ABSTRACT
Severe acute respiratory syndrome Coronavirus- 2 (SARS-CoV-2), the etiological agent of the novel coronavirus disease (COVID-19), has posed a great public health threat to the global community as a pandemic. The origin of the virus has been linked to animals, through a yet-to-be-identified intermediate host. The disease is transmitted to humans mainly through inhalation or contact with infected droplets. The variable clinical presentation of COVID-19 includes fever, cough, sore throat, breathlessness, fatigue and malaise; however, cutaneous, ocular, neurological, and gastrointestinal manifestations have also been reported. There is an urgent need to strengthen One Health surveillance, intervention, and management strategies to understand the ecology of coronaviruses and to prevent epidemics in the future. Global attention toward the development of treatments, immunotherapies, vaccines, and control options to combat the COVID-19 pandemic has been on an increasing trend. Here, we review the current epidemiological status, public health concerns, and mitigation strategies for COVID-19.
Introduction
Ever since 2000 A.D., at least one new event of an emerging disease has occurred from time to time, including Nipah, severe acute respiratory syndrome (SARS), Ebola, Middle East respiratory syndrome (MERS) and Zika. The ongoing episode of the coronavirus disease (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus- 2 (SARS-CoV-2) has posed a great public health threat as a pandemic. Therefore, the question of how these pathogens evolve and cause severe or mild infections arises.Citation1 A spillover of viruses from wild birds, bats, and monkeys to humans has been reported. Human activities including deforestation, illegal wildlife trade, and hunting of bush meat may be responsible for the spillover effect linked to animal-human interplay.Citation2 Besides, altered ecosystems, intensive animal rearing, and large-scale distribution of uncontrolled foods of animal origin may also contribute to such species jump.
The dread and terror brought about by the COVID-19 pandemic has repeated the history of zoonotic diseases over time.Citation3 SARS-CoV-2, emerged in 2019 at Wuhan, China, is the third highly pathogenic coronavirus (CoV) to infect humans. The enduring emergence of CoVs at regular intervals poses a significant threat to the public health and global economy. The pandemic has been suggested to have a zoonotic origin as the early case-patients reported had a history of visiting the Huanan Seafood Wholesale Market, where the wildlife merchandise were reported.Citation4 Upon its pandemicity, the World Health Organization (WHO) has declared COVID-19 as a public health emergency of international concern (PHEIC). The COVID-19 pandemic has taken the lives of more than 100,000s people out of more than few millions confirmed human cases and has affected the society, environment, and global economy, as a whole.
The symptoms of COVID-19 usually include fever, cough, sore throat, breathlessness, fatigue, and malaise. In most cases, the disease is mild; however, in the elderly and those with co-morbidities, it may progress to pneumonia, acute respiratory distress syndrome, and multi-organ dysfunction.Citation5
Paradoxically, even after a decade of research on CoVs, there are still no licensed vaccines or therapeutic agents to treat CoV infections, thus highlighting an urgent need to develop effective vaccines or post-exposure prophylaxis to prevent future epidemics.Citation6 Although potential vaccine candidates have been identified and are undergoing various phases of clinical trials, a successful outcome has not yet been established. Several similarities exist between the clinical, genetic, and epidemiological features of SARS-CoV-2 and those of SARS-CoV infections. Therefore, research advancements on SARS treatment might help the scientific community quickly understand the pathogenesis of COVID-19 and develop effective therapeutic/prophylactic agents to treat and prevent this infection. Monoclonal antibodies represent the major class of bio-therapeutics for passive immunotherapy to fight against viral infections. The therapeutic potential of monoclonal antibodies has been well recognized in the treatment of many diseases.Citation7 A number of review articles on the current status of COVID-19 epidemiology, pathogenesis, and vaccine development are available.Citation8-10 In this mini-review, we discuss the epidemiological aspects of COVID-19, the associated clinical features, public health concerns, vaccine developments underway, and immunotherapy approaches.
Epidemiology
Coronavirus (CoV) belongs to the subfamily Orthocoronavirinae of Coronaviridae family under the Order Nidovirales, whose members are named after their crown-like appearance under the electron microscope. CoVs are reported to manifest respiratory, enteric, hepatic and neurologic diseases.Citation11 Based on the genotypic and serotypic characteristics, subfamily Orthocoronavirinae has four genera namely, alpha-corona virus (α- CoV), beta-corona virus (β- CoV), gamma-corona virus (γ- CoV) and delta-corona virus (δ- CoV).Citation12 Mammals are frequently infected with α- and β- CoV, while, birds with γ- and δ- CoV. Human pathogenic coronaviruses include SARS-CoV, the Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and the presently recoded SARS-CoV-2, which are all betacoronaviruses.Citation1,Citation12,Citation13
Virology
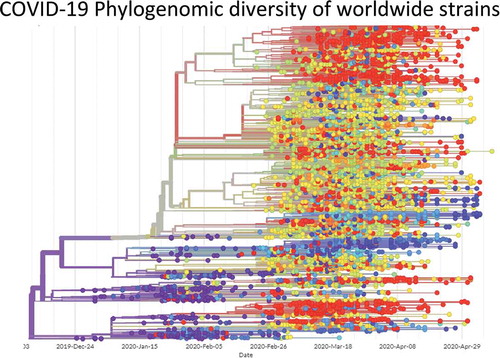
Coronaviruses (CoVs) have the largest genome among known RNA viruses. CoVs are enveloped and consists of a positive-sense, single-stranded RNA with the size varying between 26 and 32 kb. The genome of SARS-CoV-2 virion is about 29.9 kb with a nucleocapsid buried inside the phospholipid bilayer covered by two different types of spike proteins, namely, the spike glycoprotein trimmer (S) with membrane (M) protein and envelope (E) protein. The spike proteins are found in all CoVs, whereas hemagglutinin-esterase (HE) is found only in certain CoVs.Citation14 The phylogenetic analysis revealed that SARS-CoV-2 shares 79.50% and 50% sequence identity with SARS-CoV and MERS-CoV, respectively.Citation13,Citation15 Nevertheless, the sequence identity between the conserved replicase domains in ORF1ab of SARS-CoV-2 and SARS-CoV (94.60%) and between those in SARS-CoV-2 and other β-CoVs (less than 90%) clearly indicates that SARS-CoV-2 belongs to the lineage B (Sarbecovirus) of β-CoVs.Citation14 The phylogenomic diversity of COVID-19 viruses from the coronavirus pandemic is given in , while the features of COVID-19 genome are given in .
