1792–99. doi:10.1093/cid/civ872. PMID: 26449565.
- Gastañaduy PA, Contreras-Roldán I, Bernart C, López B, Benoit SR, Xuya M, Muñoz F, Desai R, Quaye O, Tam KI, et al. Effectiveness of monovalent and pentavalent Rotavirus vaccines in Guatemala. Clin Infect Dis. 2016;62(Suppl 2):S121–6. doi:10.1093/cid/civ1208. PMID: 27059345.
- Patel M, Pedreira C, De Oliveira LH, Tate J, Leshem E, Mercado J, Umaña J, Balmaceda A, Reyes M, Kerin T, et al. Effectiveness of pentavalent Rotavirus vaccine against a diverse range of circulating strains in Nicaragua. Clin Infect Dis. 2016;62(Suppl 2):S127–32. doi:10.1093/cid/civ1017. PMID: 27059346.
- Hemming-Harlo M, Vesikari T, Uhari M, Renko M, Salminen M, Torcel-Pagnon L, Hartwig S, Simondon F, Bricout H. Sustained high effectiveness of RotaTeq on hospitalizations attributable to Rotavirus-associated Gastroenteritis during 4 years in Finland. J Pediatric Infect Dis Soc. 2017;6:317–23. doi:10.1093/jpids/piw061. PMID: 27760800.
- Muhsen K, Anis E, Rubinstein U, Kassem E, Goren S, Shulman LM, Ephros M, Cohen D. Effectiveness of rotavirus pentavalent vaccine under a universal immunization programme in Israel, 2011-2015: a case-control study. Clin Microbiol Infect. 2018;24:53–59. doi:10.1016/j.cmi.2017.04.018. PMID: 28442435.
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of Rotavirus vaccination: a systematic review of the first decade of global Postlicensure data, 2006-2016. Clin Infect Dis. 2017;65:840–50. doi:10.1093/cid/cix369. PMID: 28444323.
- Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines. 2018;17:593–606. doi:10.1080/14760584.2018.1489724. PMID: 29909693.
- Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, De Vos B. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–43. doi:10.1097/01.inf.0000141722.10130.50. PMID: 15602194.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–63. doi:10.1016/s0140-6736(07)61744-9. PMID: 18037080.
- Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, da Silva VB, Mascarenhas JD, Noronha VL, Clemens R, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. J Pediatr (Rio J). 2007;83:217–24.doi:10.2223/jped.1600. PMID: 17380232.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine. 2010;28:5272–79. doi:10.1016/j.vaccine.2010.05.057. PMID: 20538094.
- Linhares AC, Ruiz-Palacios GM, Guerrero ML, Salinas B, Perez-Schael I, Clemens SA, Innis B, Yarzabal JP, Vespa G, Cervantes Y, et al. A short report on highlights of world-wide development of RIX4414: a Latin American experience. Vaccine. 2006;24:3784–85. doi:10.1016/j.vaccine.2005.07.027. PMID: 16098636.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi:10.1056/NEJMoa052434. PMID: 16394298.
- Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, Lopez P, Macias-Parra M, Ortega-Barria E, Rivera-Medina DM, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–89. doi:10.1016/s0140-6736(08)60524-3. PMID: 18395579.
- Justino MC, Araujo EC, van Doorn LJ, Oliveira CS, Gabbay YB, Mascarenhas JD, Miranda YS, Guerra Sde F, Silva VB, Linhares AC. Oral live attenuated human rotavirus vaccine (Rotarix) offers sustained high protection against severe G9P[8] rotavirus gastroenteritis during the first two years of life in Brazilian Children. Mem Inst Oswaldo Cruz. 2012;107:846–53. doi:10.1590/s0074-02762012000700002. PMID: 23147138.
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27:5936–41. doi:10.1016/j.vaccine.2009.07.098. PMID: 19679216.
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, van Doorn LJ, Teoh YL, Tang H, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30:4552–57. doi:10.1016/j.vaccine.2012.03.030. PMID: 22497874.
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29:6335–41. doi:10.1016/j.vaccine.2011.05.017. PMID: 21640780.
- Kim JS, Bae CW, Lee KY, Park MS, Choi YY, Kim KN, Kim JD, Park WS, Sin JB, Kim EA, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double-blind, placebo-controlled, phase IV study. Hum Vaccin Immunother. 2012;8:806–12. doi:10.4161/hv.19853. PMID: 22699440.
