ABSTRACT
SARS-CoV-2, which causes coronavirus disease 2019 (COVID-19), is suspected to have been first contracted via animal-human interactions; it has further spread across the world by efficient human-to-human transmission. Recent reports of COVID-19 in companion animals (dogs and cats) and wild carnivores such as tigers have created a dilemma regarding its zoonotic transmission. Although in silico docking studies, sequence-based computational studies, and experimental studies have shown the possibility of SARS-CoV-2 infection and transmission in cats, ferrets, and other domestic/wild animals, the results are not conclusive of infection under natural conditions. Identifying the potential host range of SARS-CoV-2 will not only help prevent the possibility of human-to-animal and animal-to-human transmission but also assist in identifying efficient animal models that can mimic the clinical symptoms, transmission potential, and pathogenesis of the disease. Such an efficient animal model will accelerate the process of development and evaluation of vaccines, immunotherapeutics, and other remedies for SARS-CoV-2.
Introduction
SARS-CoV-2 is the third zoonotic coronavirus (CoV) after SARS-CoV and MERS-CoV that has caused an epidemic outbreak in the past two decades. Preliminary evidence suggests that SARS-CoV-2 emerged from Wuhan, China, via zoonotic (animal-to-human) transmission. Genome analysis has identified the bat as the most probable reservoir host of SARS-CoV-2 infection.Citation1 All three zoonotic CoVs, i.e., SARS-CoV, MERS-CoV, and SARS-CoV-2, are reported to originate from bats and were transmitted to humans through an intermediate animal host.Citation2 A report dated February 7, 2020, indicated the pangolin as the prime intermediate host.Citation3 SARS-CoV-2 exhibits higher transmissibility and lower pathogenicity than its sibling virus, SARS-CoV.Citation2 Nonetheless, the coronavirus disease named COVID-19 is assumed to have originated from an animal host (zoonotic) and spread by human-to-human transmission; however, other possibilities such as food-borne transmission cannot be ruled out.Citation4 Live-animal markets in China represent ideal conditions that facilitate inter-species interactions between wild and domestic animals, thereby increasing the probability of inter-species transmission of CoVs. These CoVs later undergo adaptive genetic recombination and finally infect humans by jumping the species barrier.Citation4 Sun et al. (2020)Citation5 assessed the natural and social factors that may have predisposed humans to the severe acute respiratory syndrome (SARS) epidemiology. Their evaluation points toward seasonal (cold dry winters) and geographical restrictions leading to a nutritive taste for wild animals. Another report, while pinpointing the putative origin of SARS-CoV-2, based on genomic and codon usage analysis tracking of a number of animal species sequences, put forward a hypothesis of the high resemblance of SARS-CoV-2 with bat CoVs and a codon usage bias toward snakes.Citation6
Recently, it was found that SARS-CoV-2 has evolved into two major types (L and S). These two subtypes differ greatly in their transmission ability and disease severity. They also exhibit differences in their geographical distribution, with the L type (∼70%) being more prevalent than the S type.Citation7 The spike glycoprotein (S protein) of this CoV is considered the major inducer of neutralizing antibodies. Most vaccines and drugs against this CoV are directed against the S protein.Citation8 Genomic analysis of 103 SARS-CoV-2 strains identified the presence of 149 mutation sites spread across the genome.Citation7 This may create further difficulties in our search for vaccines against SARS-CoV-2. Before the successful commercialization of newly developed COVID-19 vaccines as well as their use in humans, regulators need to evaluate their safety in more than one animal model.Citation9
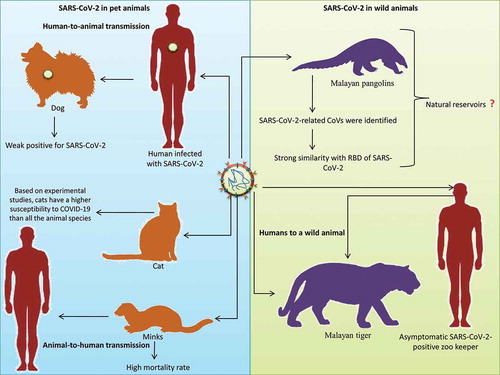
Serological investigation of domestic and wild animal populations residing in close proximity to humans can help predict and prevent the possibility of spill-overs of other SARS-CoV-2-like CoVs in the future.Citation10 In addition to analyzing the pandemic potential of SARS-CoV-2, it is equally important to gather knowledge regarding its spread in multiple animal species, including domestic and wild animals. As per reports, COVID-19 transmission to cats, tigers, and lions has been documented, with clinical signs such as vomiting, diarrhea, difficulties in breathing, dry cough, and wheezing; the exception has been dogs, which contract the disease but show none of these symptoms.Citation11–15 Additionally, since cats, ferrets, pigs, and non-human primates have the same receptor for SARS-COV-2 binding as humans,Citation16 the random mutations in the genome of the virus during its replication suggest its endemicity in these species.Citation17 The recent reports of COVID-19 in companion, as well as wild animals, have caused great concern among the scientific community and general public. Hence, the present situation calls for an in-depth analysis of the myths and facts associated with this topic. This review aims to analyze the possibility of human-to-animal as well as animal-to-human transmission based on the available evidence. We have also discussed the potential animal models available for the evaluation of vaccines and therapeutics against SARS-CoV-2 and their future prospects.
Potential for animal-to-human and human-to-animal transmission
Currently, SARS-CoV-2 is spreading at an alarming rate within the human population. Infected individuals often produce high viral loads that increase the possibility of spill-over to other animal species, including pigs. Our previous experience with SARS-CoV suggests the possibility of detecting SARS-CoV-2 RNA in pigs. Although the possibility of viral amplification in pigs is unlikely, such instances should be monitored very closely to prevent any spill-over.Citation18 Taking into consideration the possibility of SARS-CoV-2 transmission between human beings and animals, the US Centers for Disease Control and Prevention (CDC) recommended laboratory-confirmed COVID-19 cases to limit their contact with companion animals such as dogs and cats.Citation19 Despite the lack of proof indicating virus spread from COVID-19-infected animals, the information was conveyed to the public and pet-owners, which put them in a state of anxiety, resulting in pets being abandoned at many places, which negatively impacted animal welfare.Citation20 Consequently, to dispel this fear, Deng et al. (2020)Citation21 commenced a large serosurvey to screen 1914 samples, covering 35 animal species, for SARS-CoV-2-specific antibodies; none of the animal species under survey were found to be carrying virus-specific antibodies, dismissing the notion that they could be intermediate hosts for this virus. Notably, pet animals (cats, stray dogs, and three dogs that were close to a SARS-CoV-2 patient) were also found to be free from SARS-CoV-2 infection.
Owing to the uncertainty regarding the origin of SARS-CoV-2, general precautions should be taken while visiting live-animal markets, wet markets, and animal product markets. These include general hygiene measures such as regular handwashing with soap after coming into contact with animals and animal products. Special precautions should also be taken to avoid contact with diseased animals or spoiled animal products.Citation22 It is also advisable to avoid contact with stray animals (cats and dogs), rodents, birds, and bats.
Zoonotic coronaviruses in animals: lessons from SARS and MERS
The zoonotic link of SARS-CoV was revealed when a CoV that closely resembled SARS-CoV was isolated from masked palm civets (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides) in a live-animal market.Citation23 Antibodies against SARS-CoV were also detected in Chinese ferret badgers (Melogale moschata) found in the same market.Citation23 The SARS-CoV outbreak was immediately followed by speculation that it originated in domestic animals. Researchers attempted to infect domestic species of animals such as pigs and chickens to study their susceptibility to this virus. Although viral RNA was detected in the blood samples of both species using RT-PCR, none of them developed any clinical signs or gross pathological changes, ruling out the possibility that they acted as amplifying hosts for SARS-CoV.Citation24
For retrieving the genetic relatedness of animal origin and human CoVs, we analyzed the whole genome sequences of SARS-CoV-2 (n = 28) and other animal origin CoVs, which were retrieved from the NCBI GenBank portal. Strains representing different subgenus of Betacoronaviruses including SARS-CoV and MERS-CoV were included based on previous literature (). The canine and feline origin Alphacoronaviruses were also included in the analysis. The phylogenetic analyzes was performed using MEGA 7.0 and the construction of phylogenetic trees were conducted using GTR+G substitution model applying the Maximum Composite Likelihood method.