Figure 1. The genome and genomic features of the COVID-19 genome. Open reading frames are shown at the bottom, while mutations (nucleotide-diversity) as compared to the reference strain (Wuhan-Hu-1/2019) at the respective chromosomal locus are shown in the above panel. The top three most common mutations are annotated. The image is constructed from GISAID Next hCoV-19 app. The date of isolation of strains is mentioned at the bottom line. The data was accessed on 17th May 2020
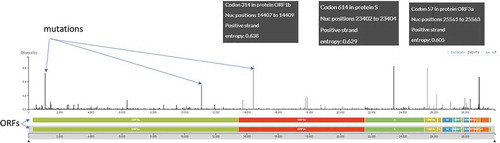
Figure 2. Phylogenomic diversity of COVID-19 viruses from the coronavirus pandemic. Each dot represents a viral strain, and different colours represent different countries. The date of isolation of strains is mentioned at the bottom line. The image is constructed from GISAID Next hCoV-19 app. The date of isolation of strains is mentioned at the bottom line. The data was accessed on 17th May 2020
Origin and hosts
The origin of SARS-CoV-2 remains obscure. SARS-CoV-2 has shown high genome sequence identities (87.6%–87.8%) to SARSr-Rp-BatCoV-ZXC21/ZC45 detected in Rhinolophus pusillus bats in 2015Citation16 and to SARSr-Ra-BatCoV-RaTG13 (96.1% genome identity with SARS-CoV-2) detected in Rhinolophus affinis bats in 2013.Citation13 Subsequently, the two viral strains exhibiting 85.3% and 89.7% genome identities to SARS-CoV-2 were detected in smuggled pangolins in 2017.Citation17,Citation18 However, none of the existing SARSr-CoVs represent its immediate ancestor, despite the close relatedness of SARS-CoV-2 to strains isolated from bat and pangolin. The SARS-CoV-2 strains are closely related. It has been suggested that the Wuhan outbreak might have originated from a point source with subsequent human-to-human transmission.Citation4
Whereas, identification of potential recombination sites around the receptor-binding domain (RBD) region suggested that SARS-CoV-2 might be a recombinant virus, with the evolution of its genome backbone from the Yunnan bat virus–like SARSr-CoVs and acquisition of its RBD region from the pangolin virus–like SARSr-CoVs.Citation4 It could also be possible that the pangolin SARSr-CoVs originated from bat viruses as a result of animal mixing.
The RBD is considered as a hot spot for the construction of recombinant CoVs for receptor and viral replication studies. Therefore, the suspicion of an artificial recombinant virus has been raised owing to the presence of evolutionarily distinct SARS-CoV-2 RBD and the unique insertion of S1/S2 cleavage site among Sarbecovirus species.Citation4 However, currently, no evidence exists to prove that SARS-CoV-2 is an artificial recombinant virus. Further surveillance studies to identify the possible source and evolutionary path of SARS-CoV-2 in bats are warranted.
COVID-19 is postulated to have emerged from animals, although its exact source is not clear.Citation13 SARS-CoV-2 has been shown to replicate poorly in dogs, pigs, chickens, and ducks; however, ferrets and cats were permissive to the infection.Citation19 Experimentally, cats were found to be susceptible to airborne infection,Citation19 providing important insights into the animal models for SARS-CoV-2. Further, Shi et al.Citation19 explained that the ferrets are widely used as animal models for investigating human respiratory viruses. Ferrets infected with SARS-CoV-2 exhibited elevated body temperatures and virus replication, and shed virus in nasal washes, saliva, urine, and feces up to 8 days post-infection.Citation20 All naive direct contact ferrets were positive for SARS-CoV-2 at 2 days post-contact and a few naive indirect contact ferrets were positive for viral RNA, suggesting its air-borne transmission. The detection of viral antigens was reported in the nasal turbinate, trachea, lungs, and intestine with acute bronchiolitis present in infected lungs.Citation20
Transmission of the virions from humans to dogs, domestic cats, tigers, and lions has also been recently reported.Citation21 Pigs, cats, ferrets, and primates have been identified as good candidates for susceptibility to SARS-CoV-2. The potential implications indicate the need for One Health surveillance, intervention, and management strategies to mitigate the effects on animal populations and prevent a second preparedness failure during this health emergency.
Interestingly, all human CoVs may be potentially zoonotic, thus making bats the most likely natural hosts for the known CoVs.Citation12,Citation22 For instance, during the SARS pandemic of 2002–2003, the preliminary observations pointed to a zoonotic origin RS-CoV, with civets as the foci of human infection.Citation23 However, the roosting sites of bats were located away from the human activity areas; hence, the virus could have been probably transmitted to humans from another animal host after mutation or recombination in animal hosts. For instance, in SARS-CoV and MERS-CoV, civets and dromedary camels served as the animal hosts, prior to the human transmission. In SARS-CoV-2, 99% identity has been identified between pangolin origin CoV and SARS-CoV-2, which could be inferred that SARS-CoV-2 might have originated from pangolins.Citation24
The screening of serum samples (n = 1914) from 35 animal species for SARS‐CoV‐2‐specific antibodies using double‐antigen sandwich ELISA could not reveal the presence of SARS‐CoV‐2‐specific antibodies in the tested samples, which excluded the possibility of 35 animal species as the intermediate host for SARS‐CoV‐2.Citation25 The samples included those from companion animals, including pet dogs and cats, street dogs, and cats, which dispelled public concerns about pets being the carriers of SARS‐CoV‐2.