- Li RC, Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10:11–18. doi:10.4161/hv.26319. PMID: 24013441.
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian Children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine. 2012;30(Suppl 1):A36–43. doi:10.1016/j.vaccine.2011.09.120. PMID: 22520135.
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl 1):A44–51. doi:10.1016/j.vaccine.2011.08.080. PMID: 22520136.
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta SK, Suryakiran PV, Delem A, Han HH, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5:414–19. doi:10.4161/hv.5.6.8176. PMID: 19276664.
- Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, Kang G. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine. 2014;32(Suppl 1):A129–33. doi:10.1016/j.vaccine.2014.03.002. PMID: 25091666.
- Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, da Silva VB, Sanchez N, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J. 2011;30:396–401. doi:10.1097/INF.0b013e3182055cc2. PMID: 21150692.
- de, Palma O, Cruz L, Ramos H, de, Baires A, Villatoro N, Pastor D, de, Oliveira LH, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi:10.1136/bmj.c2825. PMID: 20551120.
- Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L, Iniguez V, Rivera R, Tam KI, Quaye O, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ. 2013;346:f3726. doi:10.1136/bmj.f3726. PMID: 23783434.
- Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, Mulligan C, Diedericks R, Cohen C, Fleming JA, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14:1096–104. doi:10.1016/s1473-3099(14)70940-5. PMID: 25303843.
- Willame C, Vonk Noordegraaf-Schouten M, Gvozdenović E, Kochems K, Oordt-Speets A, Praet N, van Hoorn R, Rosillon D. Effectiveness of the oral human attenuated Rotavirus vaccine: a systematic review and meta-analysis-2006-2016. Open Forum Infect Dis. 2018;5:ofy292. doi:10.1093/ofid/ofy292. PMID: 30539038.
- Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. PMID: 21675888. 2011;364:2283–92. doi:10.1056/NEJMoa1012952.
- Das BK, Gentsch JR, Hoshino Y, Ishida S, Nakagomi O, Bhan MK, Kumar R, Glass RI. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197:99–107. doi:10.1006/viro.1993.1570. PMID: 8212599.
- Dunn SJ, Greenberg HB, Ward RL, Nakagomi O, Burns JW, Vo PT, Pax KA, Das M, Gowda K, Rao CD. Serotypic and genotypic characterization of human serotype 10 rotaviruses from asymptomatic neonates. J Clin Microbiol. 1993;31:165–69. PMID: 8380181. doi:10.1128/JCM.31.1.165-169.1993.
- Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–87. doi:10.1093/infdis/168.2.282. PMID: 8393054.
- Bhandari N, Sharma P, Glass RI, Ray P, Greenberg H, Taneja S, Saksena M, Rao CD, Gentsch JR, Parashar U, et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine. 2006;24:5817–23. doi:10.1016/j.vaccine.2006.05.001. PMID: 16735085.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(Suppl 1):A110–6.doi:10.1016/j.vaccine.2014.04.079. PMID: 25091663.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–43. doi:10.1016/s0140-6736(13)62630-6. PMID: 24629994.
- John J, Kawade A, Rongsen-Chandola T, Bavdekar A, Bhandari N, Taneja S, Antony K, Bhatnagar V, Gupta A, Kabra M, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine. 2014;32(Suppl 1):A104–9. doi:10.1016/j.vaccine.2014.03.036. PMID: 25091662.
- Bajaj J, Puliyel JM. Intussusception risk with 116E rotavirus vaccine in Vellore, South India. PLoS One. 2016;34:403. doi:10.1371/journal.pone.0187446. PMID: 25800733.
- Reddy S, Nair NP, Giri S, Mohan VR, Tate JE, Parashar UD, Gupte MD, Arora R, Kang G. Safety monitoring of ROTAVAC vaccine and etiological investigation of intussusception in India: study protocol. BMC Public Health. 2018;18:898. 10.1186/s12889-018-5809-7. PMID: 30029630.
- Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen-Chandola T, Appaiahgari MB, Mishra A, Singh S, Vrati S. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–29. doi:10.1086/600104. PMID: 19545211.
- Sultana M, Mahumud RA, Van Der Meer R, Morton A, Chandola TR, Taneja S, Goyal N, Antony K, Bhatia K, More D, et al. ROTAVAC((R)) does not interfere with the immune response to childhood vaccines in Indian infants: A randomized placebo controlled trial. Hum Vaccin Immunother. 2017;3:e00302. doi:10.1016/j.heliyon.2017.e00302. PMID: 28560356.