Figure 1. The phylogenetic grouping of different animals and human origin coronaviruses. The phyloanalysis included SARS-CoV-2 isolates originating from humans, tiger and mink. It also includes closely related bat and pangolin origin CoVs of Betacoronavirus. The canine and feline origin Alphacoronaviruses are included in the phylogenetic tree. Major species of each subgenus have been depicted in front of each clade
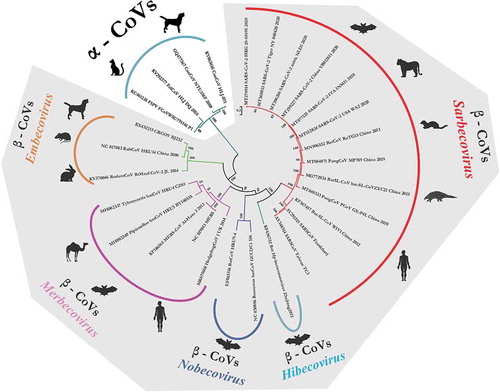
Isolates belonging to particular subgenus of Betacoronaviruses clustered in their respective clade. The canine origin, canine respiratory coronavirus (CRCoV) grouped distantly in Embecovirus subgenus along with rodent and lapine origin CoVs. Isolates belonging to SARS-CoV/SARS-CoV-2 origin clustered in Sarbecovirus subgenus clade. Canine and feline origin CoVs of genus Alphacoronavirus formed a distant clade away from all other Betacoronaviruses which showed canine and feline CoVs may share a close ancestral origin and are too diverse with respect to SARS-CoVs (). Canine and feline CoVs shared a low nucleotide similarity index with respect to SARS-CoV and SARS-CoV-2 which ranged from 44.0% to 44.5%. Whereas, SARS-CoV-2 isolated from tiger (MT065033) and mink (MT396266) shared a high nucleotide similarity percentage of 99.6% to 99.9%. Similarity index between SARS-CoV-2 and other animal origin CoV species like pangolin (MP789; MT081071), Bat-SL-CoVs (CoVZXC21; MG772934), Bat-CoV (RaTG13; MN996532) in subgenus Sarbecovirus varies between 86.6%, 88.4% and 96.3%, respectively.
Domestic cats were also found to be susceptible to SARS-CoV infection. The infected cats were found living in an apartment block occupied by individuals who had contracted SARS a year ago.Citation25,Citation26 Following this report, researchers attempted to study the susceptibility of domestic cats (Felis domesticus) and ferrets (Mustela furo) to SARS-CoV infection under experimental conditions.Citation26,Citation27 Intra-tracheal inoculations of SARS-CoV in cats and ferrets led to the shedding of the virus from the pharynx in both species. SARS-CoV-inoculated cats did not exhibit any clinical symptoms, whereas the ferrets demonstrated signs of lethargy. Researchers also found that the infected cats and ferrets had the potential to transmit SARS-CoV to susceptible animals cohabiting with them.Citation26 The pathological findings associated with experimental SARS-CoV infection in cats and ferrets closely resembled those related to humans, except for the absence of syncytia and hyaline membranes.Citation27 A study demonstrated that infected cats may remain asymptomatic but have the ability to infect other nearby cats via droplets and successfully maintain the infection chain. In this context, the CDC advised people with COVID-19 symptoms to minimize contact with their pets, including snuggling, petting, and getting licked.Citation28
Similarly, MERS-CoV that caused Middle East respiratory syndrome (MERS) in humans was transmitted from dromedary camels (Camelus dromedaries) in Saudi Arabia. MERS-CoV is another zoonotic CoV that originated from bats.Citation29 Several bat families in Asia, Africa, and Europe are identified to be the reservoir host of CoVs.Citation30 They further transmitted the CoVs to other animal species such as camels, alpaca (Vicugna pacos), and llama (Lama glama).Citation30–32 The susceptibility of livestock such as cattle, sheep, and goat to MERS-CoV vary depending on the differential expression of dipeptidyl peptidase 4 (DPP4), the receptor utilized by MERS-CoV for binding to the host cells.Citation32 Genomic analysis has identified bats as original host, camel as the reservoir host and other animals such as cattle, sheep, goat, pigs, rabbits, rhesus macaques and common marmosets acting as the susceptible or intermediate hosts. Human beings are considered as the terminal or definitive host.Citation30,Citation32–36 Transmission of CoV from animals to human has always been considered as the main concern. An increase in the host range further widens the zoonotic potential of CoVs.Citation30,Citation32 Our understanding of the zoonotic aspects of MERS-CoV will provide valuable lessons that can guide us to deal with the COVID-19 pandemic.
SARS-CoV-2 in animals
Angiotensin-converting enzyme 2 (ACE2) has been identified as the receptor of SARS-CoV-2.Citation37 The key ACE2 residues that are responsible for recognizing the spike/S protein were analyzed to identify the potential host range of SARS-CoV-2. Based on the analysis, the authors predicted that mammals such as Rhinopithecus roxellana (golden snub-nosed monkey), Macaca mulatta (rhesus macaque), Mustela erminea (stoat), Paguma larvata (masked palm civet), Rhinolophus macrotis (big-eared horseshoe bat), Rhinolophus sinicus (Chinese rufous horseshoe bat), Rousettus leschenaultii (Leschenault’s rousette), Sus scrofa (wild boar), Sus scrofa domesticus (domestic pig), Mustela putorius furo (ferret), Canis lupus familiaris (dog), Felis catus (cat), Manis javanica (pangolin), Rhinolophus pearsonii (Pearson’s horseshoe bat), Pteropus vampyrus (large flying fox), Pongo abelii (Sumatran orangutan), Equus caballus (horse), Bos taurus (cattle), Pan troglodytes (chimpanzee), Ovis aries (sheep), Papio anubis (olive baboon), Oryctolagus cuniculus (rabbit), Vulpes (red fox), Phodopus campbelli (Campbell’s hamster), Mesocricetus auratus (golden hamster), Callithrix jacchus (common marmoset), Heterocephalus glaber (naked mole-rat), Ictidomys tridecemlineatus (thirteen-lined ground squirrel), and Cricetulus griseus (Chinese hamster) possess ACE2 residues that may have the potential to bind to the S protein of SARS-CoV-2.Citation37 In another study, the amino acid sequence alignment of ACE2 was compared among species such as humans, non-human primates (gibbon, green monkey, macaque, orangutan, and chimpanzee), cats, dogs, bovines, sheep, goats, swine, horses, chickens, ferrets, civets, mice, rats, and Chinese horseshoe bats, and a high sequence similarity was found, with the exception of chickens.Citation38
Initially, snakes or turtles were suspected to be the intermediate host of SARS-CoV-2. Recent studies demonstrated that neither snakes nor turtles can be considered the intermediate hosts. Instead, they suggested the screening of animals in the Bovidae and Cricetidae families to identify the possible intermediate host of SARS-CoV-2.Citation39 This is based on the finding that ACE2 proteins from Bovidae and Cricetidae associate with the receptor-binding domain (RBD) of SARS-CoV-2.Citation39 Experimental inoculation of SARS-CoV-2 in several animal species indicated that ferrets and cats are highly susceptible to COVID-19; it was also found that dogs have comparatively less susceptibility to COVID-19.Citation40 Researchers found that animals such as chickens, pigs, and ducks are not infected by SARS-CoV-2.Citation19,Citation40
SARS-CoV-2 in dogs
On February 28, 2020, a Pomeranian dog in Hong Kong tested positive for SARS-CoV-2 without showing any signs of the disease.Citation41 The genetic sequences of SARS-CoV-2 obtained from the Pomeranian and its human contacts were very similar, indicating human-to-animal transmission.Citation42 It was the first report of the human-to-animal transmission of COVID-19. The samples obtained from the nasal and oral cavities tested “weak positive” for SARS-CoV-2 in the RT-PCR tests.Citation42 Hence, the possibility of transmitting such a weak form of the disease to pets or people, especially in the absence of any relevant clinical signs, is very low.Citation41 Similarly, SARS-CoV-2 was also detected in a German Shepherd dog in Hong Kong.Citation42 In both these cases, the dogs that tested positive were living in close contact with their COVID-19-positive owners.