Recently, respiratory disease outbreaks caused by SARS-CoV-2 with increased mortality were reported in mink farms in the Netherlands.Citation26,Citation27 An acute interstitial pneumonia with acute alveolar damage was found in the minks died at the peak of the outbreaks. The viral RNA was detected in throat swabs and by immunohistochemical detection of viral antigen in nasal conchae, trachea, and lung (Molenaar et al., 2020). The probable source of the initial infection to minks was pointed at humans based on sequence analysis of mink-derived viruses. In Denmark, three mink farms were confirmed to be positive for SARS-CoV-2. Similar mutations in the viral genome of infected minks and infected farmers were found with the possibility that the virus was transmitted to minks via infected humans.Citation28 The possible exposure of farm workers to virus excreted by minks has been postulated due to the presence of viral RNA in inhalable dust collected from the farms.Citation27
Receptor insights
The virus utilizes angiotensin-converting enzyme 2 (ACE2), a functional receptor in humans, for its entry into cells, as in the case of SARS-CoV.Citation13 ACE2 receptors, expressed in the lungs, heart, kidneys, and intestine,Citation29 bind directly to the S proteins of CoVs and undergo structural rearrangement so as to enable the fusion of viral membrane with the host cell membrane.Citation11,Citation15 Upon its entry into alveolar epithelial cells, SARS-CoV-2 replicates rapidly, which results in cytokine storm syndrome (hypercytokinemia) and damage the pulmonary tissue. The cytokine storm syndrome is a group of disorders characterized by the uncontrolled production of pro-inflammatory cytokines, which culminates in acute respiratory distress syndrome (ARDS) and multiple organ failure.Citation12
The preliminary research has shown that SARS-CoV-2 can use ACE2 from bats, civet cats, swine, cats, ferrets, non-human primates (NHPs), and humans as a receptor.Citation13,Citation30,Citation31 Infection in a pet dog in Hong Kong suggested that the canine ACE2 could also be recognized by SARS-CoV-2. Pangolins have been proposed as potential amplifying host in some studies.Citation18,Citation32
Transmission
It is currently believed that this deadly CoV strain originated from wild animals in the Huanan market of Wuhan, a city in the Hubei province, China. Bats, snakes, and pangolins have been cited as potential carriers of the virus, based on the sequence homology between the nucleic acids of CoV isolated from these animals and the viral nucleic acid isolated from SARS-CoV-2-infected patients.Citation33
The most common symptoms at the onset of illness caused by SARS-CoV-2 are given in . The major routes of transmission in humans include respiratory droplets and contact. It has been recently reported that SARS-CoV-2 could be detected in the urine and feces of laboratory-confirmed individuals; which indicates the potential of faeco-oral route as yet another possible route of virus transmission.Citation44 Nonetheless, it is uncertain whether the virus could be transmitted through the consumption of contaminated foods, aerosols, or in utero. For now, patients with COVID-19 are considered as the prime sources of infection; asymptomatic persons and patients in incubatory stage of infection have been proved to shed infectious virions and could serve as potential sources of infection.Citation45
Table 1. The most common as well as non-common symptoms at the onset of illness, SARS-CoV-2
The transmission efficiency of any respiratory virus has significant implications on its mitigation. The basic reproductive number (R0) of SARS-CoV-2, in its initial evaluation, was estimated to be 2.20 (1.40–3.90), which implies that on an average, each infected person could spread the infection to another two individuals.Citation46 However, the high viral titers observed in the oropharynx during the course of early infection aroused serious concern regarding the enhanced infectivity of the virus when the disease symptoms are minimal. The R0 of SARS-CoV and MERS-CoV in the absence of intervention was found to be 2.30–3.70; however, after the mitigation strategies were employed, the R0 dropped to less than 1.0,Citation47 explaining the basis of controlling the outbreaks. It could be worth mentioning that the R0 estimates may vary based on numerous exogenous factors (biological, socio-behavioural, and environmental) and should be cautiously interpreted.
It has been speculated that SARS-CoV-2 might have emerged as a recombinant virus between the bat coronavirus and a coronavirus of origin-unknownCitation48 which might have occurred within the viral spike glycoprotein. Additionally, it has been suggested that the genome of SARS-CoV-2 is most similar to that of bat coronavirus and its codon usage bias is most similar to that of snake. In summary, homologous recombination may have contributed to the cross-species transmission of the virus.Citation48
The case fatality rate (CFR) of SARS-CoV-2 has been reported to be age-dependent, with higher rate in the elderly population, especially men, and with an overall interim CFR of approximately 1%–3%. The number of asymptomatic individuals could be much higher than the official case number.Citation49 Persons of all ages are susceptible to COVID-19. Large droplets generated by symptomatic patients, including those generated before the onset of symptoms, as well as asymptomatic people during coughing and sneezing can transmit the infection.Citation50 The viral loads were found to be higher in the nasal cavity than in the throat with no difference in viral burden between symptomatic and asymptomatic people.Citation51 The virus can remain viable on the inanimate surfaces for days under favorable atmospheric conditions. It can be destroyed in less than a minute using common disinfectants such as sodium hypochlorite, hydrogen peroxide etc.Citation52 The virus may be present in the stools and contaminated water supply and it has been hypothesized that it may be subsequently transmitted via aerosolization or faeco-oral route.Citation53
Infection source, transmission route, and susceptible population are the three vital elements for the emergence of an infectious disease.Citation54 In the current COVID-19 pandemic, infected patients are the main source of infection and they produce a huge quantity of virus in the upper respiratory tract during the prodromal period.Citation51 As the patients exhibit mild clinical symptoms during the incubation period, their mobility leads to the spread of infection. Asymptomatic carriers can also be a source of infection.Citation50 The incubation period of the disease varies from 1–14 days and could reach up to 24 days, making it difficult to screen for infections. Additionally, the disease is mainly spread through respiratory droplets and contact, with the possibility of aerosol transmission in a closed environment.Citation55
Infections among health workers confirmed the high infectivity of the disease.Citation56 Nosocomial transmission has also been reported. Infections among health workers has accounted for 3.83% of the total infections.Citation38 Personal protective equipments (PPE), including fluid-resistant gown, gloves, eye protection, full face shield, and fit-tested N95 respirators, is necessary to ensure the safety of healthcare workers who need to be in contact with critically ill patients with confirmed or suspected SARS-CoV-2 infection.Citation57 The factors influencing the zoonotic events need to be properly understood in an attempt to limit future outbreaks.