- Ella R, Bobba R, Muralidhar S, Babji S, Vadrevu KM, Bhan MK. A Phase 4, multicentre, randomized, single-blind clinical trial to evaluate the immunogenicity of the live, attenuated, oral rotavirus vaccine (116E), ROTAVAC(R), administered simultaneously with or without the buffering agent in healthy infants in India. BMC Public Health. 2018;14:1791–99. doi:10.1080/21645515.2018.1450709. PMID: 29543547.
- Clements-Mann ML, Dudas R, Hoshino Y, Nehring P, Sperber E, Wagner M, Stephens I, Karron R, Deforest A, Kapikian AZ. Safety and immunogenicity of live attenuated quadrivalent human-bovine (UK) reassortant rotavirus vaccine administered with childhood vaccines to infants. Vaccine. 2001;19:4676–84. doi:10.1016/s0264-410x(01)00242-0. PMID: 11535316.
- Vesikari T, Karvonen AV, Majuri J, Zeng SQ, Pang XL, Kohberger R, Forrest BD, Hoshino Y, Chanock RM, Kapikian AZ. Safety, efficacy, and immunogenicity of 2 doses of bovine-human (UK) and rhesus-rhesus-human rotavirus reassortant tetravalent vaccines in Finnish children. J Infect Dis. 2006;194:370–76. doi:10.1086/505151. PMID: 16826486.
- Naik SP, Zade JK, Sabale RN, Pisal SS, Menon R, Bankar SG, Gairola S, Dhere RM. Stability of heat stable, live attenuated Rotavirus vaccine (ROTASIIL(R)). Vaccine. 2017;35:2962–69. doi:10.1016/j.vaccine.2017.04.025. PMID: 28434688.
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine. 2014;32(Suppl 1):A124–8. doi:10.1016/j.vaccine.2014.03.003. PMID: 25091665.
- Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo A, et al. Efficacy of a low-cost, heat-stable oral Rotavirus vaccine in Niger. N Engl J Med. 2017;376:1121–30. doi:10.1056/NEJMoa1609462. PMID: 28328346.
- Coldiron ME, Guindo O, Makarimi R, Soumana I, Matar Seck A, Garba S, Macher E, Isanaka S, Grais RF. Safety of a heat-stable rotavirus vaccine among children in Niger: data from a phase 3, randomized, double-blind, placebo-controlled trial. Vaccine. 2018;36:3674–80. doi:10.1016/j.vaccine.2018.05.023. PMID: 29752026.
- Kulkarni PS, Desai S, Tewari T, Kawade A, Goyal N, Garg BS, Kumar D, Kanungo S, Kamat V, Kang G, et al. A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35:6228–37. doi:10.1016/j.vaccine.2017.09.014. PMID: 29752026.
- Fu C, Wang M, Liang J, He T, Wang D, Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case-control study. Vaccine. 2007;25:8756–61. doi:10.1016/j.vaccine.2007.10.036. PMID: 18023510.
- Zhen SS, Li Y, Wang SM, Zhang XJ, Hao ZY, Chen Y, Wang D, Zhang YH, Zhang ZY, Ma JC, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg Microbes Infect. 2015;4:e64. doi:10.1038/emi.2015.64. PMID: 18023510.
- Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31:154–58. doi:10.1016/j.vaccine.2012.10.078. 23127516.
- He Q, Wang M, Xu J, Zhang C, Wang H, Zhu W, Fu C, Doherty TM. Rotavirus vaccination coverage among children aged 2-59 months: a report from Guangzhou, China. PLoS One. 2013;8(6):e68169. doi:10.1371/journal.pone.0068169. PMID: 23840828.
- Luan le T, Trang NV, Phuong NM, Nguyen HT, Ngo HT, Nguyen HT, Tran HB, Dang HN, Dang AD, Gentsch JR, et al. Development and characterization of candidate rotavirus vaccine strains derived from children with diarrhoea in Vietnam. Vaccine. 2009;27(Suppl 5):F130–8. doi:10.1016/j.vaccine.2009.08.086. PMID: 19931712.
- Dang DA, Nguyen VT, Vu DT, Nguyen TH, Nguyen DM, Yuhuan W, Baoming J, Nguyen DH, Le TL. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine. 2012;30(Suppl 1):A114–21. doi:10.1016/j.vaccine.2011.07.118. PMID: 22520120.
- Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–84. doi:10.1128/JCM.30.7.1678-1684.1992. PMID: 1321167.
- Barnes GL, Lund JS, Mitchell SV, De Bruyn L, Piggford L, Smith AL, Furmedge J, Masendycz PJ, Bugg HC, Bogdanovic-Sakran N, et al. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine. 2002;20:2950–56. doi:10.1016/s0264-410x(02)00235-9. PMID: 12126907.
- Danchin M, Kirkwood CD, Lee KJ, Bishop RF, Watts E, Justice FA, Clifford V, Cowley D, Buttery JP, Bines JE. Phase I trial of RV3-BB rotavirus vaccine: a human neonatal rotavirus vaccine. Vaccine. 2013;31:2610–16. doi:10.1016/j.vaccine.2013.04.008. PMID: 23597719.
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen MY, Barnes GL, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15:1389–97. doi:10.1016/s1473-3099(15)00227-3. PMID: 26318715.
- Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, Nirwati H, Ackland J, Standish J, Justice F, et al. Human neonatal Rotavirus vaccine (RV3-BB) to target Rotavirus from birth. N Engl J Med. 2018;378:719–30. doi:10.1056/NEJMoa1706804. PMID: 29466164.
- Barnes GL, Lund JS, Adams L, Mora A, Mitchell SV, Caples A, Bishop RF. Phase 1 trial of a candidate rotavirus vaccine (RV3) derived from a human neonate. J Paediatr Child Health. 1997;33:300–04. doi:10.1111/j.1440-1754.1997.tb01604.x. PMID: 9323616.
- Chen MY, Kirkwood CD, Bines J, Cowley D, Pavlic D, Lee KJ, Orsini F, Watts E, Barnes G, Danchin M. Rotavirus specific maternal antibodies and immune response to RV3-BB neonatal rotavirus vaccine in New Zealand. Hum Vaccin Immunother. 2017;13:1126–35. doi:10.1080/21645515.2016.1274474. PMID: 28059609.
- Cowley D, Nirwati H, Donato CM, Bogdanovic-Sakran N, Boniface K, Kirkwood CD, Bines JE. Molecular characterisation of rotavirus strains detected during a clinical trial of the human neonatal rotavirus vaccine (RV3-BB) in Indonesia. Vaccine. 2018;36:5872–78. doi:10.1016/j.vaccine.2018.08.027. PMID: 30145099.
- Barnes GL, Bines JE. Single-dose rotavirus vaccine at birth: is it effective or safe? - Authors’ reply. Lancet Infect Dis. 2018;18:948–49. doi:10.1016/s1473-3099(18)30487-0. PMID: 30152361.
- Istrate C, Hinkula J, Charpilienne A, Poncet D, Cohen J, Svensson L, Johansen K. Parenteral administration of RF 8-2/6/7 rotavirus-like particles in a one-dose regimen induce protective immunity in mice. Vaccine. 2008;26:4594–601. doi:10.1016/j.vaccine.2008.05.089. PMID: 18588935.
- Marelli B, Perez AR, Banchio C, de Mendoza D, Magni C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8 subunit induces specific immune response in mice. J Virol Methods. 2011;175:28–37. doi:10.1016/j.jviromet.2011.04.011. PMID: 21530589.
- Wen X, Cao D, Jones RW, Li J, Szu S, Hoshino Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine. 2012;30:6121–26. doi:10.1016/j.vaccine.2012.07.078. PMID: 22885016.
- Wen X, Wen K, Cao D, Li G, Jones RW, Li J, Szu S, Hoshino Y, Yuan L. Inclusion of a universal tetanus toxoid CD4(+) T cell epitope P2 significantly enhanced the immunogenicity of recombinant rotavirus DeltaVP8* subunit parenteral vaccines. Vaccine. 2014;32:4420–27. doi:10.1016/j.vaccine.2014.06.060. PMID: 24962749.
- Fix AD, Harro C, McNeal M, Dally L, Flores J, Robertson G, Boslego JW, Cryz S. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine. 2015;33:3766–72. doi:10.1016/j.vaccine.2015.05.024. PMID: 26065919.
- Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17:843–53. doi:10.1016/s1473-3099(17)30242-6. PMID: 28483414.
- Lakatos K, McAdams D, White JA, Chen D. Formulation and preclinical studies with a trivalent rotavirus P2-VP8 subunit vaccine. Hum Vaccin Immunother. 2020;1–12. 10.1080/21645515.2019.1710412. PMID: 31995444.