Goumenou et al. (2020)Citation43 put forward the possibility of dogs acting as intermediate hosts in the transmission of SARS-CoV-2 within the human population in Italy. This hypothesis was based on the finding that an exponential increase in positive cases as well as deaths continued in Italy even after implementing strict movement restrictions. This hypothesis was further supported by facts such as the close similarity of ACE2 in humans and dogs; there is one dog for every six individuals in Italy and the presence of human-to-animal transmission indicates the possibility of animal-to-human transmission.Citation43
SARS-CoV-2 in cats
SARS-CoV-2 was detected in two cats, one each from Belgium and Hong Kong.Citation14,Citation42,Citation44 Researchers at Harbin Veterinary Research Institute recently reported that cats can be infected with SARS-CoV-2 and can spread it to other cats.Citation19 However, this finding was based on the experimental inoculation of SARS-CoV-2 and hence may not reflect natural conditions. Further, notably, the infected cats did not show any signs of illness, indicating low transmissibility in cats.Citation19 In a serological study conducted on the cat population of Wuhan city (the initial epicenter of COVID-19), 15 out of 102 cats showed seropositivity. In the same study, three cats, the owners of which were COVID-19-positive, showed the presence of high-titered SARS-CoV-2 neutralizing antibodies.Citation45 However, the high titer of these antibodies was linked to the fact that the cats were living with COVID-19-positive individuals.Citation45 Other positive cats, six in number, were either strays or from cat clinics. Hence, similar to human beings, cats can also be infected with SARS-CoV-2 and mount an immune response. Under experimental conditions, SARS-CoV-2 was also found to be transmitted among cats via respiratory droplets.Citation40 Experimental studies revealed severe signs in the nasal cavities, tracheal mucosa, and lungs in cats aged less than 3 months; however, the clinical signs of infection were not detailed. Nonetheless, a cat infected through a COVID-19-positive owner exhibited clear signs of diarrhea, vomiting, and labored and short breath. This indicates that cats may not be easily infected with SARS-CoV-2 under natural conditions. Preliminary findings based on laboratory studies suggest that cats have a higher susceptibility to COVID-19 than all the animal species investigated. This is mainly because cats can be infected with the clinical form of the disease and then transmit the infection to other cats.Citation22 Experimental evidence suggests that SARS-CoV-2 transmission to cats from human COVID-19-positive patients occurs via respiratory droplets. Notably, there is no evidence yet on zoonosis from cats to humans. Although scientific evidence on the susceptibility of cats to COVID-19 is accumulating, there is an urgent need to explore the underlying patho-immunological mechanism in detail before arriving at any conclusion. Although there is no evidence of either pet-to-human transmission of SARS-CoV-2 or the development of immunity in cats, the possibility of our pets being reservoirs of SARS-CoV-2 cannot be ignored.Citation46,Citation47
SARS-CoV-2 in minks
SARS-CoV-2 was reported in minks housed in two separate farms in the Netherlands.Citation48 The infected animals exhibited signs of respiratory distress and were associated with an increase in the mortality rate.Citation48 Following the outbreak of SARS-CoV-2 in the mink farms, authorities have initiated mass culling fearing the possibility of minks acting as a viral reservoir for this pandemic virus.Citation49 The minks are suspected to be infected from COVID-19 positive farm workers.Citation49 However, the preliminary epidemiological studies indicate that at least two farm workers were infected via animal-to-human transmission by inhalation of dust and/or droplets containing the virus.Citation48 This is the first report of animal-to-human transmission of SARS-CoV-2.Citation49
SARS-CoV-2 in wild animals
Malayan pangolins
SARS-CoV-2-related CoVs were identified in Malayan pangolins (Manis javanica) seized from southern China. The genetic sequences of the identified pangolin-associated CoVs exhibited strong similarity with regard to the RBD of SARS-CoV-2 and belonged to the sub-lineages of SARS-CoV-2-related CoVs.Citation50 These findings suggest the possibility of pangolins acting as important hosts for the emergence of novel CoVs such as SARS-CoV-2.Citation51 Genomic and evolutionary studies identified the presence of a SARS-CoV-2-like CoV, which was named Pangolin-CoV, in dead Malayan pangolins. The whole-genome analysis determined that Pangolin-CoV is more closely related to SARS-CoV-2 (91.02%) than to BatCoV RaTG13 (90.55%).Citation52 These findings suggest that pangolins may be the natural reservoirs of SARS-CoV-2-like CoVs. Genetic analysis of genomic regions other than the RBD indicates that pangolin CoVs cannot be considered the direct sources of SARS-CoV-2; an analysis of the RBD region did not confirm the possibility of pangolins acting as the intermediate hosts of SARS-CoV-2.Citation53 The hypothesis suggesting that SARS-CoV-2 emerged directly from pangolins was rejected based on two major findings. SARS-CoV-2 isolated from humans has a unique peptide (PRRA) insertion that plays a role in the proteolytic cleavage of the spike protein. This RRAR motif was absent in the coronavirus isolated from pangolins. Pangolin CoVs were also found to be less similar to SARS-CoV-2 than the BetaCoV/bat/Yunnan/RaTG13/2013 virus isolated from bats.Citation54
Malayan tiger
Recently, the National Veterinary Services Laboratories (NVSLs) of the United States Department of Agriculture (USDA) diagnosed SARS-CoV-2 in a tiger maintained in the Bronx Zoo of New York City. This tiger, along with other tigers and lions, was tested immediately after showing signs of respiratory illness. This was the first report of SARS-CoV transmission from humans to a wild animal. This Malayan tiger is suspected of having been infected by an asymptomatic SARS-CoV-2-positive zookeeper.Citation55 depicts the reports of SARS-CoV-2 infection among pet and wild animals.
Animal models of SARS-CoV and MERS-CoV infection
Following the emergence of SARS, the first zoonotic CoV, in 2002, researchers have been continuously trying to design animal models to assist in the development of effective vaccines and therapeutics.Citation56 The initial efforts were directed toward developing non-human primate models that can replicate clinical diseases.Citation56–58 Although none of the available SARS animal models have the potential to fully replicate the clinical features of the disease, the most important aspects of the disease caused by SARS-CoV can be observed in experimentally infected non-human primates.Citation57 Several non-human primates such as the African green monkey, rhesus monkey, and cynomolgus monkey have been evaluated as disease models of SARS-CoV infection.Citation58,Citation59 Although apparent clinical illness was absent in all the three species of Old World monkeys described above, they could be used for evaluating the immunogenicity of vaccine candidates.Citation59 Cynomolgus macaques that were inoculated with SARS-CoV produced clinical disease with evidence of viral replication as well as the development of neutralizing antibodies. Although infection with SARS-CoV did not produce severe illness as observed in the majority of SARS-positive adult humans, the milder syndrome exhibited by macaques replicated the SARS-CoV infection observed in young children.Citation58 New World monkeys such as the common marmoset (Callithrix jacchus) have also exhibited susceptibility to SARS-CoV infection. The intra-tracheal inoculation of SARS-CoV in these animals was found to be associated with a mild form of the illness along with evidence of viral replication.Citation60 Hence, the common marmoset model will also serve as an ideal surrogate system for the assessment of therapeutic and preventive interventions.