A retrospective cohort study was conducted on 201 patients admitted to Wuhan Jinyintan Hospital with confirmed COVID-19 pneumonia.Citation58 The median age of the patients was 51 years, and 128 (63.7%) patients were men. Of the 201 patients, 84 patients (41.8%) developed ARDS, and of these 84 patients, 44 patients (52.4%) succumbed and had co-morbidities such as hypertension in 27.4% patients and in 13.7% patients and diabetes in 19.0% patients and 5.1% patients, with and without ARDS, respectively. The risk factors associated with the development of ARDS and with progression from ARDS to death included older age (hazard ratio (HR), 3.26; 95% CI, 2.08–5.11; and HR, 6.17; 95% CI, 3.26–11.67, respectively), neutrophilia (HR, 1.14; 95% CI, 1.09–1.19; and HR, 1.08; 95% CI, 1.01–1.17, respectively), and organ and coagulation dysfunction (e.g., higher lactate dehydrogenase (HR, 1.61; 95% CI, 1.44–1.79; and HR, 1.30; 95% CI, 1.11–1.52, respectively). High fever (≥39°C) was also associated with higher likelihood of development of ARDS and lower likelihood of death. Older age was associated with greater risk of development of ARDS and death likely owing to less rigorous immune response. Although high fever was associated with the development of ARDS, it was also associated with better outcomes among patients with ARDS.Citation58
Travel-related cases were the main source of COVID-19 cases during the early stages of the current epidemic in Italy;Citation59 however, later it became dominated by local transmission. The CFRs in China and Italy were identical at 2.30.
Status of vaccine development
Given the severity of COVID-19, vaccines and therapeutics are urgently needed to tackle this novel virus. Currently, no human CoV vaccine has been approved. In addition, the safety of many technologies used (production platforms, vectors, and so on) need to be tested thoroughly; 23 candidate vaccines are under clinical evaluation, while 140 are under pre-clinical evaluation stage. Moreover, as of now, 75 countries have expressed their partnering interests to protect the populations through joining the COVAX- a unique facility to guarantee fair and rapid access to COVID vaccines globally. The target for the vaccine, the S protein, has been identified. This is usually followed by two important steps typically needed before bringing a vaccine into clinical trials. The vaccine needs to be tested in appropriate animal models to study its protection ability. However, animal models for SARS-CoV-2 might be complex to develop. The virus did not grow in wild-type mice, and it has been found to induce mild disease in transgenic animals expressing human angiotensin-converting enzyme 2 (ACE2).Citation60 Pathogenicity studies are ongoing in ferrets and non-human primates. In the absence of a suitable animal model, serum from vaccinated animals can be tested in in vitro neutralization assays. Secondly, the toxicity of vaccines needs to be tested in animals, e.g., in rabbits. This testing has to be performed in research facilities; however, good laboratory practice compliance may take 3–6 months to complete.
The results obtained from the trials of SARS-CoV vaccines, performed with an inactivated virus vaccine and a spike-based DNA vaccine were safe and induced neutralizing antibody (NAb) titers.Citation61,Citation62 Some neutralizing monoclonal antibodies (nMAbs) isolated against SARS-CoV, like CR3022,Citation63,Citation64 can cross-react to the RBD of SARS-CoV-2 suggesting that SARS-CoV-1 vaccines might cross-protect against SARS-CoV-2.
The sequence identity of the RBD is reported to be 73.5% between SARS-CoV-1 and SARS-CoV-2.Citation65 However, only 47.8% identity has been reported in the most variable region of RBD, viz., receptor-binding motif irrespective of the similar receptor-binding mechanism of SARS-CoV-2 and SARS-CoV.Citation66-68 However, the conserved amino acid sequences between the RBDs of SARS-CoV-2 and SARS-CoV suggest that these RBDs may produce cross-reactive antibodies; but the production of antibodies with cross-reactive potential is unknown to date.Citation69 In addition, the RBD of SARS-CoV-2 is considered as a potential antigen with the probability of inducing abundant Nabs against SARS-CoV-2; hence, may be used as a crucial candidate for subunit vaccine development. Moreover, RBM-specific nMAbs prevent SARS-CoV-2 infection by blocking ACE2 receptor interactions, potentially making it a promising passive antibody-based agent in the absence of a COVID-19 vaccine.Citation69
The SARS-CoV-2 vaccines underway
A recombinant subunit vaccine based on the trimeric S protein (S-Trimer) of SARS-CoV-2 is under pre-clinical testing.Citation70 Subunit vaccines using the “molecular clamp” (a polypeptide that stabilizes a surface protein and improves recognition of the correct antigen) are being developed at The University of Queensland.Citation71 Furthermore, a DNA plasmid-based vaccine, viz., INO-4800, encoding the S protein is being developed by Inovio Pharmaceuticals. Moreover, the INO-4800 vaccine is delivered in healthy individuals by two intradermal injections followed by electroporation of the vaccine.Citation72 Pre-clinical trials for the DNA vaccine (INO-4800) against COVID-19, which induces activation of T cells by delivering DNA plasmids that express the SARS-CoV-2 spike,Citation73 have been started by Inovio Pharmaceuticals in collaboration with Beijing Advaccine Biotechnology with the advantages to produce therapeutic antibodies and activate immune cells via intradermal administration into the patient. In addition, Inovio pharmaceuticals enrolled 40 healthy individuals for the Phase 1 clinical trial of the INO-4800 vaccine. Furthermore, after attaining immunogenicity data along with safety evaluation from Phase 1 trial, Inovio pharmaceuticals is planning to advance the INO-4800 vaccine to Phase 2 trial as soon as possible along with the production of one million doses by the end of the year 2020 for emergency use and additional trials if required (INO-4800 DNA Coronavirus Vaccine, 2020).Citation74 Another mRNA vaccine mRNA-1273, encoding viral spike (S) protein of SARS-CoV-2 has entered Phase 1 clinical trials.Citation75 This has been designed in silico, which would enable rapid development and evaluation of vaccine efficacy.Citation76 A COVID-19 vaccine using the Hyleukin-7 platform technology, which enhances immune responses by the fusion of interleukin-7 (IL-7) to hyFc is being developed.Citation77
An mRNA-based vaccine (mRNA1273-COVID-19 vaccine) expressing target antigen in vivo in the vaccine after injection of mRNA encapsulated in lipid nanoparticles is currently under Phase 1 clinical trial (ClinicalTrials.gov: NCT04283461). Moreover, the mRNA1273-COVID-19 vaccine encodes a full length, prefusion stabilized S protein, and reached directly to a clinical trial in record 69 days without any pre-clinical testing due to its highly safe nature.Citation72 Additional approaches in the pre-clinical stage include recombinant-protein-based vaccines (focused on the S protein), viral-vector-based vaccines (focused on the S protein), DNA vaccines (focused on the S protein), live-attenuated vaccines, and inactivated virus vaccines. All these platforms have advantages and disadvantages, and it is not possible to predict which strategy will be faster or more successful.Citation78