- Lappalainen S, Pastor AR, Malm M, López-Guerrero V, Esquivel-Guadarrama F, Palomares LA, Vesikari T, Blazevic V. Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine. Arch Virol. 2015;160:2075–78. doi:10.1007/s00705-015-2461-8. PMID: 26016444.
- Jiang B, Wang Y, Saluzzo JF, Bargeron K, Frachette MJ, Glass RI. Immunogenicity of a thermally inactivated rotavirus vaccine in Mice. Hum Vaccin. 2008;4:143–47. doi:10.4161/hv.4.2.5263. PMID: 18382129.
- Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine. 2010;28:5432–36. doi:10.1016/j.vaccine.2010.06.006. PMID: 20558244.
- Zhang B, Yi S, Ma Y, Zhang G, Zhang Y, Xie T, Li H, Sun M. Immunogenicity of a scalable inactivated rotavirus vaccine in mice. Hum Vaccin. 2011;7:248–57. doi:10.4161/hv.7.2.14121. PMID: 21307650.
- Jiang B, Wang Y, Glass RI. Does a monovalent inactivated human rotavirus vaccine induce heterotypic immunity? Evidence from animal studies. Hum Vaccin Immunother. 2013;9:1634–37. doi:10.4161/hv.24958. PMID: 23744507.
- Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–402. doi:10.1016/j.vaccine.2012.11.027. PMID: 23174199.
- Hashim AS, Aboshanab KM, El-Sayed AF. Developing an inactivated Rotavirus vaccine and evaluating the immunogenicity against a commercially available attenuated Rotavirus vaccine using a Mice animal model. Viral Immunol. 2016;29:565–71. doi:10.1089/vim.2016.0073. PMID: 27860553.
- Resch TK, Wang Y, Moon SS, Joyce J, Li S, Prausnitz M, Jiang B. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. PLoS One. 2018;8:561. doi:10.1038/s41598-017-18973-9. PMID: 29330512.
- Shahrudin S, Chen C, David SC, Singleton EV, Davies J, Kirkwood CD, Hirst TR, Beard M, Alsharifi M. Gamma-irradiated rotavirus: A possible whole virus inactivated vaccine. PLoS One. 2018;13:e0198182. 10.1371/journal.pone.0198182. PMID: 29879130.
- Wu JY, Zhou Y, Zhang GM, Mu GF, Yi S, Yin N, Xie YP, Lin XC, Li HJ, Sun MS. Isolation and characterization of a new candidate human inactivated rotavirus vaccine strain from hospitalized children in Yunnan, China: 2010-2013. World J Clin Cases. 2018;6:426–40. doi:10.12998/wjcc.v6.i11.426. PMID: 30294607.
- Herrmann JE, Chen SC, Fynan EF, Santoro JC, Greenberg HB, Robinson HL. DNA vaccines against rotavirus infections. Arch Virol Suppl. 1996;12:207–15. doi:10.1007/978-3-7091-6553-9_22. PMID: 9015117.
- Herrmann JE, Chen SC, Fynan EF, Santoro JC, Greenberg HB, Wang S, Robinson HL. Protection against rotavirus infections by DNA vaccination. J Infect Dis. 1996;174(Suppl 1):S93–7. doi:10.1093/infdis/174.supplement_1.s93. PMID: 8752297.
- Chen SC, Fynan EF, Robinson HL, Lu S, Greenberg HB, Santoro JC, Herrmann JE. Protective immunity induced by rotavirus DNA vaccines. Vaccine. 1997;15:899–902. doi:10.1016/s0264-410x(96)00272-1. PMID: 9234543.
- Choi AH, McNeal MM, Basu M, Flint JA, Stone SC, Clements JD, Bean JA, Poe SA, VanCott JL, Ward RL. Intranasal or oral immunization of inbred and outbred mice with murine or human rotavirus VP6 proteins protects against viral shedding after challenge with murine rotaviruses. Vaccine. 2002;20:3310–21. doi:10.1016/s0264-410x(02)00315-8. PMID: 12213401.
- Choi AH, Smiley K, Basu M. Induction of immune responses and partial protection in mice after skin immunization with rotavirus VP6 protein and the adjuvant LT(R192G). Vaccine. 2005;23:2290–93. doi:10.1016/j.vaccine.2005.01.028. PMID: 15755613.