Considering the finding that pet cats can be infected with SARS-CoV, they were also considered a potential animal model for SARS-CoV infection.Citation25 Experimental inoculation of SARS-CoV in cats and ferrets resulted in an infection characterized by the shedding of the virus from the pharynx. The SARS-CoV-infected cats and ferrets were also found to transmit the disease to susceptible animals cohabiting with them.Citation26 The SARS-CoV ferret model was used to evaluate the immunoprophylactic potential of neutralizing human monoclonal antibodies. The results suggested that the administration of monoclonal antibodies prevented the development of SARS-CoV-induced macroscopic lung pathology and abolished viral shedding via pharyngeal secretions.Citation61
Murine models of SARS can be used to evaluate vaccines, antiviral agents, and immune responses. Combined oral and intranasal inoculation of BALB/c mice with infective doses of SARS-CoV resulted in infection characterized by viral replication in both lung and intestinal tissues.Citation62 Similarly, a human angiotensin-converting enzyme 2 (hACE2) transgenic mouse model was produced by inserting the hACE2 gene into the mouse genome. SARS-CoV was found to replicate more efficiently in the lungs of hACE2 transgenic mice and was associated with severe pathological changes that resembled SARS-CoV infection in humans.Citation63 However, post-vaccine pathology in response to hyper accentuated immune responses after SARS-CoV has been reported in murine models; hence, the assessment of post-vaccine disease irrespective of the model used is crucial.Citation64,Citation65
Intranasal inoculation of SARS-CoV in golden Syrian hamsters resulted in infection with high viral titers in the lungs and nasal turbinates. Neutralizing antibodies that protected the hamsters from further challenges were also detected post-infection.Citation66 Compared to murine models, SARS-CoV infection in hamsters produced viremia as well as extra-pulmonary spread of the virus to the spleen and liver. The hamster models were also characterized by high viral titers that replicated for a longer period in the respiratory tract, accompanied by significant pathology.Citation66 Guinea pigs inoculated with SARS-CoV developed interstitial pneumonitis alone, whereas simultaneous coinfection with reovirus and SARS-CoV produced severe lung pathologies that resulted in death between day 4 and day 7 post-inoculation.Citation67 Hence, simultaneous coinfection models in guinea pigs can replicate the pathological changes associated with SARS-CoV infection.
Two species of cotton rats (Sigmodon hispidus and Sigmodon fulviventer) were also evaluated as models for SARS-CoV. The experimental inoculation of SARS-CoV was not associated with any signs of disease. The researchers also failed to detect the virus in the blood as well as in the tissue samples, and no associated histopathological changes were observed.Citation68 These findings prove that cotton rats cannot be considered useful models for SARS-CoV infection.
Over the years, several animal models of SARS-CoV, such as monkeys, ferrets, hamsters, guinea pigs, rats, mice, cats, swine, and chickens, have been evaluated. Most of these models did not replicate the acute pneumonia-like symptoms associated with SARS-CoV infection in humans. Some of them exhibited only mild forms of the disease while clearing the virus. Among the available animal models, ferrets and non-human primates exhibit the strongest clinical symptoms.Citation69 The selection of animal models should be always based on the goals of each study. Depending on the research, some animal models may be preferred over other available models.Citation70 At present, there is no single preferred animal model for SARS-CoV in which all therapeutic or preventive strategies may be evaluated.
MERS-CoV has been reported in several animal species including camelids (dromedary camels, alpacas, and llamas), non-camelid domestic species (pigs and rabbits), non-human primates (rhesus macaques and common marmosets), and rodents (rats and mice).Citation30,Citation71,Citation72 The commonly used laboratory animals such as mice, Syrian hamsters, and ferrets are not susceptible to MERS-CoV. This is mainly because of the differences in DPP4, the receptor of MERS-CoV.Citation73 The presence of DDP4 receptors in these animal species makes them susceptible to MERS-CoV, however with variable success in establishing a satisfactory infection.Citation30,Citation74,Citation75 The mice lacked susceptibility to MERS-CoV. However, the expression of the human DPP4 in mice resolved this issue.Citation73 Some of the animals develop proper disease manifestations while others exhibit a few manifestations. Depending on the type of animal model, advantages and disadvantages vary. Larger animal species are considered to be the most appropriate models but are associated with difficulty in handling, require large biosafety labs besides being costly and having ethical issues. Although small animal models are considered less appropriate, they can be handled easily and less costly. The immunological response in small animal models is quite variable, however can be easily modified by genetic manipulations e.g. development of transgenic animals.Citation30,Citation74,Citation75 Transgenic mice are being developed as animal models for MERS-CoV infection.Citation76 Transgenic hDPP4 mice that were inoculated with MERS-CoV developed severe and lethal respiratory disease. The transgenic mice model is considered as the ideal animal model for testing the efficacy of therapeutic and prophylactic countermeasures against MERS-CoV.Citation73 These animal models could help in evaluating various aspects of COVID-19 that can help in better understanding of the epidemiology, pathogenesis, and can further be elaborated to evaluate diagnostic, prophylactic, and therapeutic modalities.