In addition, a Chimpanzee Adenovirus Vector (ChAdOx1) based vaccine developed against SARS-CoV-2 by Oxford’s Jenner Institute has progressed to Phase 3 clinical trials. However, the trials mainly aimed to study its reactogenicity, tolerability, and safety along with immunogenicity in 510 volunteers but the vaccine is also being evaluated for its efficacy to prevent SARS-CoV-2 infection (NCT04324606).Citation72,Citation79 Moreover, the ChAdOx1 is a non-replicating virus with one or a few encoded antigens and the vaccine may generate a strong immune response even after one dose hence it can be safely used in older individuals, children, and people with co-morbidities.Citation72,Citation79 As per reports, another adenovirus vector-based vaccine, viz., Ad5-nCoV is being developed by CanSino Biologics of China, which is a genetically engineered vaccine candidate and uses a replication-defective adenovirus type 5 (Ad5) as a vector to deliver the S protein gene of SARS-CoV-2. Moreover, the Ad5-nCoV is reported to be the most advanced DNA vaccine candidate at present and has already completed the Phase 2 trial. Furthermore, the company has started enrolling healthy volunteers of more than 18 years of age for the next phase, randomized, double-blinded, and placebo-controlled clinical trials.Citation80 However, the Ad5-nCoV vaccine has been declared a top contender for SARS-CoV-2 vaccine by the WHO but the scientists are worried about the immunity among the people against the Ad5 vector attributed to the possibility of vaccine failure along with associated harmful effects as observed earlier in a trial conducted by Merck for an Ad5-based HIV vaccine.Citation80,Citation81
An inactivated-adsorbed COVID vaccine manufactured by Sinovac in healthcare professionals (Profiscov) are under Phase 3 clinical trials (NCT04456595). The study will be estimated to be conducted over 8870 participants as a double-blind trial with randomly allocated participants to placebo as well as vaccine groups (1:1). The vaccine candidate will be tested for its efficacy, safety and immunogenicity.Citation82
Immunotherapy
Immunotherapy is regarded as an effective method for the clinical treatment of infectious diseases. Various attempts to develop immunotherapy for COVID-19 have included plasma therapy, polypeptide hormone for the maturation of T cells, immunoglobulins, ACE2 immunoadhesin, and a monoclonal antibody against interleukin-6.Citation9 Several approaches () have been suggested to control infections of SARS-CoV-2, including vaccines, viral-vectors, nanoparticles, inactivated whole virus, DNA as vaccines, monoclonal antibodies, oligonucleotides, peptides, interferon, and small molecule drugs.Citation89,Citation90 The humoral immune response is crucial for preventing viral infections.
Table 2. Vaccines and immunotherapy potentials of different approaches
Passive immunotherapy is important in the short-term protection and prevention of viral infections. Many drawbacks associated with serum therapy and intravenous immunoglobulin preparations in terms of specificity, purity, low risk of blood-borne pathogen contamination, and safety may be overcome by the use of monoclonal antibodies.Citation91-93 The effective treatment options against SARS-CoV-2 can directly interrupt any stage of the viral life cycle or the receptor proteins located in the host cell surface to restrain the virus from binding, thereby blocking viral attachment and entry. These can be accomplished by using peptidic fusion inhibitors, anti-SARS-CoV-2 nMAbs, anti-ACE2 monoclonal antibodies, and protease inhibitors.Citation7
Passive antibody therapy can be an important approach to limit COVID-19 epidemics. The viral replication and disease severity can be reduced by passive immunization with an antibody that can recognize epitopic regions in the foreign virus particle.Citation7 Monoclonal antibodies represent the major class of biotherapeutics for passive immunotherapy to fight against viral infection. The therapeutic potential of monoclonal antibodies has been well-recognized in the treatment of many diseases. The blood of infected patients can be the source of antibodies for passive immunotherapy. The convalescent sera of infected patients may be effective in neutralizing the virus and preventing further infection in humans. The early administration of convalescent plasma or hyper-immune immunoglobulin from patients having significant antibody titers can likely reduce the viral load and disease mortality as evidenced by prior experience in treating other viral infections such as influenza, SARS, MERS, and Ebola.Citation94-97 It has been proven that SARS-CoV-2 uses host receptor, ACE2, for its attachment and entryCitation28 as reported earlier in SARS-CoV.Citation98 Hence, the therapies for SARS-CoV can be extrapolated and used for SARS-CoV-2. The virus entry could be blocked by specific nMAbs either against the RBD in the spike protein or specific antibody that binds to ACE2. In addition, RBM-specific nMAbs were reported to block the ACE2 receptor interactions and subsequently the SARS-CoV-2 infection, hence could be potentially used as a crucial passive antibody-based agent for COVID-19 in the absence of a specific vaccine.Citation66 Suitable expression systems such as mammalian, yeast, or plant could be used to clone and express the sequences of monoclonal antibodies effective against SARS-CoV, and recombinant monoclonal antibodies could be tested against SARS-CoV-2. Plant expression systems could be considered for the rapid production of monoclonal antibodies in a short time at an affordable cost.Citation7,Citation99,Citation100
Monoclonal antibody therapy is one of the best types of passive immunotherapy. A human IgG1 monoclonal antibody, CR3014, has been generated and found to be reactive with whole inactivated SARS-CoV and could be used as prophylaxis for SARS-CoV infection in ferrets.Citation60
Viral infectivity is reduced by NAbs, which bind the surface epitopes of viral particles and block the entry of the virus to an infected cell.Citation101 NAbs elicit their protective activities by preventing the attachment of the virion to their receptors on targeted cells, causing aggregation of virus particles and by lysis of viruses through the constant (C) region of antibody-mediated opsonization or complement activation.Citation102
Explication of the immunopathogenesis of SARS-CoV-2 is useful for developing passive antibody therapy, designing vaccines, and understanding of clinical drug interventions. The human-to-human transmission of SARS-CoV-2 may be enhanced by the high affinity of the S protein for human ACE2, and the S protein might be the main target for antibody-mediated neutralization.