- Zhou B, Zhang Y, Wang X, Dong J, Wang B, Han C, Yu J, Li D. Oral administration of plant-based rotavirus VP6 induces antigen-specific IgAs, IgGs and passive protection in mice. Vaccine. 2010;28:6021–27. doi:10.1016/j.vaccine.2010.06.094. PMID: 20637305.
- Lappalainen S, Tamminen K, Vesikari T, Blazevic V. Comparative immunogenicity in mice of rotavirus VP6 tubular structures and virus-like particles. Hum Vaccin Immunother. 2013;9:1991–2001. doi:10.4161/hv.25249. PMID: 23777748.
- Li T, Lin H, Zhang Y, Li M, Wang D, Che Y, Zhu Y, Li S, Zhang J, Ge S, et al. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine. 2014;32:1921–31. doi:10.1016/j.vaccine.2014.01.093. PMID: 24530406.
- Pastor AR, Rodriguez-Limas WA, Contreras MA, Esquivel E, Esquivel-Guadarrama F, Ramirez OT, Palomares LA. The assembly conformation of rotavirus VP6 determines its protective efficacy against rotavirus challenge in Mice. Vaccine. 2014;32:2874–77. doi:10.1016/j.vaccine.2014.02.018. PMID: 24583002.
- Afchangi A, Jalilvand S, Mohajel N, Marashi SM, Shoja Z. Rotavirus VP6 as a potential vaccine candidate. Rev Med Virol. 2019;29:e2027. doi:10.1002/rmv.2027. PMID: 30614135.
- Estes MK, Crawford SE, Penaranda ME, Petrie BL, Burns JW, Chan WK, Ericson B, Smith GE, Summers MD. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987;61:1488–94. doi:10.1128/JVI.61.5.1488-1494.1987. PMID: 3033276.
- Changotra H, Vij A. Rotavirus virus-like particles (RV-VLPs) vaccines: an update. Rev Med Virol. 2017;27:e1954. doi:10.1002/rmv.1954. PMID: 29048711.
- Luna EJ, Frazatti-Gallina NM, Timenetsky MC, Cardoso MR, Veras MA, Miraglia JL, Escobar AM, Grisi SJ, Raw I, Precioso AR. A phase I clinical trial of a new 5-valent rotavirus vaccine. Vaccine. 2013;31:1100–05. doi:10.1016/j.vaccine.2012.12.020. PMID: 23261048.
- Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, Bhattacharya MK, Ghosh R, Kumar R, Sur D, et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1-G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in healthy Indian adults and infants. Vaccine. 2014;32(Suppl 1):A117–23. doi:10.1016/j.vaccine.2014.03.069. PMID: 25091664.
- Chinese Clinical Trial Registry. A phase 1 study for the Hexavalent BRV vaccine. (2018) [ Accessed 2019 Aug 27]. http://www.chictr.org.cn/showproj.aspx?proj=14914
- Huang T, Li Y, Zhou X, Guo T, Liu T, Li Z. The safety and immunogenicity of dometic trivalent rotavirus vaccine. Applied Prev Med. 2014;20:349–54.195.
- Anil K, Desai S, Bhamare C, Dharmadhikari A, Madhusudhan RL, Patel J, Kulkarni PS. Safety and tolerability of a liquid bovine rotavirus pentavalent vaccine (LBRV-PV) in adults. Vaccine. 2018;36:1542–44. doi:10.1016/j.vaccine.2018.02.024. PMID: 29439867.
- Vesikari T. Trials of oral bovine and rhesus rotavirus vaccines in Finland: a historical account and present status. Arch Virol Suppl. 1996;12:177–86. doi:10.1007/978-3-7091-6553-9_19. PMID: 9015114.
- Debellut F, Clark A, Pecenka C, Tate J, Baral R, Sanderson C, Parashar U, Kallen L, Atherly D. Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. Lancet Glob Health. 2019;7:e1664–e74. doi:10.1016/s2214-109x(19)30439-5. PMID: 31708147.
- Rozenbaum MH, Mangen M, Fau - Giaquinto C, Giaquinto C, Fau - Wilschut JC, Wilschut J, Fau - Hak E, Hak E, Fau - Postma MJ, Postma MJ. Cost-effectiveness of rotavirus vaccination in the Netherlands; the results of a consensus model. BMC Public Health. 2011;11(1). doi:10.1186/1471-2458-11-462. PMID: 21663620.