Animal models of SARS-CoV-2 infection
Animal models are considered essential tools for evaluating the efficacy of vaccines during the preclinical phase of a study. They are used to assess vaccine safety, protection against challenge infection, dose and formulation of the vaccine (efficacy of adjuvant addition), route of vaccine delivery, type of immunity, and onset, magnitude, and duration of the immune response.Citation8,Citation77 The efficacy of a vaccine in preventing an infection can be correlated with the induction of specific antibodies. However, CD4+ responses, key to B-cell help and cytokine production, are sometimes considered as the better correlates of protection than antibody titers.Citation78 Only a few animal models are currently available for the evaluation of vaccines against SARS-CoV-2 since it is a new pathogen.Citation79 Animal models that can mimic SARS-CoV-2 infection in human beings by producing similar clinical characteristics are essential for evaluating the efficacy of the immunity induced by vaccination.Citation80 Owing to their close similarity to human beings, non-human primates can efficiently simulate viral diseases that affect humans, making them an ideal choice for vaccine evaluation. However, small animal models have advantages such as comparatively low research and development costs and ease of handling; they can also be used in large numbers, thereby improving statistical correctness.Citation80
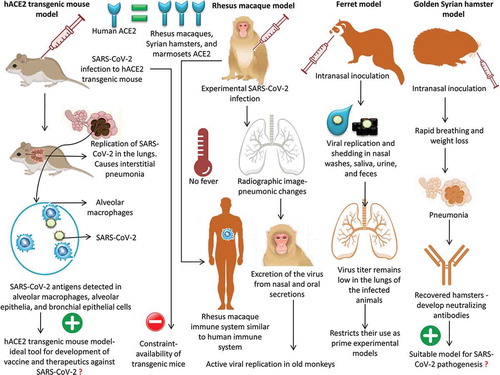
The ACE2 receptors of rhesus macaques, Syrian hamsters, and common marmosets were found to be highly similar to that of humans. Among them, the ACE2 receptor of rhesus macaques is 100% identical, whereas Syrian hamsters and common marmosets exhibit 3–4 mutations at the interface region.Citation81 Animals that develop mild forms of the infection are appropriate for evaluating vaccines and therapeutics.Citation82 Molecular docking studies have been conducted to evaluate the potential of the surface spike/S protein to bind to ACE2 of common laboratory mammals, to identify the ideal laboratory animal model for SARS-CoV-2.Citation81 The hamster ACE2 exhibited the highest binding affinity to both the SARS-CoV-2 and SARS-CoV spike proteins among all the species evaluated other than the rhesus macaque.Citation81
Human angiotensin-converting enzyme 2 (hACE2) transgenic mouse model
Recently, a hACE2 transgenic mouse model was used to study the pathogenicity of SARS-CoV-2. The infected hACE2 transgenic mice exhibited weight loss as well as viral replication in the lung tissue; histopathological findings indicated the presence of interstitial pneumonia. SARS-CoV-2 viral antigens were detected in the alveolar macrophages, alveolar epithelia, and bronchial epithelial cells.Citation83 All of these features make the hACE2 transgenic mouse model an ideal tool for the development of vaccines and therapeutics against SARS-CoV-2. Two major disadvantages were reported for this model; non-physiological expression of hACE2 had to be performed and, being a transgenic animal, availability was a major constraint.Citation81 The hACE2 transgenic mice are produced by the Jackson Laboratory breeding facility in Bar Harbor.Citation82 Following the COVID-19 outbreak, there exists a very high demand for hACE2 transgenic mice, which limits their use in research. At present, several other attempts are being made to develop mouse models that are susceptible to infection by SARS-CoV-2. Scientists are attempting to insert the human version of ACE2 into mice using a viral carrier.Citation82
Rhesus macaque model
The genetic sequences of ACE2 in humans and non-human primates are highly similar. Owing to this close similarity, non-human primate systems can recognize SARS-CoV-2 and mediate clinical infection similar to humans.Citation38 When rhesus macaques were experimentally infected with SARS-CoV-2, a fairly mild form of the disease ensued. Although none of the macaques developed fevers, radiographic findings indicated pneumonic signs in the lungs, similar to those in COVID-19-positive humans.Citation84 Since the immune systems of monkeys show close similarity to that of humans, experimental studies in primates will provide an insight into the manner in which our bodies cope with the infection.Citation82
To study the age-related pathogenic mechanism of SARS-CoV-2 in non-human primate models, rhesus macaques of 3–5 (n = 3) and 15 (n = 2) years of age were infected intra-tracheally, and the clinical signs, replication of the virus, chest images, immunological responses, and histopathological findings were analyzed. The results revealed more active viral replication in old monkeys than in young monkeys along with the development of more severe interstitial pneumonia in old monkeys.Citation85 Furthermore, Rockx et al. (2020)Citation86 showed the applicability of macaques as suitable animal models to assess the potential of newly emerging therapeutics and vaccines against COVID-19 infection. In a detailed comparative pathological investigation using SARS-CoV-2, SARS-CoV, and MERS-CoV, infection was established in cynomolgus monkeys that showed excretion of the virus from nasal and oral secretions without apparent clinical signs. Nonetheless, SARS-CoV causes more severe lung damage than MERS-CoV.
Ferret model
Ferrets are considered good models to study human respiratory viral infections owing to the close physiological similarity of the lungs.Citation80 Experimental infection of SARS-CoV-2 via intranasal inoculation in ferrets was found to be associated with viral replication and shedding in nasal washes, saliva, urine, and feces. The infected animals exhibited airborne transmission of infection to naïve indirect-contact ferrets, thereby acting as an infection and transmission animal model of COVID-19.Citation87 Notably, the infected ferrets did not show the complete clinical signs of COVID-19 infection but exhibited a rather mild form of the condition. Furthermore, the virus titer remains low in the lungs of the infected animals, which restricts their use as prime experimental models for studying COVID-19-preventive therapeutics and vaccines.
Golden Syrian hamster model
The hamster model is considered a more stable and predictable model of coronavirus infection than murine models.Citation66 Intranasal inoculation of SARS-CoV-2 in golden Syrian hamsters was found to be associated with clinical infection characterized by rapid breathing and weight loss. The virus challenge led to clinical and pathological signs of pneumonia. The recovered hamsters had developed serum neutralizing antibodies.Citation81 Furthermore, it was also demonstrated that close contact between infected and susceptible hamsters promoted the transmission of the disease. Hence, the golden Syrian hamster model of COVID-19 can be used to study the transmission characteristics and pathogenesis of SARS-CoV-2, which will, in turn, help in the development of vaccines and therapeutics against this novel pathogen. Based on the available literature on SARS-CoV-2, hamsters, ferrets, and cats can be considered as an attractive alternative animal model for SARS-CoV-2 infection as well as transgenic mice models. For studying the efficacy of various vaccine platforms, hamsters and transgenic mice models are ideal since they exhibit severe clinical symptoms of SARS-CoV-2 infection.Citation88 Due to the superior intraspecies transmission of SARS-CoV-2, cat and ferret models are ideal for studying the transmissibility and the effectiveness of antivirals to limit spread. However, the non-human primate models are the best to evaluate and assess antiviral and vaccine effectiveness before they are rapidly deployed for treating humans.Citation88
An overview of experimentation studies in animal models of SARS-CoV-2 infection is presented in
Limitations of animal models
The animal models can be used in the fundamental research involving the development of vaccines, immunotherapeutics, and drugs.Citation72,Citation89 They can help in attaining a better understanding of the disease progression, pathogenesis, transmission, and immune response. The challenge studies using suitable animal models are considered to be an important stage in the initial evaluation of human vaccines. However, the translation of results obtained from a single animal model to a disease having various outcomes in humans might not only challenging but also potentially misleading.Citation88 Despite the considerable benefits, there may be several lacunae that limit the use of such models for product development. Vaccine candidates when used in animal models can elicit neutralizing antibodies (NAbs) that provide immunity by inhibiting viral replication in non-human primates, mice, ferrets, and hamsters and hence can help in studying the immune response in such animal models.Citation72 However, sometimes this immune response is not enough as sufficient NAbs are not being generated against the vaccine candidate in some of the animal models.Citation55 Further cross-protection may not be generated against various strains of the virus.Citation72 This can be overcome by using live or inactivated vaccines which can develop sufficient immune response in mice and ferrets. However, there are chances of reversion to pathogenic viruses.Citation90