Both the innate and adaptive immune responses are involved in resistance to SARS-CoV infections.Citation103 Excessive proinflammatory cytokine responses are induced because of the activation of dendritic cells and macrophages by SARS-CoV.Citation104 Generally, the levels of IFN-γ, IL-1β IL-6, IL-12, IL-8, MCP-1, and IP-10 are increased in the early phase of infection and later reduced in the recovery stage.Citation105 Notably, T- cytopenia was recorded in the CD4+ and the CD8+ populations as evidenced by flow cytometry analysis, which inversely correlated with increased serum levels of the proinflammatory cytokines IL-6, IL-10, and TNF-α. A progressive increase in the expression of programmed cell death marker-1 (PD-1) and T cell immunoglobulin and mucin domain 3 (Tim-3) was observed as patients (n = 14) deteriorated from prodromal to symptomatic COVID-19 requiring intensive care.Citation106
Uncontrolled systemic inflammation or cytokine storm results in severe illness, which has been observed in SARS-CoV-2 infection. The inflammatory cytokines and chemokines (IL-1β, IFN-γ, IP-10, and MCP-1) were upregulated.Citation107,Citation108 T cell-mediated responses in SARS-CoV infection have been well elucidatedCitation109 and both CD4+ and CD8 + T-cells have been proven to provide broad and long-term protection. Elucidation of T-cell-mediated response in SARS-CoV-2 infection may provide important hints for the design of a vaccine composed of viral structural proteins.
A model in which complement activation in the lung and in other organs is a critical host mediator of SARS-CoV-2-induced development of atypical ARDS and thromobotic microangiopathy (TMA) has been suggested.Citation110 The complement activation has been said to occur primarily in the lower airways and result in the release of C5a into the circulation. It activates proinflammatory immune cells as a key mechanism that drives the “cytokine and chemokine storm” associated with fatal lung injury and TMA development. Thus, for alleviation of the proinflammatory effects, reduce lung pathology, and increase the survival of COVID-19 patients, C5a should be targeted.Citation110
Perspectives on the development of neutralizing antibodies against SARS-CoV-2
Convalescent plasma is chosen when there are no specific vaccines or drugs available for emerging infection‐related diseases.Citation111 The feasibility of convalescent plasma therapy as well as its safety and clinical efficacy in critically ill MERS patients was testedCitation96 and observed to be of immunotherapeutic potential for the treatment of MERS-CoV infection. The use of convalescent plasma from recovered SARS patients had been used for treating other SARS patients.Citation112,Citation113 Notably, the World Health Organization has suggested the use of convalescent plasma or serum under Blood Regulators Network when vaccines and antiviral drugs were unavailable for an emerging virus.Citation83 Use of plasma from the convalescent patients might be the simplest and most direct approach to combat SARS-CoV-2 during the outbreak.Citation85 Polyclonal NAbs that are induced in convalescent patients would be effective in treating SARS-CoV-2.Citation34 Earlier, SARS and Ebola patients were treated using convalescent plasma.Citation112,Citation114 The SARS-CoV-2 ‘S’ protein forms an important target for developing NAbs to block binding and fusion of SARS-CoV-2.
A cocktail antibody approach for SARS-CoV-2 could be undertaken as concoction of NAbs has shown the stronger neutralization than alone in treatment of both Ebola and SARS viruses.Citation115,Citation116 Therefore, generation of NAbs targeting different epitopes on SARS-CoV-2 would be a practical approach.
Effects of COVID-19 on current vaccinations
The COVID-19 pandemic has encouraged intense debates on current (and future) preventive measures, including vaccination. A topic of intense debate and a matter of scientific interest for future research is the role of bacillus Calmette-Guérin (BCG) vaccination in this situation.Citation117 An analysis of data on COVID-19 in countries with BCG vaccination and countries without such a program revealed a daily incidence of 0.8 per million compared with 34.8 per million, respectively. The respective data on the mortality were 0.08 and 34.8 per million, the crude case fatality rate was 4.1% and 5.1% in countries with BCG vaccination and countries without BCG vaccination.Citation117
The innate immune system and trained immunity can be considered in the fight against viruses including COVID-19.Citation118 It has been shown that BCG vaccination before influenza vaccination in healthy individuals resulted in a pronounced antibody response against influenza A (H1N1) compared to the placebo.Citation119 Based on these observations, BCG vaccination trials have been initiated to fight infections such as COVID-19, particularly in the elderly population, and to prevent severe COVID-19 infection in health care workers.Citation117 Two randomized-controlled trials are currently testing BCG vaccination for COVID-19 prevention in Australia (NCT04327206) and the Netherlands (NCT04328441).
Conclusions and perspective
Animal health surveillance systems have an important role in anticipating, detecting and CONTAINING outbreaks of emerging diseases. There is an urgent need to integrate these with human public health surveillance systems and ecological systems, underlining the essence of ‘one health’ concept. In the era of globalization, it is difficult for any country to neglect or hide an emerging epidemic. The recent and past occurrences of coronavirus outbreaks do not seem to be inconsequential but a result of inappropriate human activities. To have a better understanding on the ecology of CoVs and further to prevent its animal-to-human transmission and epidemics in the future, continuous surveillance in mammals and birds is essential.
Approximately 60% of emerging transferable diseases originate from animals, and 70% of these are reported to originate from wild animals, indicating that the unrestricted wildlife trade might enhance the risks of emerging infections. There have been rising calls from different countries to permanently ban wildlife markets and trades. These actions, partly, would help to protect human lives from future pandemics, like COVID-19. Therefore, it is indispensable to globally ban wildlife markets and trades considering national security, biosafety, and public health.
The development of therapeutic NAbs against SARS-CoV-2 may offer benefits for the control of the current pandemic and the possible reemergence of the virus in the future, and their development, therefore, remains a high priority. Vaccines are being developed rapidly; however, they will likely arrive too late to affect the first wave of the pandemic. Establishing an animal model of infection and disease pathogenesis is imperative for understanding several essential elements of a viral disease in the infected host, including host tropism, immune responses, and modes of transmission, as well as for the progression of therapeutic development.