- Shakerian S, Moradi Lakeh M, Esteghamati A, Zahraei M, Yaghoubi M, Esteghamati A. Cost-effectiveness of Rotavirus vaccination for under-five Children in Iran. Iranian Journal of Pediatrics. 2015;25(4). doi:10.5812/ijp.2766. PMID: 26396704.
- Smith ER, Rowlinson E, Fau - Iniguez V, Iniguez V, Fau - Etienne KA, Etienne KF, Rivera R, Rivera RF, Mamani N, Mamani NF, et al. Cost-effectiveness of rotavirus vaccination in Bolivia from the state perspective. Vaccine. 2011;29(38):6704–11. doi:10.1016/j.vaccine.2011.05.038. PMID: 21624421.
- From the centers for disease control and prevention. Intussusception among recipients of rotavirus vaccine–United States. 1998-1999; Jama. 1999; 282:520–21. PMID: 10450702.
- Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, Cortese M, Braun MM, Belongia EA, Miller E, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States. Pediatrics.February 1, 2006, to September 2520072008;121:1206–12. 10.1542/peds.2007-3793. PMID: 18519491.
- Belongia EA, Irving SA, Shui IM, Kulldorff M, Lewis E, Yin R, Lieu TA, Weintraub E, Yih WK, Li R, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29:1–5. doi:10.1097/INF.0b013e3181af8605. PMID: 19907356.
- Meeting of the Global advisory committee on vaccine safety. Wkly Epidemiol Rec. 2011; December 2010; 86:38–43. PMID: 21299033.
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the national immunization program in Australia. Vaccine. 2011;29:3061–66. doi:10.1016/j.vaccine.2011.01.088. PMID: 21316503.
- Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, Mujuru H, Mpabalwani E, Ngwira B, Cortese MM, et al. Evaluation of intussusception after monovalent Rotavirus vaccination in Africa. N Engl J Med. 2018;378:1521–28. doi:10.1056/NEJMoa1713909. PMID: 29669224.
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi:10.1002/rmv.448. PMID: 15484186.
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4:1303–16. doi:10.2217/fmb.09.96. PMID: 19995190.
- Tiku VR, Sharma S, Verma A, Kumar P, Raghavendhar S, Aneja S, Paul VK, Bhan MK, Ray P. Rotavirus diversity among diarrheal children in Delhi, India during 2007-2012. Vaccine. 2014;32(Suppl 1):A62–7. doi:10.1016/j.vaccine.2014.03.005. PMID: 25091683.
- Hungerford D, Allen DJ, Nawaz S, Collins S, Ladhani S, Vivancos R, Iturriza-Gomara M. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England. Euro Surveill. September 2006 to August 2016; 2019; 24. 10.2807/1560-7917.Es.2019.24.6.1700774. PMID: 30755297.
- Jain S, Vashistt J, Changotra H. Rotaviruses: is their surveillance needed? Vaccine. 2014;32:3367–78. doi:10.1016/j.vaccine.2014.04.037. PMID: 24793942.
- Kollaritsch H, Kundi M, Giaquinto C, Paulke-Korinek M. Rotavirus vaccines: a story of success. Clin Microbiol Infect. 2015;21:735–43. doi:10.1016/j.cmi.2015.01.027. PMID: 25680314.
- Biryahwaho B. Trial of an attenuated bovine rotavirus vaccine (RIT 4237). Lancet. 1987;2:344. doi:10.1016/s0140-6736(87)90943-3. PMID: 2886814.
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics. 2008;122:e1062–6. doi:10.1542/peds.2008-1059. PMID: 18977955.
- Ciarlet M, He S, Lai S, Petrecz M, Yuan G, Liu GF, Mikviman E, Heaton PM, Panzer F, Rose T, et al. Concomitant use of the 3-dose oral pentavalent rotavirus vaccine with a 3-dose primary vaccination course of a diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type b vaccine: immunogenicity and reactogenicity. Pediatr Infect Dis J. 2009;28:177–81. doi:10.1097/INF.0b013e31818c0161. PMID: 19209092.
- Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, Luby S, Breiman RF, Neuzil K, Datta SK, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–39. doi:10.1016/j.vaccine.2008.12.059. PMID: 19162114.
- Steele AD, De Vos B, Tumbo J, Reynders J, Scholtz F, Bos P, de Beer MC, Van der Merwe CF, Delem A. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28:6542–48. doi:10.1016/j.vaccine.2008.08.034. PMID: 18786585.