Similarly, animal models can help in elucidating novel putative drug molecules and evaluating their therapeutic potential. However, the in vivo efficacy may vary between actual species of target.Citation71 Every animal model utilized for product development has its own advantages and disadvantages. Sometimes some vaccine candidates are immunogenic only in few animal species and certain viral vectors do not replicate well or not at all replicate in some animal models.Citation72 Hence the selection of animal models should be always based on the purpose or utility. African green monkeys, rhesus macaques, cynomolgus macaques, BALB/c, C57B6 mice, 129S6/SvEv mice, ferrets, and rabbits are being used for evaluating inactivated vaccines.Citation72 They are rapid and easy for development, safety, high-titer of Nabs, and protective when used along with adjuvant. However, the limitations include the induction of inflammatory immune pathology and antibody-dependent enhancement (ADE), possibly incomplete protection.Citation72 Similarly, for vector-based vaccines, BALB/c mice, 129S6/SvEv mice, hamsters, ferrets, hDPP4-Tg or transfected mice, and dromedary camels are currently being used. Advantages include comprehensive, stronger, and specific activation of host immunity, high-titer of Nabs, and safety. For virus like particle, BALB/c mice and Ad-hDPP4 mice are being used. They help in the induction of antiviral T cell responses and S protein-specific NAbs; reduce viral titers in the lungs to nearly undetectable levels by one after inoculation with MERS-CoV. Both classes have disadvantages of varied and skewed immune responses; and possibly incomplete protection.Citation72
BALB/c mice, hACE2-Tg mice, and hamsters are employed for live-attenuated particle vaccines. They are inexpensive; develop quick immunity; less adverse effect; comprehensive activation of host immunity; multiple targets. Limitations associated are the risk of phenotypic or genotypic reversion and disseminated infection in immunocompromised patients. For subunit vaccines, rhesus macaques, BALB/c mice, hDPP4 transgenic mice, and rabbits are used. There is high safety, consistent production, comprehensive, stronger, and specific activation of host immunity, and high-titer of Nabs. Concerns are uncertain cost-effectiveness, mild immunogenicity, need appropriate adjuvants, and risk of ADE. For DNA based vaccines rhesus macaques and BALB/c mice, Ad-hDPP4 mice are helpful as they are easier to design, high safety, and have high-titer Nabs. However, there may be lower and skewed immune responses and possibly delayed-type hypersensitivity.Citation72
Similarly, for drug trials, rhesus macaques, common marmoset, mice, rabbits, ferrets, and hamsters are being used to study safety and efficacy.Citation72,Citation89,Citation91 For raising antibodies as immunotherapeutics and for vaccine development rhesus macaques and common marmoset, mice, rabbits, ferrets, and hamsters have been employed.Citation92 However, abnormal immune responses and ADE effects induced by the antibodies need to be properly evaluated.Citation92
Conclusion and future prospects
It is interesting to note the uncanny similarity that exists between SARS-CoV-2 and SARS-CoV, such as the susceptibility of cats and ferrets, transmission of infection to cage mates, and resistance of chickens and pigs to infection. The animal models that are currently being used for SARS-CoV-2 are those that were established for SARS-CoV. The production of hACE2 transgenic mice, which was stopped after the SARS outbreak ended, has been resumed to facilitate and promote research on SARS-CoV-2. Significant advances have already been made in the development of several animal models for SARS-CoV and SARS-CoV-2 infection. However, their practical utility has been significantly hampered by the lack of pathological similarity with the human diseases produced by these viruses. Although non-human primate models simulate almost similar clinical forms of the disease observed in humans, they have several disadvantages that limit their extensive use in evaluating vaccines and therapeutics against SARS-CoV-2 infection. Extended COVID-19 surveillance in animal species is needed to reach a consensus about the role of animals in the emergence and maintenance of SARS-CoV-2 in the ecosystem.Citation93 The experiences gained from research on animal CoVs will possibly assist in addressing the origin of the virus and its spread and may support upcoming research in humans by enabling progress in the development of immunogenic, effective, and harmless vaccines and therapeutics.Citation94 A multidisciplinary approach involving specialists in medicine, veterinary science, virology, and microbiology helped identify the putative intermediate hosts for previous zoonotic CoVs (SARS-CoV and MERS-CoV).Citation95 A similar One-Health approach may assist in identifying the origin of SARS-CoV-2.Citation96 Hence, there is an urgent need for such an approach to enhance our knowledge on the transmission of the disease from humans to domestic, companion, and wild animals as well as our preparedness for unanticipated emergencies in the future.
At present, the susceptibility of domestic and wild animal species to SARS-CoV-2 has major implications in the development of preventive and control strategies against this pandemic. Although significant pieces of experimental evidence show possible SARS-CoV-2 infection in cats, ferrets, or other domestic/wild animals, none of them conclusively prove infection and transmission among animals or spill-over to humans under natural conditions. To date, there are no reports of SARS-CoV-2 transmission from companion or wild animals to humans. Even if such transmission has occurred, the identification of such a case is very difficult based on the evidence, since it will be masked by the aggressive human-to-human transmission that is characteristic of this disease. The increase in the number of reports of SARS-CoV-2 infection in companion and wild animals warrants in silico docking studies as well as sequence-based computational studies to identify host susceptibility to COVID-19. Such a study will not only help evaluate the risk of animal-to-human transmission but also assist in identifying suitable animal models for the evaluation of vaccines and therapeutics against SARS-CoV-2.
Acknowledgments
The authors acknowledge and thank their respective Institutes and Universities.
Disclosure of potential conflicts of interest
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Additional information
Funding
References
- Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40(1):68–76. doi:https://doi.org/10.1080/01652176.2020.1727993.
- Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–97. doi:https://doi.org/10.7150/ijbs.45472.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–73. doi:https://doi.org/10.1038/s41586-020-2012-7.
- Dhama K, Sharun K, Tiwari R, Sircar S, Bhat S, YS M, KP S, Chaicumpa W, DK B-A, AJ R-M. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020a;33(4):e00028–20. doi:https://doi.org/10.1128/CMR.00028-20.
- Sun Z, Thilakavathy K, Kumar SS, He G, Liu SV. Potential factors influencing repeated SARS outbreaks in China. Int J Environ Res Public Health. 2020;17(5):1633. Published 2020. doi:https://doi.org/10.3390/ijerph17051633
- Ji W, Wang W, Zhao X, Zai J, Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–40. doi:https://doi.org/10.1002/jmv.25682.
- Tang X, Wu C, Li X, Song Y, Yao X, Wu X, Lu J. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev. 2020. doi:https://doi.org/10.1093/nsr/nwaa036.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020b;18:1–7. doi:https://doi.org/10.1080/21645515.2020.1735227.
- Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579(7799):321. doi:https://doi.org/10.1038/d41586-020-00751-9.
- Dhama K, Patel SK, Sharun K, Pathak M, Tiwari R, Yatoo MI, Malik YS, Sah R, Rabaan AA, Panwar PK, et al. SARS-CoV-2: jumping the species barrier, lessons from SARS and MERS, its zoonotic spillover, transmission to humans, preventive and control measures and recent developments to counter this pandemic virus. Preprints 2020c, 2020040011. doi: https://doi.org/10.20944/preprints202004.0011.v1
- The Government of the Hong Kong Special Administrative Region - Press Releases. 2020a. Pet dog tests positive for COVID-19 virus. [cited 2020 Apr 1]. Avaialble from: https://www.info.gov.hk/gia/general/202003/19/P2020031900606.htm
- The Government of the Hong Kong Special Administrative Region - Press Releases. 2020b. Pet dog further tests positive for antibodies for COVID-19 virus. [cited 2020 Apr 1]. Avaialble from: https://www.info.gov.hk/gia/general/202003/26/P2020032600756.htm
- The Government of the Hong Kong Special Administrative Region - Press Releases. 2020c. Pet cat tests positive for COVID-19. [cited 2020 Feb 4]. Avaialble from: https://www.info.gov.hk/gia/general/202003/31/P2020033100717.htm
- Chini M. Coronavirus: belgian cat infected by owner. The Brussels Times. cited April 20, 2020. Avaialble from: www.brusselstimes.com/all-news/belgium-allnews/103003/coronavirus-belgian-woman-infected-her-cat
- WCS Newsroom. A tiger at Bronx zoo tests positive for COVID 19; The tiger and the zoo’s other cats are doing well at this time [Internet]. [cited 2020 April 5; cited April 20, 2020. Avaialble from: https://newsroom.wcs.org/News Releases/articleType/ArticleView/articleId/14010/A Tiger at Bronx Zoo Tests Positive for COVID 19 The Tiger and the Zoos Other Cats Are Doing Well at This Time.aspx
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–20. doi:https://doi.org/10.1128/JVI.00127-20.
- Gollakner R, Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet Ital. 2020. doi:https://doi.org/10.12834/VetIt.2246.12523.1.
- Opriessnig T, Huang YW. Coronavirus disease 2019 (COVID-19) outbreak: could pigs be vectors for human infections? Xenotransplantation. 2020:e12591. doi:https://doi.org/10.1111/xen.12591.
- Mallapaty S. Coronavirus can infect cats - dogs, not so much. Nature. 2020. doi:https://doi.org/10.1038/d41586-020-00984-8.