A detailed understanding of the pathogenesis of COVID-19 might increase opportunities for the realistic design of vaccines and immunotherapies for the novel SARS-CoV-2.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Disclosure of potential conflicts of interest
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
Additional information
Funding
References
- Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, thrusting corona viruses into the spotlight. Viruses. 2019;11:59.
- Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, Rubino S. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. 2020;14:254–64.
- Kelvin DJ, Rubino S. Fear of the novel coronavirus. J Infect Dev Ctries. 2020;14:1–2.
- Lau SKP, Luk HKH, Wong ACP, Li KSM, Zhu L, He Z, Fung J, Chan TTY, Fung KSC, Woo PCY. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1542.
- Singhal T. A review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87:281–86.
- Shanmugaraj B, Malla A, Phoolcharoen W. Emergence of novel Coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9:148.
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38:10–18.
- Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30:313–24.
- Amin Jafari A, Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int Immunopharmacol. 2020;83:106455.
- Cevik M, Bamford C, Ho A. COVID-19 pandemic – A focused review for clinicians. Clin Microbiol Infect. 2020. doi:https://doi.org/10.1016/j.cmi.2020.04.023.
- de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ Host factors in coronavirus replication. Current Topics in Microbiology and Immunology. 2018; 419:1–42
- Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:10591.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Hu Y, Song ZG, Tao ZW, Tian JH, Pei YY, et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. BioRxiv. 2020. doi:https://doi.org/10.1101/2020.01.24.919183
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372.
- Hu D, Zhu C, Ai L, He T, Wang Y, Ye F, Yang L, Ding C, Zhu X, Lv R, et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 2018;7:1–10.
- Liu P, Chen W, Chen J-P. Viral metagenomics revealed sendai virus and Coronavirus infection of Malayan Pangolins (Manis javanica). Viruses. 2019;11:979.
- Lam TT, Jia N, Zhang YW, Shum MH, Jiang JF, Zhu HC, Tong YG, Shi YX, Ni XB, Liao YS, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020;583:282–85.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368:1016–20.
- Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, et al. Infection and rapid transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. 2020;27:704–09.
- Gollakner R, Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Vet Ital. 2020;56:11–12.
- Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y, Wang X. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27:119–29.
- Paules CI, Marston HD, Fauci AS. Coronavirus infections - more than just the common cold. JAMA. 2020;323:707.
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–66.
- Deng J, Jin Y, Liu Y, Sun J, Hao L, Bai J, Huang T, Lin D, Jin Y, Tian K. Serological survey of SARS‐CoV‐2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transbound Emerg Dis. 2020;00:1–5.
- Molenaar RJ, Vreman S, Hakze-van der Honing RW, Zwart R, de Rond J, Weesendorp E, Smit LA, Koopmans M, Bouwstra R, Stegeman A, et al. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Vet Pathol. 2020;300985820943535. doi:https://doi.org/10.1177/0300985820943535.
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020;25:2001005.
- OIE. Letter to OIE about the COVID-19 situation in Denmark;2020 July 3 [accessed 2020 July 18]. https://www.oie.int/fileadmin/Home/MM/Update_1_Letter_to_OIE_about_the_COVID-19_situation_in_Denmark.pdf
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1−e9.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–69.
- Wan Y, Shang J, Graham R, Baric RS, Li F. receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol. 2020;94:e00127−20.
- Zhang T, Wu Q, Zhang Z. Pangolin homology associated with 2019-nCoV. bioRxiv. 2020. doi:https://doi.org/10.1101/2020.02.19.950253.
- Yang Y, Peng F, Wang R, Guan K, Jiang T, Xu G, Sun J, Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
- Kluytmans-van den Bergh M, Buiting A, Pas S, Bentvelsen R, Bijllaardt W, van Den, Oudheusden AV, Rijen MV, Verweij J, Koopmans M, Kluytmans J. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv. 2020. 10.1101/2020.03.23.20041913.
- Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q, Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–43.
- Zhang J, Dong X, Cao Y, Yuan Y, Yang Y, Yan Y, Akdis CA, Gao Y. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730–41.
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92:568–76.
- Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858.
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90.
- Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory Coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–90.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:212–13.
- Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of ocular findings of patients with Coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575.
- General Office of National Health Commission; General Office of National Administration of Traditional Chinese Medicine. Diagnostic and treatment protocol for Novel Coronavirus Pneumonia; (Trial version 6); 2020. [accessed 2020 February 20]. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml
- Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D, Behrens P, Böddinghaus B, Götsch U, Naujoks F, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–80.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–207.
- Sun J, He W-T, Wang L, Lai A, Ji X, Zhai X, Li G, Suchard MA, Tian J, Zhou J, et al. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26:483–95.
- Ji W, Wang W, Zhao X, Zai J, Li X. Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. J Med Virol. 2020;92:433–40.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY. Early transmission dynamics in Wuhan, China, of Novel Coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207.
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–71.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–79.
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–51.
- World Health Organization. Situation reports; [accessed 2020 April 22]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- Yang Y, Shang W, Rao X. Facing the COVID‐19 outbreak: what should we know and what could we do? J Med Virol. 2020;92:536–37.
- Medicine, G.O.o.N.H.C.O.o.S.A.o.T.C., The diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (Trial Version 6). 2020: p. 1–5.
- Li X, Zai J, Wang X, Li Y. Potential of large “first generation” human‐to‐human transmission of 2019‐nCoV. J Med Virol. 2020;92:448–54.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth Can D’anesthésie. 2020;67:568–76.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43.
- Porcheddu R, Serra C, Kelvin D, Kelvin N, Rubino S. Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020;14:125–28.
- Bao L, Deng W, Huang B, Gao H, Ren L, Wei Q, Yu P, Xu Y, Liu J, Qi F, et al. The pathogenicity of 2019 Novel Coronavirus in hACE2 transgenic mice. bioRxiv. 2020. doi:https://doi.org/10.1101/2020.02.07.939389.
- Lin JT, Zhang JS, Su N, Xu JG, Wang N, Chen JT, Chen X, Liu YX, Gao H, Jia YP, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12:1107–13.
- Martin JE, Louder MK, Holman LA, Gordon IJ, Enama ME, Larkin BD, Andrews CA, Vogel L, Koup RA, Roederer M, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–43.
- Ter Meulen J, van den Brink EN, Poon LLM, Marissen WE, Leung CSW, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, et al. Human monoclonal antibody combination against SARS Coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237.
- Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–85.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–24.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–63. doi:https://doi.org/10.1126/science.abb2507.
- Yi C, Sun X, Ye J, Ding L, Liu M, Yang Z, Lu X, Zhang Y, Ma L, Gu W, et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17:621–30.
- Anon. Clover biopharmaceuticals vaccines programs. [accessed 2020 April 28]. http://www.cloverbiopharma.com/index.php?m=content&c=index&a=lists&catid=42.
- Significant step in COVID-19 vaccine quest. University of Queensland; 2020. [accessed 2020 April 28] https://www.uq.edu.au/news/article/2020/02/significant-step%E2%80%99-covid-19-vaccine-quest.
- Kim YC, Dema B, Reyes-Sandoval A. COVID-19 vaccines: breaking record times to first-in-human trials. NPJ Vaccines. 2020;5:1–3. doi:https://doi.org/10.1038/s41541-020-0188-3.
- Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800; [accessed 2020 Mar 3]. http://ir.inovio.com/news-andmedia/news/press-release-details/2020/Inovio-Accelerates-Timeline-for-COVID-19-DNA-Vaccine-INO-4800/default.aspx.
- INO-4800 DNA Coronavirus Vaccine; 2020 [accessed 2020 May 18]. https://www.precisionvaccinations.com/vaccines/ino-4800-dna-coronavirus-vaccine.
- Moderna’s pipeline; [accessed 2020 April 28] https://www.modernatx.com/pipeline.
- mRNA platform: enabling drug discovery & development; [accessed 2020 April 28] https://www.modernatx.com/mrna-technology/mrna-platform-enabling-drug-discovery-development.
- hyFc platform; [accessed 2020 April 28]. http://www.genexine.com/m21.php.
- Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583‐589.
- COVID-19 vaccine development; 2020; [accessed 2020 May 18]. https://www.ovg.ox.ac.uk/news/covid-19-vaccine-development
- CanSino Bio enrols for Ph 2 trial of COVID-19 vaccine; [accessed 2020 May 18] https://www.biospectrumasia.com/news/37/15850/cansino-bio-enrols-for-ph-2-trial-of-covid-19-vaccine.html
- Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368:14–16. doi:https://doi.org/10.1126/science.368.6486.14.
- World Health Organisation. Draft landscape of COVID-19 candidate vaccines; 2020 July 15 [accessed 2020 July 18]. www.who.int/docs/default-source/coronaviruse/novel-coronavirus-landscape-covid-19-e72692323504f4ce8bb2fe20709e830bd.pdf?sfvrsn=4c9a242c_5&download=true.
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–90.
- Baruah V, Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020;92:495–500.
- Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9:72.
- Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against middle east respiratory syndrome-Coronavirus. Front Microbiol. 2019;10:1781.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254.
- Clover Biopharmaceuticals vaccines programs. Anon 2020; [accessed 2020 April 28] http://www.cloverbiopharma.com/index.php?m
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149–50.
- Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–32.
- Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–34.
- Sui J, Li W, Roberts A, Matthews LJ, Murakami A, Vogel L, Wong SK, Subbarao K, Farzan M, Marasco WA. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J Virol. 2005;79:5900–06.
- Both L, Banyard AC, van Dolleweerd C, Wright E, Ma JKC, Fooks AR. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine. 2013;31:1553–59.
- Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PKS, Peng MY, Wan HL, Chen JH, Hu BS, Perng CL, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–22.
- Chan KH, Chan JFW, Tse H, Chen H, Lau CCY, Cai JP, Tsang AKL, Xiao X, To KKW, Lau SKP, et al. Cross-reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect. 2013;67:130–40.
- Arabi Y, Balkhy H, Hajeer AH, Bouchama A, Hayden FG, Al-Omari A, Al-Hameed FM, Taha Y, Shindo N, Whitehead J, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–54.
- Hiatt A, Whaley KJ, Zeitlin L. Plant-Derived monoclonal antibodies for prevention and treatment of infectious disease. Microbiol Spectr. 2014;2:AID-0004-2012.
- Sainsbury F. Innovation in plant-based transient protein expression for infectious disease prevention and preparedness. Curr Opin Biotechnol. 2020;61:110–15.
- Klasse PJ. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol. 2014;2014:1–24.
- Coughlin MM, Prabhakar BS. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev Med Virol. 2012;22:2–17.
- Li C, Xu X. Host immune responses to SARS Coronavirus in humans. In: S. K. Lal, editor.Molecular biology of the SARS-Coronavirus. Heidelberg, Germany: Springer; 2010. p. 259–78.
- Tseng CTK, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. 2005;174:7977–85.
- Thiel V, Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine Growth Factor Rev. 2008;19:121–32.
- Chiappelli F, Khakshooy A, Greenberg G. CoViD-19 Immunopathology and immunotherapy. Bioinformation. 2020;16:219–22.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061.
- Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, Deng Y, Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–08.
- Oh HL J, Ken-En Gan S, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg Microbes Infect. 2012;1:1–6.
- Jodele S, Köhl J. Tackling COVID-19 infection through complement-targeted immunotherapy. Br J Pharmacol. 2020. doi:https://doi.org/10.1111/bph.15187.
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152‐157.
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46.
- Soo YOY, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, Ng MH, Chan P, Cheng G, Sung JJ. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676‐78.
- Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, Varkey JB, Mehta AK, Lyon III GM, Friedman-Moraco RJ, et al. the use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the united states. Clin Infect Dis. 2015;61:496–502.
- Elshabrawy HA, Coughlin MM, Baker SC, Prabhakar BS. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS One. 2012;7:e50366.
- Davey RT Jr., Dodd L, Proschan MA, Neaton J, Neuhaus Nordwall J, Koopmeiners JS, Beigel J, Tierney J, Lane HC, Fauci AS, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375: 1448–56.
- Hegarty PK, Sfakianos JP, Giannarini G, DiNardo AR, Kamat AM. COVID-19 and Bacillus Calmette-Guérin: what is the Link? Eur Urol Oncol. 2020;3:259–61.
- Daza J, Charap A, Wiklund PN, Sfakianos JP. Role of the innate immune system in the development, progression, and therapeutic response of bladder cancer. Eur Urol Focus. 2020;6:650–52.
- Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, Rimmelzwaan GF, Pickkers P, Netea MG. BCG Vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212:1930–38.