- Lim FS, Phua KB, Lee BW, Quak SH, Teoh YL, Ramakrishnan G, Han HH, Van Der Meeren O, Jacquets JM, Bock HL. Safety and reactogenicity of DTPa-HBV-IPV/Hib and DTPa-IPV/I-Hib vaccines in a post-marketing surveillance setting. Southeast Asian J Trop Med Public Health. 2011;42:138–47. PMID: 21323176.
- Li RC, Huang T, Li Y, Wang LH, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum Vaccin Immunother. 2016;12:785–93. doi:10.1080/21645515.2015.1085143. PMID: 27149266.
- Zaman K, Fleming JA, Victor JC, Yunus M, Bari TI, Azim T, Rahman M, Mowla SM, Bellini WJ, McNeal M, et al. Noninterference of Rotavirus vaccine with Measles-Rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J Infect Dis. 2016;213:1686–93. doi:10.1093/infdis/jiw024. PMID: 26823338.
- Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, Anand A, Zaman K. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent Rotavirus vaccine immunogenicity in rural Bangladesh. Clin Infect Dis. 2016;62:150–56. doi:10.1093/cid/civ807. PMID: 26349548.
- Van, der Wielen M, Van Damme P. Pentavalent human-bovine (WC3) reassortant rotavirus vaccine in special populations: a review of data from the Rotavirus efficacy and safety trial. Eur J Clin Microbiol Infect Dis. 2008;27:495–501. doi:10.1007/s10096-008-0479-5. PMID: 18351405.
- Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM, Kuter BJ. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J. 2008;27:656–58. doi:10.1097/INF.0b013e318168d29e. PMID: 18520448.
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl 1):A134–9. doi:10.1016/j.vaccine.2014.04.078. PMID: 25091668.
- Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, Jiang B, McNeal MM, Steele D, Bhutta Z, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine–a randomized trial. PLoS One. 2015;10:e0127622. doi:10.1371/journal.pone.0127622. PMID: 26035743.
- Krawczyk A, Lewis MG, Venkatesh BT, Nair SN. Effect of exclusive breastfeeding on Rotavirus infection among Children. Indian J Pediatr. 2016;83:220–25. doi:10.1007/s12098-015-1854-8. PMID: 26307755.
- Shen J, Zhang BM, Zhu SG, Chen JJ. No direct correlation between rotavirus diarrhea and breast feeding: a meta-analysis. Pediatr Neonatol. 2018;59:129–35. doi:10.1016/j.pedneo.2017.06.002. PMID: 28958831.
- Steele AD, Madhi SA, Louw CE, Bos P, Tumbo JM, Werner CM, Bicer C, De Vos B, Delem A, Han HH. Safety, reactogenicity, and immunogenicity of human Rotavirus vaccine RIX4414 in human immunodeficiency virus-positive infants in South Africa. Pediatr Infect Dis J. 2011;30:125–30. doi:10.1097/INF.0b013e3181f42db9. PMID: 20842070.
- Laserson KF, Nyakundi D, Feikin DR, Nyambane G, Cook E, Oyieko J, Ojwando J, Rivers SB, Ciarlet M, Neuzil KM, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq((R)), in Kenya, including among HIV-infected and HIV-exposed infants. Vaccine. 2012;30(Suppl 1):A61–70. doi:10.1016/j.vaccine.2011.09.026. PMID: 22520138.
- Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, Diaz Z, Parashar UD, Kirkwood CD. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Hum Vaccin Immunother. 2019;15:1215–27. doi:10.1080/21645515.2018.1553593. PMID: 30735087.
- Phua KB, Quak SH, Lee BW, Emmanuel SC, Goh P, Han HH, De Vos B, Bock HL. Evaluation of RIX4414, a live, attenuated rotavirus vaccine, in a randomized, double-blind, placebo-controlled phase 2 trial involving 2464 Singaporean infants. J Infect Dis. 2005;192(Suppl 1):S6–s16. doi:10.1086/431511. PMID: 16088807.
- Phua KB, Lim FS, Quak SH, Lee BW, Teoh YL, Suryakiran PV, Han HH, Bock HL. Efficacy, immunogenicity and safety of a human Rotavirus vaccine RIX4414 in Singaporean infants. Ann Acad Med Singapore. 2016;45:44–50. PMID: 27125345.
- Lau YL, Nelson EA, Poon KH, Chan PK, Chiu S, Sung R, Leung CW, Ng D, Ma YM, Chan D, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine. 2013;31:2253–59. doi:10.1016/j.vaccine.2013.03.001. PMID: 23499605.