- Parry MAN. COVID-19 and pets: when pandemic meets panic. Forensic Sci Int Rep. 2020;2:100090. doi:https://doi.org/10.1016/j.fsir.2020.100090.
- Deng J, Jin Y, Liu Y, Sun J, Hao L, Bai J, Huang T, Lin D, Jin Y, Tian K. Serological survey of SARS-CoV-2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound Emerg Dis. 2020;67(4):1745–49. doi:https://doi.org/10.1111/tbed.13577.
- OIE, 2020. Questions and answers on the 2019 coronavirus disease (COVID-19). cited April 11, 2020. Avaialble from: https://www.oie.int/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/
- Cyranoski D, Abbott A. Virus detectives seek source of SARS in China’s wild animals. Nature. 2003;423(6939):467–467. doi:https://doi.org/10.1038/423467a.
- Weingartl HM, Copps J, Drebot MA, Marszal P, Smith G, Gren J, Andova M, Pasick J, Kitching P, Czub M. Susceptibility of pigs and chickens to SARS coronavirus. Emerg Infect Dis. 2004;10(2):179–84. doi:https://doi.org/10.3201/eid1002.030677.
- Abbott A. Pet theory comes to the fore in fight against SARS. Nature. 2003;423(6940):576. doi:https://doi.org/10.1038/423576b.
- Martina BE, Haagmans BL, Kuiken T, Fouchier RA, Rimmelzwaan GF, Van Amerongen G, Peiris JS, Lim W, Osterhaus AD. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425(6961):915. doi:https://doi.org/10.1038/425915a.
- Van den Brand JM, Haagmans BL, Leijten L, van Riel D, Martina BE, Osterhaus AD, Kuiken T. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet Pathol. 2008;45(4):551–62. doi:https://doi.org/10.1354/vp.45-4-551.
- CDC, 2020. Coronavirus Disease 2019 (COVID-19). Accessed on April 11, 2020. Avaialble from: https://www.cdc.gov/coronavirus/2019-ncov/daily-lifecoping/animals.html
- Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819–23. doi:https://doi.org/10.3201/eid1911.131172.
- Vergara-Alert J, Vidal E, Bensaid A, Segalés J. Searching for animal models and potential target species for emerging pathogens: experience gained from middle east respiratory syndrome (MERS) coronavirus. One Health. 2017;3:34–40. doi:https://doi.org/10.1016/j.onehlt.2017.03.001.
- Adney DR, van Doremalen N, Brown VR, Bushmaker T, Scott D, de Wit E, Bowen RA, Munster VJ. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis. 2014;20(12):1999–2005. doi:https://doi.org/10.3201/eid2012.141280.
- Vergara-Alert J, van den Brand JM, Widagdo W, Muñoz M 5th, Raj S, Schipper D, Solanes D, Cordón I, Bensaid A, BL H, et al. Livestock susceptibility to infection with middle east respiratory syndrome coronavirus. Emerg Infect Dis. 2017;23(2):232–40. doi:https://doi.org/10.3201/eid2302.161239.
- De WE, AL R, Falzarano D, Bushmaker T, Feldmann F, DL B, ER F, Martellaro C, Okumura A, Chang J, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A. 2013;110(41):16598–603. doi:https://doi.org/10.1073/pnas.1310744110.
- Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368(16):1560–62. doi:https://doi.org/10.1056/NEJMc1215691.
- Yao Y, Bao L, Deng W, Xu L, Li F, Lv Q, Yu P, Chen T, Xu Y, Zhu H, et al. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis. 2014;209(2):236–42. doi:https://doi.org/10.1093/infdis/jit590.
- Falzarano D, de Wit E, Feldmann F, Rasmussen AL, Okumura A, Peng X, Thomas MJ, van Doremalen N, Haddock E, Nagy L, et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10(8):e1004250. doi:https://doi.org/10.1371/journal.ppat.1004250.
- Luan J, Lu Y, Jin X, Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020a;526(1):165–69. doi:https://doi.org/10.1016/j.bbrc.2020.03.047.
- Li R, Qiao S, Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect. 2020a;80(4):469–96. doi:https://doi.org/10.1016/j.jinf.2020.02.013.
- Luan J, Jin X, Lu Y, Zhang L. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J Med Virol. 2020b. doi:https://doi.org/10.1002/jmv.25817.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020a:eabb7015. doi:https://doi.org/10.1126/science.abb7015.
- Almendros A. Can companion animals become infected with Covid-19? Vet Rec. 2020;186(12):388–89. doi:https://doi.org/10.1136/vr.m1194.
- American Veterinary Medical Association (2020). SARS-CoV-2 in animals, including pets. cited April 11, 2020: Avaialble from: https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets
- Goumenou M, Spandidos DA, Tsatsakis A. [Editorial] Possibility of transmission through dogs being a contributing factor to the extreme Covid-19 outbreak in North Italy. Mol Med Rep. 2020. doi:https://doi.org/10.3892/mmr.2020.11037.
- Li X. Can cats become infected with Covid-19? Vet Rec. 2020;186(14):457–58. doi:https://doi.org/10.1136/vr.m1455.
- Zhang Q, Zhang H, Huang K, Yang Y, Hui X, Gao J, Jin M. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 2020a. doi:https://doi.org/10.1101/2020.04.01.021196.
- Rodriguez-Morales AJ, Dhama K, Sharun K, Tiwari R, Bonilla-Aldana DK. Susceptibility of felids to coronaviruses. Vet Rec. 2020;186(17):e21–e21. vetrecm1671. doi:https://doi.org/10.1136/vr.m1671
- Thomson GA. COVID-19: leaving lockdown - Of Schrodinger, cats, testing and masks. Int J Clin Pract. 2020:e13519. doi:https://doi.org/10.1111/ijcp.13519.
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill. 2020;25(23). doi:https://doi.org/10.2807/1560-7917.ES.2020.25.23.2001005.
- Enserink M. Coronavirus rips through Dutch mink farms, triggering culls. Science. 2020;368(6496):1169. doi:https://doi.org/10.1126/science.368.6496.1169.
- Lam TT, Shum MH, Zhu HC, Tong YG, Ni XB, Liao YS, Wei W, Cheung WY, Li WJ, Li LF, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020. doi:https://doi.org/10.1038/s41586-020-2169-0.
- Tiwari R, Dhama K, Sharun K, Iqbal Yatoo M, Malik YS, Singh R, Michalak I, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020:1–22. doi:https://doi.org/10.1080/01652176.2020.1766725.
- Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020b;S0960-9822(20):30360–62. doi:https://doi.org/10.1016/j.cub.2020.03.022.
- Han G-Z. Pangolins harbor SARS-CoV-2-related coronaviruses. Trends Microbiol. 2020;28(7):515–17. doi:https://doi.org/10.1016/j.tim.2020.04.001.
- Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020b. doi:https://doi.org/10.1002/jmv.25731.
- USDA, 2020. United States Department of Agriculture. Statement on the Confirmation of COVID-19 in a Tiger in New York. cited on April 7, 2020. Avaialble from: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/ny-zoo-covid-19
- Enserink M. Infectious diseases. SARS researchers report new animal models. Science. 2003;302(5643):213. doi:https://doi.org/10.1126/science.302.5643.213a.
- Haagmans BL, Osterhaus AD. Nonhuman primate models for SARS. PLoS Med. 2006;3(5):e194. doi:https://doi.org/10.1371/journal.pmed.0030194.
- Lawler JV, Endy TP, Hensley LE, Garrison A, Fritz EA, Lesar M, Baric RS, Kulesh DA, Norwood DA, Wasieloski LP, et al. Cynomolgus macaque as an animal model for severe acute respiratory syndrome. PLoS Med. 2006;3(5):e149. doi:https://doi.org/10.1371/journal.pmed.0030149.
- McAuliffe J, Vogel L, Roberts A, Fahle G, Fischer S, Shieh WJ, Butler E, Zaki S, St Claire M, Murphy B, et al. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology. 2004;330(1):8–15. doi:https://doi.org/10.1016/j.virol.2004.09.030.
- Greenough TC, Carville A, Coderre J, Somasundaran M, Sullivan JL, Luzuriaga K, Mansfield K. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol. 2005;167(2):455–63. doi:https://doi.org/10.1016/S0002-9440(10)62989-6.
- Ter MJ, Bakker AB, van den Brink EN, Weverling GJ, Martina BE, Haagmans BL, Kuiken T, de Kruif J, Preiser W, Spaan W, et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–41. doi:https://doi.org/10.1016/S0140-6736(04)16506-9.
- Wentworth DE, Gillim-Ross L, Espina N, Bernard KA. Mice susceptible to SARS coronavirus. Emerg Infect Dis. 2004;10(7):1293–96. doi:https://doi.org/10.3201/eid1007.031119.
- Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, Gao H, Huang L, Liu YL, Ma CM, et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–59.
- Yasui F, Kai C, Kitabatake M, Inoue S, Yoneda M, Yokochi S, Kase R, Sekiguchi S, Morita K, Hishima T, et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181(9):6337–48. doi:https://doi.org/10.4049/jimmunol.181.9.6337.
- Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–15. doi:https://doi.org/10.1128/JVI.06048-11.
- Roberts A, Vogel L, Guarner J, Hayes N, Murphy B, Zaki S, Subbarao K. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol. 2005;79(1):503–11. doi:https://doi.org/10.1128/JVI.79.1.503-511.2005.
- Liang L, He C, Lei M, Li S, Hao Y, Zhu H, Duan Q. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA Cell Biol. 2005;24(8):485–90. doi:https://doi.org/10.1089/dna.2005.24.485.
- Watts DM, Peters CJ, Newman P, Wang N, Yoshikawa N, Tseng CK, Wyde PR. Evaluation of cotton rats as a model for severe acute respiratory syndrome. Vector Borne Zoonotic Dis. 2008;8(3):339–44. doi:https://doi.org/10.1089/vbz.2007.0210.
- Gong SR, Bao LL. The battle against SARS and MERS coronaviruses: reservoirs and animal models. Animal Model Exp Med. 2018;1(2):125–33. doi:https://doi.org/10.1002/ame2.12017.
- Subbarao K, Roberts A. Is there an ideal animal model for SARS? Trends Microbiol. 2006;14(7):299–303. doi:https://doi.org/10.1016/j.tim.2006.05.007.
- Skariyachan S, Challapilli SB, Packirisamy S, Kumargowda ST, Sridhar VS. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for middle east respiratory syndrome coronavirus infections. Front Microbiol. 2019;10:569. doi:https://doi.org/10.3389/fmicb.2019.00569.
- Yuan L, Tang Q, Cheng T, Xia N. Animal models for emerging coronavirus: progress and new insights. Emerg Microbes Infect. 2020;9(1):949–61. doi:https://doi.org/10.1080/22221751.2020.1764871.
- van Doremalen N, Munster VJ. Animal models of middle east respiratory syndrome coronavirus infection. Antiviral Res. 2015;122:28–38. doi:https://doi.org/10.1016/j.antiviral.2015.07.005.
- Coleman CM, Matthews KL, Goicochea L, Frieman MB. Wild-type and innate immune-deficient mice are not susceptible to the middle east respiratory syndrome coronavirus. J Gen Virol. 2014;95(Pt 2):408–12. doi:https://doi.org/10.1099/vir.0.060640-0.
- de Wit E, Prescott J, Baseler L, Bushmaker T, Thomas T, Lackemeyer MG, Martellaro C, Milne-Price S, Haddock E, Haagmans BL, et al. The middle east respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters. PLoS One. 2013;8(7):e69127. doi:https://doi.org/10.1371/journal.pone.0069127.
- Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. Generation of a transgenic mouse model of middle east respiratory syndrome coronavirus infection and disease. J Virol. 2015;89(7):3659–70. doi:https://doi.org/10.1128/JVI.03427-14.
- Gerdts V, Wilson HL, Meurens F, Van D, Littel-van den Hurk S, Wilson D, Walker S, Wheler C, Townsend H, AA P. Large animal models for vaccine development and testing. Ilar J. 2015;56(1):53–62. doi:https://doi.org/10.1093/ilar/ilv009.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010 Jul;17(7):1055–65. doi:https://doi.org/10.1128/CVI.00131-10.
- Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel). 2020c;8(2):E153. doi:https://doi.org/10.3390/vaccines8020153.
- Shi Y, Wang N, Zou QM. [Progress and challenge of vaccine development against 2019 novel coronavirus (2019-nCoV)]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020b;54:E029. Chinese. doi:https://doi.org/10.3760/cma.j.cn112150-20200317-00366.
- Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020:ciaa325. doi:https://doi.org/10.1093/cid/ciaa325.
- Callaway E. Labs rush to study coronavirus in transgenic animals-some are in short supply. Nature. 2020;579(7798):183. doi:https://doi.org/10.1038/d41586-020-00698-x.
- Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. bioRxiv. 2020. doi:https://doi.org/10.1101/2020.02.07.939389.
- Shan C, Yao YF, Yang XL, Zhou YW, Gao G, Peng Y, Yang L, Hu X, Xiong J, Jiang RD, et al. Infection with novel coronavirus (SARS-CoV-2) Causes pneumonia in the rhesus macaques, 2020, Preprint (Version 1) available at Research Square. 2020;30:670–677. doi:https://doi.org/10.1038/s41422-020-0364-z.
- Yu P, Qi F, Xu Y, Li F, Liu P, Liu J, Bao L, Deng W, Gao H, Xiang Z, et al. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med. 2020;3(1):93–97. doi:https://doi.org/10.1002/ame2.12108.
- Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012–15. doi:https://doi.org/10.1126/science.abb7314.
- Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709.e2. doi:https://doi.org/10.1016/j.chom.2020.03.023.
- Lakdawala SS, Menachery VD. The search for a COVID-19 animal model. Science. 2020;368(6494):942–43. doi:https://doi.org/10.1126/science.abc6141.
- Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–13. doi:https://doi.org/10.1093/infdis/jiv392.
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–48. doi:https://doi.org/10.1038/nrmicro3143.
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. doi:https://doi.org/10.1038/s41467-019-13940-6.
- Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. doi:https://doi.org/10.1172/jci.insight.123158.
- Foddai A, Lindberg A, Lubroth J, Ellis-Iversen J. Surveillance to improve evidence for community control decisions during the COVID-19 pandemic - Opening the animal epidemic toolbox for public health. One Health. 2020:100130. doi:https://doi.org/10.1016/j.onehlt.2020.100130.
- Decaro N, Martella V, Saif LJ, Buonavoglia C COVID-19 from veterinary medicine and one health perspectives: what animal coronaviruses have taught us [published online ahead of print, 2020 Apr 7]. Res Vet Sci. 2020;131:21–23. doi: https://doi.org/10.1016/j.rvsc.2020.04.009
- Hemida MG, Elmoslemany A, Al-Hizab F, Alnaeem A, Almathen F, Faye B, Chu DK, Perera RA, Dromedary PM. Camels and the transmission of middle east respiratory syndrome coronavirus (MERS-CoV). Transbound Emerg Dis. 2017;64(2):344–53. doi:https://doi.org/10.1111/tbed.12401.
- Zhai SL, Wei WK, Lv DH, Xu ZH, Chen QL, Sun MF, Li F, Wang D. Where did SARS-CoV-2 come from? Vet Rec. 2020;186(8):254. doi:https://doi.org/10.1136/vr.m740.